Back to Journals » International Journal of Women's Health » Volume 16
A 12 Week Fetus with Anophthalmia, Limb Anomalies and Infratemporal Teratoma
Received 7 October 2023
Accepted for publication 28 December 2023
Published 10 January 2024 Volume 2024:16 Pages 41—46
DOI https://doi.org/10.2147/IJWH.S441452
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Yu Lu,1 Jing-Jing Zhao,2 Ping He1
1Department of Ultrasonography, Shanghai Key Laboratory of Maternal Fetal Medicine, Shanghai Institute of Maternal-Fetal Medicine and Gynecologic Oncology, Shanghai First Maternity and Infant Hospital, School of Medicine, Tongji University, Shanghai, 200092, People’s Republic of China; 2Department of Pathology, Shanghai Key Laboratory of Maternal Fetal Medicine, Shanghai Institute of Maternal-Fetal Medicine and Gynecologic Oncology, Shanghai First Maternity and Infant Hospital, School of Medicine, Tongji University, Shanghai, 200092, People’s Republic of China
Correspondence: Ping He, Department of Ultrasonography, Shanghai First Maternity and Infant Hospital, School of Medicine, Tongji University, No. 2699 Gaoke West Road, Pudong District, Shanghai, 201204, People’s Republic of China, Tel +86-2120261000, Email [email protected]
Background: Microphthalmia is a rare autosomal recessive condition commonly known as Waardenburg anophthalmia syndrome (WAS) or oculo-acromal formation syndrome (MIM#206920).
Case Description: Here, we report the case of a woman whose fetal ultrasonography at 12 weeks of pregnancy revealed multiple fetal abnormalities. These included the absence of the left upper limb, an unclear display of the right orbit, a visible maxillary space, and a round, echoless appearance measuring 4 mm in diameter in the middle of the forehead. There was also a significant echo in the sac wall. The possibility of a frontal meningocele or a proboscis-like nose was considered. The fetus was delivered with absence of the left upper limb, absence of the right eye, a cleft lip on the right side, and a milky white sac with a diameter of 5 mm on the forehead after the pregnancy was terminated at the hospital. Pathological investigation revealed a mature cystic teratoma. The conclusion was microphthalmia with limb anomalies (MLA) after missing limbs, absence of eyes, and cleft lip were input into the Online Mendelian Inheritance in Man database. The case was diagnosed with fetal microphthalmia with limb anomalies and an interfrontal teratoma.
Conclusion: In this case, the entire exon analysis was not conducted, and as a result, the final diagnosis remains unclear. Based exclusively on the phenotype of the induced fetus, MLA was diagnosed. It is proposed that cases satisfying the requirements for a pathological diagnosis should undergo a pathological examination to establish a definitive diagnosis.
Keywords: cleft lip, limb anomalies, microphthalmia, teratoma
Introduction
Microphthalmia, also known as Waardenburg anophthalmia syndrome (WAS) or oculo-acromal formation syndrome (MIM#206920), is a rare autosomal recessive condition associated with a variety of congenital abnormalities. Waardenburg described it for the first time in 1935. It is characterized by ocular malformations (microphthalmia or anophthalmia), abnormal skeletal development (oligodactyly, syndactyly, fusion of the fourth and fifth metacarpals), and is associated with hypoplasia of long bones, horseshoe kidney, venous malformations, vertebral malformations, developmental delays, and intellectual disability. Approximately 90% of parents of infants with microphthalmia with limb anomalies (MLA) are biologically related.1 It is caused by a biallelic pathogenic variant of the SMOC1 gene.2 However, in some families with typical acromegaly syndrome, SMOC1 mutations cannot be identified, indicating limitations in existing SMOC1 mutation analysis.3,4 We report a case of microphthalmia (anophthalmia) with limb anomalies and an interfrontal teratoma.
Case Report
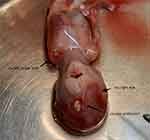
Thirty-year-old female, pregnant for 12 weeks. The couple is in good health and denies any family history of genetic diseases or close relatives having children. Working as a freelancer, with a history of non-toxic contact during pregnancy and taking teratogenic drugs, without taking additional folic acid. The fetal ultrasonography revealed multiple anomalies. These included the absence of the left upper limb, an unclear display of the right orbit, a visible maxillary space, and a round, echoless appearance measuring 4 mm in diameter in the middle of the forehead. Additionally, the sac wall exhibited a significant echo. The possibility of a frontal meningocele or a proboscis-like nose was considered. The woman was admitted to the hospital to have her pregnancy terminated, where she delivered a male fetus measuring 9 cm in length and 0.3 cm in plantar length. The fetus had a missing left upper limb, a protrusion on the left frontal area, a missing right eye, and a cleft lip. There was a milky white sac on the forehead with a diameter of 5 mm. Transparent, membrane-like tissue connected it to a longitudinal strip of tissue in the middle of the forehead. The white cyst was pathologically examined and diagnosed as a mature cystic teratoma. The fetal tissue was analyzed using a low-density microarray, and no clinically significant chromosome microdeletions or microduplications were detected. The conclusion was MLA after missing limbs, absence of eyes, and cleft lip were input into the Online Mendelian Inheritance in Man database. The case was diagnosed with fetal MLA and an interfrontal teratoma. The pregnant woman naturally became pregnant again one year after induced abortion, and the results of Down’s screening during early pregnancy were normal. No abnormalities were found during routine prenatal examinations. After 28 weeks of prenatal examination in our hospital, the newborn was delivered abroad and healthy.
