Back to Journals » International Journal of Women's Health » Volume 16
A Case of Rectal Endometriosis Misdiagnosed as Rectal Malignancy on Three Colonoscopies and Biopsies Sharing a Combined Literature Review
Authors Liang Y, Mei L, Ning Q, Zhang J, Fei J, Dong J
Received 4 November 2023
Accepted for publication 11 January 2024
Published 26 January 2024 Volume 2024:16 Pages 163—174
DOI https://doi.org/10.2147/IJWH.S445280
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Everett Magann
Yufei Liang,1 Lina Mei,2 Qipeng Ning,2 Jiao Zhang,2 Jingying Fei,3 Jie Dong1
1Department of Gynaecology and Obstetrics, Huzhou Maternity & Child Health Care Hospital, Huzhou, People’s Republic of China; 2Department of Digestive, Huzhou Maternity & Child Health Care Hospital, Huzhou, People’s Republic of China; 3Department of Ultrasound, Huzhou Maternity & Child Health Care Hospital, Huzhou, People’s Republic of China
Correspondence: Jie Dong, Department of Gynaecology and Obstetrics, Huzhou Maternity & Child Health Care Hospital, No. 2 East Street, Wuxing District, Huzhou City, Zhejiang Province, People’s Republic of China, Email [email protected]
Background: Endometriosis involves the intestine, and its clinical manifestations are nonspecific and lack of etiological manifestations. The diagnosis is difficult, which often leads to misdiagnosis. We report a case of endometriosis which was misdiagnosed as intestinal malignant tumor after colonoscopy and three biopsies.
Case Presentation: We reported a 42-year-old woman who went to see a doctor because of anal distension. She was examined by three gastrointestinal endoscopists at different levels in different hospitals and underwent biopsy at the same time. Combined with clinical manifestations, imaging examination, endoscopic examination and pathological examination, she was misdiagnosed as intestinal malignant tumor, and partial intestinal resection was performed according to the surgical principle of malignant tumor.
Conclusion: Although there are advanced gastrointestinal endoscopy and imaging techniques, intestinal endometriosis is still easy to be misdiagnosed. As our case report shows, after three colonoscopy and biopsy, it is still misdiagnosed as intestinal malignant tumor. Further research is needed to improve the ability of preoperative diagnosis, which deserves the attention of gastroenterologists and obstetricians and gynecologists.
Keywords: endometriosis, intestinal malignant tumor, colonoscopy
Background
Endometriosis (EM) is the presence of functional endometrial glands and stroma outside the uterine cavity, including the skin, lungs, gastrointestinal tract, urinary system, and central nervous system. Approximately 3–15% of women of reproductive age have varying degrees of EM,1,2 affecting approximately 190 million women worldwide,3 with the majority of EM located in the genital organs, the most abnormal sites being the ovaries, uterosacral ligament, greater ligament, and pelvic peritoneum. The most common site of extra-genital endometriosis is the gastrointestinal tract, accounting for 3.8%–37%.4 Colorectal endometriosis (CEM), a form of deeply infiltrating endometriosis, occurs when endometriosis lesions invade the colorectum and infiltrate the plasma layer by more than 5 mm. Although CEM does not represent a significant proportion of the colonoscopy population, its prevalence is as high as 1% in women of childbearing age.5 Rectal endometriosis, first reported by Dr. Sampson in 1922,6 is a deep infiltrating endometriosis that invades the colorectum and can cause lower gastrointestinal symptoms such as diarrhea, blood in the stool, and periodic abdominal pain, which can seriously affect the quality of life of women of childbearing age. Also due to its infiltrative nature and tendency to produce strictures leading to obstruction, the clinical presentation of intestinal endometriosis is often misdiagnosed as malignancy, especially during surgery, and its appearance may also be indistinguishable from malignancy,7 which may lead to misdiagnosis. In this paper, we report a case of intestinal endometriosis that was misdiagnosed as intestinal malignancy on three colonoscopies and tissue biopsies.
Case Presentation
Patient, female, 42 years old, height 159 cm, weight 61 kg, usually regular menstruation, cycle 30 days, period 3–4 days, medium volume, red color, denied history of dysmenorrhea. 2006 full-term cesarean section, intraoperative hemorrhage, treated with blood transfusion, 2010 cholecystectomy due to “gallbladder stones”. In 1999, she delivered a baby girl at term; in 2006, she delivered a baby girl by cesarean section. On September 22, 2020, she was admitted to the hospital with “lower abdominal pain for 8 hours after vigorous activity”. Emergency ultrasound showed multiple fibroids in the uterus (with ruptured fibroids at the base of the ectopia?) The uterus was irregularly enlarged as large as 2+ months of pregnancy, and a completely convex mass was seen at the base of the uterus, about 9*8*5 cm in size, hard, with anisotropic vascular hyperplasia on the surface and twisted and exposed blood vessels at the tip of the mass. The left anterior wall of the uterus showed a completely convex mass, about 7*6*4 cm in size, soft and cystic in nature, with clear borders, the right wall of the uterus showed a slightly convex mass about 4 cm in diameter, hard and clear borders, the rectal fossa of the uterus was closed, some intestinal tubes and bilateral ovarian adhesions with the posterior wall of the uterus were seen, the bilateral fallopian tubes had no obvious abnormalities with the naked eye, and the umbilical ends were visible. The intraoperative diagnosis: uterine fibroids (multiple) and intestinal adhesions. Intraoperative diagnosis: uterine fibroids (multiple) and intestinal adhesions. After the operation, there was obvious abdominal distension and vomiting, and the standing abdominal plain film suggested: abdominal intestinal distension with fluid flattening (in the shape of “spring” change). The postoperative pathology report suggested: uterine smooth muscle tumor (several), one with edema and deformation, and was discharged 11 days after surgery.
On August 3, 2022 (2-years postoperative), the patient visited the clinic for six months due to anal swelling, the symptoms were not related to menstrual cycle, and a fecal imaging was performed: the distal part of the rectal jug abdomen was seen to be mildly protruding anteriorly during forceful evacuation, with a cystic pouch-like change, about 2.8 cm in length and 0.8 cm in depth. The local intestinal lumen of the sigmoid colon was slightly narrowed, with an upper and lower range of about 3.3 cm, and the margin The lumen of the sigmoid colon was slightly narrowed, with an upper and lower range of about 3.3 cm, and the margin was not well defined, See Figure 1. The intestinal lumen was slightly narrowed, and the endoscope could barely pass through. Conclusion: colonic occupying lesion (whole abdomen enhanced CT is recommended). The intraoperative pictures are shown in Figure 2. Further CT examination suggested that: the intestinal wall at the junction of sigmoid colon and rectum was thickened with mass-like changes, the range was about 4.4cm×2.3cm, the measured CT value was about 49HU, after enhancement, moderate enhancement was seen, the CT value was about 75HU, the plasma membrane surface of the intestinal canal corresponding to the lesion was still smooth; the distribution and morphology of the remaining abdominal intestinal canal did not show obvious abnormal changes, the mesenteric fat gap was still clear, and no obvious enlarged lymph nodes were seen. No obvious enlarged lymph nodes were seen. The bladder was full and no significant abnormal density foci were seen in the bladder. A small cystic hypodense foci, about 2.4 cm × 1.7 cm in size, could be seen in the right adnexal area, and no significant abnormal enhancement was seen after enhancement. The size and shape of the uterus were acceptable, and no significant abnormal enhancement was seen after enhancement. The biopsy report after colonoscopy suggested tubular adenoma (rectum) with mild heterogeneous hyperplasia of the glandular epithelium (low-grade intraepithelial neoplasia). The pathological report is shown in Figure 3. The patient was referred to a general hospital for gastrointestinal surgery, where colon cancer was highly considered and a biopsy was performed, which showed tubular adenoma (rectum) with low-grade intraepithelial neoplasia. The pathological report is shown in Figure 4. The patient refused further treatment and returned to the hospital 2 months later with no significant change in the symptoms of anal distention. After MTD discussion, colonoscopy was performed again with the patient’s informed consent, and the endoscopist was changed. The pathological report of the third colonoscopy biopsy is the same as before, and the pathological report is shown in Figure 5. The lesion was partially excised from the colon with adequate information, and endometriosis was considered in the intraoperative frozen section, and the excised tissue is shown in Figure 6. Immunohistochemical results: CK (AE1/AE3) (+), CK20 (-), CDX-2 (-), CD10 (+), ER (3+), PR (2+), WT-1 (+), p53 (~5%+), Ki-67 (~10%+). The routine pathological report is shown in Figure 7, and the immunohistochemistry is shown in Figure 8. Postoperative symptomatic treatment and recovery was good. Postoperative follow-up until the writing of this manuscript the patient was generally well with no significant discomfort.
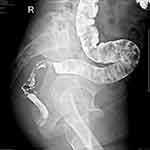 |
Figure 1 Defecography image. |
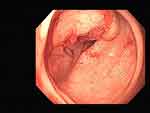 |
Figure 2 First colonoscopy image. |
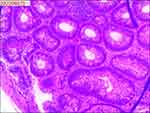 |
Figure 3 Pathological examination of the first colonoscopy biopsy. |
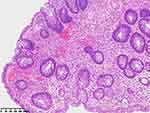 |
Figure 4 Pathological examination of the second colonoscopy biopsy. |
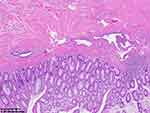 |
Figure 5 Pathological examination of the third colonoscopy biopsy. |
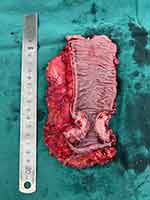 |
Figure 6 Surgical removal of some intestinal tissue samples. |
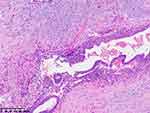 |
Figure 7 Histopathological analysis following partial intestinal resection. |
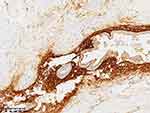 |
Figure 8 Immunohistochemical investigation subsequent to partial intestinal resection. |
Discussion and Conclusion
Endometriosis is defined as the implantation of the endometrium in a location other than the uterine cavity. The pathogenesis of endometriosis is unknown. Several hypotheses have been established to explain the development of endometriosis, including retrograde menstruation and ectopic transplantation, corpora cavernosa chemotaxis, medically induced injury, autoimmunity, embryonic theory and stem cell origin, genetic susceptibility, and hormones.8,9 The most common sites of endometriosis are the ovaries (60%), uterosacral ligament (60%), and broad ligament (15%).10 Extrapelvic endometriosis can occur in several sites throughout the body such as the liver, lungs, and pericardium11 (Table 1 shows some of the specific sites of endometriosis reported outside the relevant pelvis), but the most frequent site outside the pelvis is the gastrointestinal tract. Deep endometriosis (DE) is defined as endometrial tissue infiltrating the peritoneum to a depth of at least 5 mm12 and is one of the most aggressive subtypes of endometriosis, often in combination with ovarian and peritoneal endometriosis, with DE lesions prevalent in the posterior pelvis, often involving multiple sites, most commonly the uterosacral ligament, and other relatively common sites including the vaginal-rectal septum, urinary tract, and intestinal canal, etc.13
 |
Table 1 Selected Extra-Pelvic Specific Sites of Endometriosis |
Intestinal endometriosis refers to the infiltration of endometrial glands and stroma from the plasma layer of the intestinal canal to at least the subplasma adipose tissue, whereas colorectal endometriosis is often located in the subplasma and intrinsic muscular layer, with possible involvement of the submucosal layer, but lesions involving the mucosal layer are relatively rare.5 The exact prevalence is not known, but some retrospective studies have shown that intestinal involvement in patients with endometriosis ranges from 5.3% to 12%, with the sigmoid colon and rectum being the most frequently involved sites in the GI tract,28 accounting for 50%–70%.29 Also in general, ectopic endometrial tissue in the rectum is usually located in the muscular layer and rarely in the submucosa, and the rectal mucosa is usually not involved.30 The pathology did not suggest endometriosis in any of the 3 colonoscopies + tissue biopsies, which was considered to be caused by invasion into the intestinal lumen but not penetration of the mucosal layer.
Endometriosis invading specific organs is often associated with corresponding symptoms. The clinical manifestations of intestinal endometriosis are mainly blood in the stool, abdominal masses, intestinal obstruction, followed by paroxysmal lower abdominal pain, urgency, diarrhea, and changes in bowel habits.31 Colorectal endometriosis is rare and usually presents with nonspecific abdominal pain, dyspareunia, dry stools, catamenial or painful defecation.32 In the initial stage of colorectal involvement, bowel symptoms such as painful defecation, abdominal discomfort and diarrhea may occur; when the lesion is large or invades the intestinal mucosa, symptoms such as constipation and bloody stools may occur, mostly cyclical, aggravated 1 day before or during menstruation, and sometimes blood in the stool is not cyclical; in advanced patients, complete intestinal obstruction may develop.33 The patient presented with symptoms of intestinal obstruction after emergency dissection, which cannot be completely excluded in the current analysis as being due to intestinal endometriosis, while the patient had anal cramping but no other obvious clinical symptoms, and the association between anal cramping and menstrual cycle changes was not obvious, and these may also be the reasons why endometriosis was not first considered in the preliminary diagnosis. The obstruction in rectal endometriosis is mainly associated with transmural involvement forming a stricture or mass, which is mainly due to severe smooth muscle hypertrophy around the endometrial lesion present in the intrinsic myometrium. This phenomenon is similar to the formation of uterine adenomyosis.28 The authors reviewed the relevant literature and made a demonstration for the location, symptoms and treatment modalities of some intestinal endometriosis, as detailed in Table 2. Table 2 Some reported sites, symptoms and treatment modalities of intestinal endometriosis.
 |
Table 2 Some Reported Sites, Symptoms and Treatment Modalities of Intestinal Endometriosis |
The fact that endometriosis involves the intestine, whether it occurs in the colorectum or penetrates the muscular layer to reach the mucosal layer, its clinical manifestations are nonspecific and therefore its diagnosis can be very difficult. The lack of etiologic manifestations of intestinal endometriosis makes its differential diagnosis with other diseases challenging47 and needs to be differentiated from chronic enteritis, ulcerative colitis, Crohn’s disease, appendicitis, isolated rectal ulcer syndrome and especially malignancy, because although they share many clinical symptoms, the treatment approaches are very different.29 For rectal and sigmoid endometriosis diagnosis can be made by transvaginal ultrasound (TVUS), transrectal ultrasound (TRUS) and, when a rectal mass is present, abdominal CT scan can be used to evaluate rectal endometriosis with variable sensitivity. In the patient reported in this paper, CT suggested thickening of the intestinal wall but the mucosal surface was intact. Combined with the relevant imaging indexes CT diagnostic opinion also considered the possibility of malignancy, which also had some influence on the late misdiagnosis. And other related reports suggest that magnetic resonance imaging and ultrasound may be more sensitive in detecting rectal endometriosis,48 especially pelvic MRI, which is more commonly used abroad in recent years in the examination of deeply infiltrating endometriosis because of its superiority to CT in imaging the soft tissues of the pelvis, which can provide information on the depth of intestinal wall infiltration, the extent of intestinal wall lesions, and the distance between intestinal lesions and the anal verge,49 Del Frate C et al50 summarize the solid mass type presents with low-intensity signal on T1-weighted images mixed with small patchy areas of strong signal that are small foci of hemorrhage and homogeneous low signal on T2-weighted images that intensify on enhancement, consistent with a large amount of fibrous tissue in the lesion on histologic examination. In a 2016 review51 concluded that no imaging test is superior to surgery in the diagnosis of endometriosis. However, with the development of imaging techniques such as ultrasound, imaging is becoming more sensitive in the diagnosis of endometriosis.52
With the development of intestinal endoscopic techniques, new advances have been made in the diagnosis of intestinal endometriosis. Since the site of colorectal endometriosis is the anterior rectal wall and the anterior lower border of the sigmoid colon, growing from the plasma membrane layer into the intestinal lumen, enteroscopic manifestations are mostly signs of submucosal or extra-mural masses, which may be normal on the mucosal surface, but may show congestion, edema and superficial ulcers, sometimes inflammatory polyps, occasionally dark purple hemorrhagic spots in the submucosal layer, eccentric mucosal wrinkles, aggregates in the invaded intestinal canal, and the presence of fibrosis in Jiang W et al53 described 15 patients with intestinal mucosal endometriosis, whose mucosal changes were mainly characterized by (1) replacement of the intestinal mucosal surface epithelium by endometrial glands, (2) mingling of intestinal mucosal glands with endometrial glands, and (3) mixing of endometrial glands with intestinal mucosal glands. The glands are surrounded by endometrial interrogative cells, and the interrogative mass is seen to have hemorrhagic and edematous changes. The endometrial glandular epithelial luminal margin may be mildly serrated and irregular, lined with a single layer of epithelium, with tall columnar cells with cigar-shaped growing nuclei, acidophilic cytoplasm, no cellular secretion, ciliated (tubal epithelium) metaplasia, and other metaplasia such as squamous metaplasia, or significantly dilated glandular lumen with flattened lining epithelium. However, some endoscopic presentations lack specificity, so some physicians choose to perform simultaneous biopsies at endoscopy for suspicious sites. Endoscopic biopsies often obtain tissue that only reflects chronic injury but may lack a basis for a definitive diagnosis of endometriosis lesions, so this may lead to misdiagnosis.47 Kim et al54 reported a positive rate of colonoscopic biopsies of only 47.0% (8/17), but their study hypothesized that increasing the number of biopsies may help to improve the positive rate of biopsies. The superficial mucosa of endometriosis lesions has more obvious inflammatory manifestations, and in some cases cryptitis, crypt abscesses and other pathological manifestations similar to inflammatory bowel disease can be seen,53 thus having an impact on the interpretation of pathological findings. The colonoscopic presentation and biopsy pathology of endometriosis lesions can be indistinguishable from inflammatory bowel disease, ischemic bowel disease, or even colonic malignancy, which may lead to unnecessary surgical treatment. Studies have reported that immunohistochemistry (CK7, ER, CK20, and CDX2) can help improve the diagnosis in cases of diagnostic difficulties.31 The patient underwent 3 colonoscopies, and although the endoscopists were different on all three occasions, the microscopic manifestations all showed raised intestinal mucosal lesions, and the microscopic manifestations all first considered intestinal malignancy, because the lesions invaded the muscular layer but did not reach the mucosal layer, so although the specificity of the endoscopic manifestations was not obvious compared with malignancy, and the pathological results of all 3 biopsies did not suggest endometriosis, they were all diagnosed as low-grade This is consistent with reports suggesting some difficulties in pathological diagnosis. In addition, we need to pay attention to another thing. Some literatures reported that there was stool bleeding after surgical resection of ovarian endometrial cyst, and colonoscopy suggested that there was a lump blocking the intestinal canal, and finally hysterectomy, double appendectomy, rectal excision and colostomy were performed, but postoperative pathology did show endometriosis with adenocarcinoma.55 We know that although the malignant transformation of pelvic endometriosis affects less than 1% of endometriosis women, there is a lack of reliable biomarkers in the process of ovarian atypical endometriosis developing into ovarian cancer,56 so it is difficult to diagnose. Therefore, when patients with ovarian endometriosis have intestinal related diseases, it is necessary to consider whether there is a possibility of malignant transformation of ovarian endometriosis.
The treatment of endometriosis aims to reduce and eliminate lesions, alleviate and eliminate pain, improve and promote fertility, and reduce and avoid recurrence,57 Currently, drugs available for the treatment of endometriosis include denogestrel, oral contraceptives, GnRH-A and non-steroidal anti-inflammatory analgesics, especially denogestrel because it can effectively control pain without producing hypoestrogenemia. It is now the first-line treatment drug.58 However, medications can reduce symptoms but do not completely cure the disease and are often associated with side effects, while progesterone and gonadotropin-releasing hormone analogs can be used for women with intestinal strictures <60% and who want to avoid surgery.59 When hormonal therapy fails, surgery is the treatment of choice. Especially when the patient presents with intestinal obstruction or severe endometriosis, the only treatment is surgical excision due to myocyte proliferation and fibrosis of the endometriotic tissue in the intestinal muscular layer.7 For the treatment of intestinal endometriosis, total anterior resection is feasible for single infiltrating nodules, approximately 30 mm in size and less than one-third of the intestinal circumference, whereas more extensive infiltrating lesions require formal excision and anastomosis. The most common treatment for endometriosis in the rectosigmoid colon is currently partial resection.60 Bowel resection is also considered the recommended treatment for patients with bleeding and suspected malignancy,61 the goal of surgical treatment is to remove all visible areas of endometriosis lesions and for some authors it should be as radical as possible, just like cancer surgery,62 including rectal segmental resection,63 but there are no more scientific studies reporting on the extent of bowel resection and whether the extent of resection is required is the same as for colon There is no uniformity in the extent of resection for rectal cancer, and we know that inappropriate radical surgery can result in permanent stoma and reduced quality of life, so it is more important to be able to distinguish intestinal endometriosis from intestinal cancer preoperatively, and adequate biopsy specimens should be examined histopathologically to confirm the diagnosis and guide treatment. In contrast, another opinion is that when endometriosis invades the mucosal layer, conservative treatment such as lesion excision (ie, removal of endometriosis nodules in contact with the intestinal plasma membrane without opening the rectal lumen) should be preferred to reduce the associated functional sequelae.64 And in a study of 26 patients with rectosigmoid endometriosis, issue 42.3% had lymph node involvement and 36.3% had lymphovascular invasion,65 while on rapid frozen section examination, endometriosis in the rectum may be mistaken for cancer because glands in the rectal muscularis may be mistaken for cancer infiltration,66 which further increases the difficulty of clinical diagnosis and treatment. However, we need to know that the chance of malignant endometriosis is about 0.7% to 1% and it occurs less frequently in the colorectum,67 so the choice of surgical approach for this group of patients should be made carefully to avoid avoidable sequelae for the patients.
The clinical symptoms of intestinal endometriosis lack specificity, while the endoscopic performance lacks specificity and the positive pathology rate is low, so it is easy to be misdiagnosed. The case was finally misdiagnosed as intestinal malignant tumor by 3 times of colonoscopy and biopsy, and segmental bowel resection was performed according to malignant tumor. This case reminds clinicians to pay attention to the diagnosis and treatment of intestinal endometriosis, especially to improve the diagnosis before treatment, and reduce the physical trauma and economic burden of patients.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author.
Ethical Standards
Written informed consent was obtained from the patient. This paper was approved by Huzhou Maternity & Child Health Care Hospital (number: 2023-J-072), and we were in accordance with the 1975 Helsinki declaration and its later amendments.
Consent for Publication
Patient informed consent has been obtained.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Zanelotti A, Decherney AH. Surgery and endometriosis. Clin Obstet Gynecol. 2017;60(3):477–484. doi:10.1097/GRF.0000000000000291
2. Arafat S, Alsabek MB, Almousa F, et al. Rare manifestation of endometriosis causing complete recto-sigmoid obstruction:A case report. Int J Surg Case Rep. 2016;26:30–33. doi:10.1016/j.ijscr.2016.07.004
3. Horne AW, Missmer SA. Pathophysiology, diagnosis, and management of endometriosis. BMJ. 2022;379:1756–1833.
4. Nezhat C, Li A, Falik R, et al. Bowel endometriosis:diagnosis and management. Am J Obstet Gynecol. 2018;218(6):549–562. doi:10.1016/j.ajog.2017.09.023
5. Remorgida V, Ferrero S, Fulcheri E, et al. Bowel endometriosis: presentation, diagnosis, and treatment. Obstet Gynecol Surv. 2007;62(7):461–470. doi:10.1097/01.ogx.0000268688.55653.5c
6. Sampson JA. Intestinal adenomas of endometrial type: their importance and their relation to ovarian hematomas of endometrial type (perforating hemorrhagic cysts of the ovary). Arch Surg. 1922;5(1):217–280.
7. Jarmin R, Idris MA, Shaharuddin S, et al. Intestinal obstruction due to rectal endometriosis:a surgical enigma. Asian J Surg. 2006;29(3):149–152. doi:10.1016/S1015-9584(09)60075-0
8. Liu K, Zhang W, Liu S, et al. Hepatic endometriosis: a rare case and review of the literature. Eur J Med Res. 2015;20(1):48. doi:10.1186/s40001-015-0137-1
9. Yang J, Song RJ, C. X, et al. Renal endometriosis tends to be misdiagnosed as renal tumor: a rare case report. Int Surg. 2015;100(2):376–380. doi:10.9738/INTSURG-D-13-00190.1
10. Oats JJ, Boyle J. Llewellyn-Jones Fundamental of Obstetrics and Gynecology.
11. Law YY, Patel R, Yorke R, et al. A case of infiltrative cecal endometriosis with appendiceal obliteration and lymph node involvement. J Surg Case Rep. 2020;2020(10):rjaa396. doi:10.1093/jscr/rjaa396
12. Ledu N, Rubod C, Piessen G, et al. Management of deep infiltrating endometriosis of the rectum: is a systematic temporary stoma relevant? J Gynecol Obstet Hum Reprod. 2018;47(1):1–7. doi:10.1016/j.jogoh.2017.10.005
13. Nicolaus K, Reckenbeil L, Bräuer D, et al. Cycle-related diarrhea and dysmenorrhea are independent predictors of peritoneal endometriosis, cycle -related dyschezia is an independent predictor of rectal involvement. Geburtshilfe Frauenheilkd. 2020;80(3):307–315. doi:10.1055/a-1033-9588
14. Heijink T, Bogers H, Steensma A. Endometriosis of the Bartholin gland: a case report and review of the literature. J Med Case Rep. 2020;14(1):85. doi:10.1186/s13256-020-02424-7
15. Oner A, Karakucuk S, Serin S. Nasolacrimal endometriosis. A case report. Ophthalmic Res. 2006;38(5):313–314. doi:10.1159/000095776
16. Pascoal E, Rogers S, Leonardi M, et al. Case report: extrapelvic endometriosis in the medial thigh. Front Reprod Health. 2021;3:692249. doi:10.3389/frph.2021.692249
17. Aboujaoude N, Iskandar M, Tannouri F. Catamenial hemoptysis: a case report of pulmonary endometriosis. Eur J Radiol Open. 2021;8:100302. doi:10.1016/j.ejro.2020.100302
18. Sherif AM, Musa ER, Kedar R, et al. Subcapsular hepatic endometriosis: case report and review of the literature. Radiol Case Rep. 2016;11(4):303–308. doi:10.1016/j.radcr.2016.08.004
19. Bacher H, Schweiger W, Cerwenka H, et al. Use of anal endosonography in diagnosis of endometriosis of the external anal sphincter: report of a case. Dis Colon Rectum. 1999;42(5):680–682. doi:10.1007/BF02234150
20. Yokota N, Yoshida H, Sakakibara H, et al. A severe vaginal hemorrhage caused by cervical endometriosis. Am J Obstet Gynecol. 2008;199(1):e12–e13. doi:10.1016/j.ajog.2008.02.012
21. Wolthuis AM, Aelvoet C, Bosteels J, et al. Diaphragmatic endometriosis: diagnosis and surgical management--A case report. Acta Chir Belg. 2003;103:5.
22. Christable E, Shivkumaran S, Venkitaraman B, et al. Endometriosis of para-aortic node masquerading a malignancy: a rare occurrence. BMJ Case Rep. 2021;14(7):1757–790X. doi:10.1136/bcr-2020-240750
23. Nigam A. Subcutaneous endometriosis: a rare cause of deep dyspareunia. BMJ Case Rep. 2014;2014(1):1757–790X. doi:10.1136/bcr-2013-202230
24. Newme K, Hajong R, Newme R, et al. A case report on umbilical endometriosis. J Family Med Prim Care. 2021;10(7):2716–2717. doi:10.4103/jfmpc.jfmpc_2375_20
25. Charpentier E, Petit E, Beranger S, et al. Presumption of pericardial endometriosis using MRI:Case report and review of the literature. J Gynecol Obstet Hum Reprod. 2019;48(1):71–73. doi:10.1016/j.jogoh.2018.06.009
26. Patel VC, Samuels H, Abeles E, et al. Endometriosis at the knee. A case report. Clin Orthop Relat Res. 1982;171:140–144.
27. Yang Y, Zhao X, Huang Y, et al. Renal endometriosis mimicking cystic renal tumor: case report and literature review. Front Med. 2021;8:684474. doi:10.3389/fmed.2021.684474
28. Tarjanne S, Sjöberg J, Heikinheimo O. Rectovaginal endometriosis characteristics of operative treatment and factors predicting bowel resection. J Minim Invasive Gynecol. 2009;16(3):302–306. doi:10.1016/j.jmig.2008.12.019
29. Zhao LJ, Wang YH, Zhang JD. A case report:Rectal endometriosis mimicking rectal cancer. Int J Surg Case Rep. 2018;53:137–139. doi:10.1016/j.ijscr.2018.10.021
30. Shi X, Fan C. Endometriosis in the rectum accompanied by hemorrhoids leading to diagnostic pitfalls: a rare case report. BMC Womens Health. 2018;18(1):120. doi:10.1186/s12905-018-0615-z
31. Hui C, Qiuping L, Shaoyuan L, et al. Rectal mucosal endometriosis primarily misinterpreted as adenocarcinoma: a case report and review of literature. Int J Clin Exp Pathol. 2015;8(5):5902–5907.
32. Pramateftakis MG, Psomas S, Kanellos D, et al. Large bowel obstruction due to endometriosis. Tech Coloproctol. 2010;14(Suppl 1):S87–S89. doi:10.1007/s10151-010-0616-x
33. González-Pezzat I, Soto-pérez-de-celis E, García-Lascurain JL. Bowel endometriosis as an unusual cause of rectal bleeding. Am Surg. 2011;77(2):239–241. doi:10.1177/000313481107700233
34. Buldanlı MZ, Özemir İA, Yener O, et al. A rare case of acute mechanical intestinal obstruction:Colonic endometriosis. Ulus Travma Acil Cerrahi Derg. 2020;26(1):148–151. doi:10.5505/tjtes.2018.62705
35. Gregorić P, Doklestić K, Pandurović M, et al. Distal ileal endometriosis as a cause of ileus: a case report. Srp Arh Celok Lek. 2012;140(3–4):225–228. doi:10.2298/SARH1204225G
36. Yilmaz B, Cukur S, Sahin R. A case of rectal bleeding caused by digestive endometriosis resembling colon cancer. Endoscopy. 2014;46(Suppl 1 UCTN):E357–8. doi:10.1055/s-0034-1377378
37. Indraccolo U, Trevisan P, Gasparin P, et al. Cecal endometriosis as a cause of ileocolic intussusception. JSLS. 2010;14(1):140–142. doi:10.4293/108680810X12674612015229
38. Marklund A, Sjövall A, Blomqvist L, et al. Endometriosis, the great imitator-A successful case of fertility preservation in a woman receiving Endometriosis, the great imitator-A successful case of fertility preservation in a woman receiving sterilizing treatment due to a diagnosis of rectosigmoid carcinoma. Gynecol Endocrinol. 2019;35(11):945–948.
39. Alvarado LER, Bahmad H, Mejia O, et al. Rectal endometriosis presenting as toxic megacolon. Autops Case Rep. 2021;11:e2021319. doi:10.4322/acr.2021.319
40. Allan Z. A case of endometriosis causing acute large bowel obstruction. Int J Surg Case Rep. 2018;42:247–249. doi:10.1016/j.ijscr.2017.12.031
41. Garg NK, Bagul NB, Doughan S, et al. Intestinal endometriosis--A rare cause of colonic perforation. World J Gastroenterol. 2009;15(5):612–614. doi:10.3748/wjg.15.612
42. Lee M, Yu L. Cecal endometriosis presenting as a term intrauterine fetal demise and gastrointestinal hemorrhage: a case report. Case Rep Womens Health. 2021;30:e00301. doi:10.1016/j.crwh.2021.e00301
43. Aragone L, Pasquini MT, Rebzda VS, et al. Appendiceal endometriosis: case report of a rare differential diagnosis of acute appendicitis. Int J Surg Case Rep. 2023;105:107993. doi:10.1016/j.ijscr.2023.107993
44. Alabbas H, Awidah T, Alhallaq O, et al. Small bowel endometriosis;a case report and review of literature. Ann Med Surg. 2019;47:24–26. doi:10.1016/j.amsu.2019.09.005
45. Mittelstadt S, Stäbler A, Kolb M, et al. Acute endometriosis-related sigmoid perforation in pregnancy-case report. BMC Pregnancy Childbirth. 2022;22(1):647. doi:10.1186/s12884-022-04973-9
46. Nishikawa A, Kondoh E, Hamanishi J, et al. Ileal perforation and massive intestinal haemorrhage from endometriosis in pregnancy: case report and literature review. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):20–24. doi:10.1016/j.ejogrb.2013.04.018
47. Keith JJ, Hernandez LO, Maruoka Nishi LY, et al. Catamenial rectal bleeding due to invasive endometriosis:a case report. J Med Case Rep. 2020;14(1):61. doi:10.1186/s13256-020-02386-w
48. Brosens I, Puttemans P, Campo R, et al. Noninvasive methods of diagnosis of endometriosis. Curr Opin Obstet Gynecol. 2003;15(6):519–522. doi:10.1097/00001703-200312000-00011
49. Trippia CH, Zomer MT, Terazaki CR, et al. Relevance of imaging examinations in the surgical planning of patients with bowel endometriosis. Clin Med Insights Reprod Health. 2016;10:1–8. doi:10.4137/CMRH.S29472
50. Del Frate C, Girometti R, Pittino M, et al. Deep retroperitoneal pelvic endometriosis:MR imaging appearance with laparoscopic correlation. Radiographics. 2006;26(6):1705–1718. doi:10.1148/rg.266065048
51. Young S, Burns MK, Difrancesco L, et al. Diagnostic and treatment guidelines for gastrointestinal and genitourinary endometriosis. J Turkish Ger Gynecol Assoc. 2017;18(4):200–209. doi:10.4274/jtgga.2017.0143
52. Leonardi M, Uzuner C, Mestdagh W, et al. Diagnostic accuracy of transvaginal ultrasound for detection of endometriosis using International Deep Endometriosis Analysis (IDEA) approach: prospective international pilot study. Ultrasound Obstet Gynecol. 2022;60(3):404–413. doi:10.1002/uog.24936
53. Jiang W, Roma AA, Lai K, et al. Endometriosis involving the mucosa of the intestinal tract: a clinicopathologic study of 15 cases. Mod Pathol. 2013;26(9):1270–1278. doi:10.1038/modpathol.2013.51
54. Kim KJ, Jung SS, Yang SK, et al. Colonoscopic findings and histologic diagnostic yield of colorectal endometriosis. J Clin Gastroenterol. 2011;45(6):536–541. doi:10.1097/MCG.0b013e3181fd297b
55. Wu LM, Li JK. Malignant transformation of deep infiltrating endometriosis with invasion of the rectum in a 34-year-old woman. Asian J Surg. 2023;46(7):2999–3000. doi:10.1016/j.asjsur.2023.02.024
56. Bartiromo L, Schimberni M, Villanacci R, et al. A systematic review of atypical endometriosis-associated biomarkers. Int J Mol Sci. 2022;23(8):1422–1467. doi:10.3390/ijms23084425
57. Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021;397(10276):839–852. doi:10.1016/S0140-6736(21)00389-5
58. Ruan X, Seeger H, Mueck AO. The pharmacology of dienogest. Maturitas. 2012;71(4):337–344. doi:10.1016/j.maturitas.2012.01.018
59. Vercellini P, Buggio L, Berlanda N, et al. Estrogen-progestins and progestins for the management of endometriosis. Fertil Steril. 2016;106(7):1552–1571. doi:10.1016/j.fertnstert.2016.10.022
60. Kamergorodsky G, Lemos N, Rodrigues FC, et al. Evaluation of pre- and post-operative symptoms in patients submitted to linear stapler nodulectomy due to anterior rectal wall endometriosis. Surg Endosc. 2015;29(8):2389–2393. doi:10.1007/s00464-014-3945-4
61. Abrão MS, Petraglia F. Deep endometriosis infiltrating the recto-sigmoid:Critical factors to consider before management. Hum Reprod Update. 2015;21(3):329–339. doi:10.1093/humupd/dmv003
62. Re´genet N, Me´tairie S, Cousin GM, et al. Endome´triose colorectale. diagnostic et prise en charge. Ann Chirurg. 2001;126(8):734–742. doi:10.1016/S0003-3944(01)00614-9
63. Remorgida V, Ragni N, Ferrero S, et al. How complete is full thickness disc resection of bowel endometriotic lesions?A prospective surgical and histological study. Human Reprod Hum Reprod. 2005;20(8):2317–2320. doi:10.1093/humrep/dei047
64. Roman H, Vassilieff M, Gourcerol G, et al. Surgical management of deep infiltrating endometriosis of the rectum: pleading for a symptom-guided Surgical management of deep infiltrating endometriosis of the rectum: pleading for a symptom-guided approach. Hum Reprod. 2011;26(2):274–281. doi:10.1093/humrep/deq332
65. Cheewakriangkrai C, Panggid K, Siriaungkul S, et al. Lymphovascular space invasion as a prognostic determinant in uterine cancer. Asian Pac J Cancer Prev. 2007;8(3):363–366.
66. Okazawa Y, Takahashi R, Mizukoshi K, et al. A case of clear cell adenocarcinoma arising from endometriosis of the rectum treated by laparoscopic surgery. Int J Surg Case Rep. 2014;5(12):979–983. doi:10.1016/j.ijscr.2014.10.034
67. Kobayashi S, Sasaki M, Goto T, et al. Endometrioid adenocarcinoma arising from endometriosis of the rectosigmoid. Dig Endosc. 2010;22(1):59–63. doi:10.1111/j.1443-1661.2009.00925.x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