This study was approved by the ethics committee with the patient’s consent and signed an informed consent form.
Discussion
Microphthalmia, also known as WAS or oculo-acromal formation syndrome. The clinical manifestations of this syndrome are varied, and the range of eye involvement can be microphthalmia or anophthalmia, which can be unilateral or bilateral. Distal limb abnormalities may not be combined with eye abnormalities, and vice versa.5 It is caused by a biallelic pathogenic variant of the SMOC1 gene.2 However, Rainger et al did not discover any SMOC1 anomalies in six typical families, indicating heterogeneity in the MLA locus.3 Kondo et al discovered a homozygous FNBP4 mutation in a case of MLA by sequencing the entire exome.6 The binding protein 4 encoded by FNBP4 interacts with the FH1 domain in Formin 1 (FMN1). FMN1 inhibits BMP signaling related to cytoskeletal components. BMP signals play an important role in limb and eye development. BMP4 mutations are also associated with finger abnormalities (polydactyly) and cleft lip and palate.3,7,8 In early embryogenesis, SMOC1 also acts as a BMP antagonist, which explains the limb and eye phenotypes observed in humans and mice.3,9
Fukahori et al reported a case of PORCN-related MLA caused by an inherited PORCN mutation from the mother.10 They analyzed nine male cases and found that all the identified variants were missense variants. Male patients with PORCN-related MLA typically exhibit ocular features, followed by physical manifestations and developmental delays.
In this case, the mother is 30 years old and both spouses have no major illnesses or physical deformities. Deny family history of genetic diseases and close marriage. Working as a freelancer. History of non-toxic contact during pregnancy and use of teratogenic drugs, no additional use of folic acid. The ultrasonography of the fetus revealed multiple anomalies. These included the absence of the left upper limb (Figures 1 and 2), an unclear display of the right orbit, a visible maxillary space, and a round echoless appearance in the middle of the forehead indicating a strong echo in the sac wall (Figures 3 and 4). After the pregnancy was terminated in the hospital, the male fetus was delivered with an absence of the left upper limb, a partial protrusion of the left frontal region, an absence of the right eye, a cleft lip on the right side, and a milky white sac with a diameter of 5 mm on the forehead (Figure 5). The white cyst was pathologically examined and diagnosed as a mature cystic teratoma. The eye and upper limb malformations in the fetus were consistent with the phenotype of MLA. The fetal tissue was analyzed using a low-density microarray, and no clinically significant chromosome microdeletions or microduplications were detected. The conclusion was MLA after missing limbs, absence of eyes, and cleft lip were input into the Online Mendelian Inheritance in Man database. In addition, the fetus had an interfrontal teratoma. There are no reports of patients with MLA located in the frontal region and protruding in vitro. The majority of teratomas occur in the midline area of the human body.
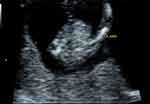 |
Figure 1 Two-dimensional ultrasound: the left upper limb of the fetus is not visible. |
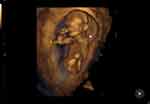 |
Figure 2 Three-dimensional ultrasound: the left upper limb of the fetus is not visible (indicated by the white arrow). |
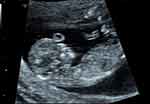 |
Figure 3 Two-dimensional ultrasound: a round, echoless appearance in the center of the fetal forehead. |
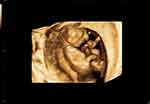 |
Figure 4 Three-dimensional ultrasound: a circular protrusion (indicated by a white arrow) in the middle of the fetus’ forehead. |
The incidence rate of congenital teratomas ranges from 20,000 to one in 40,000, with 54% occurring in the sacrococcygeal region and only 3% in the head and neck region.11 Prenatal examination revealed that most teratomas were located in the sacrococcygeal region, while facial teratomas were very rare and accounted for approximately 1.6% of all teratomas.12 In this case, the fetal frontal teratoma protruded from the body and was accompanied by MLA, which has not been reported in previous literature.
Lenz microphthalmia syndrome (LMS) (MIM#300166/309800) is a differential diagnosis for MLA. LMS is characterized by microphthalmia or anophthalmia, as well as other eye diseases, including mental disability, abnormalities in teeth, ears, and fingers, as well as heart defects, bone (limbs and/or spine), and various malformations of the genitourinary system. It is an inherited disorder with an X-linked recessive pattern caused by mutations in the BCL-6 corepressor gene (BCOR).13 The genetic patterns of MLA and LMS differ, and MLA does not exhibit any cardiac abnormalities.
In early pregnancy, if fetal malformation is detected during an examination, most pregnant women choose to induce labor, while pathological examination is rarely selected. Low density chip examination was performed on the fetal tissue of this case, and no clinically significant chromosomal microdeletions or duplications were found. Unfortunately, the entire exon analysis was not performed in this case, and as a result, the final diagnosis remains unclear. It is suggested that cases satisfying the requirements for a pathological diagnosis may undergo a pathological examination and molecular diagnosis to establish a definitive diagnosis. If the phenotype of the fetus makes clinicians highly suspicious of a certain syndrome, it is recommended to directly choose the corresponding molecular examination, which not only reduces the patient’s examination items, reduces the economic burden, but also improves the detection rate of the cause. In this case, the pregnant woman underwent prenatal genetic counseling after induced abortion. The doctor suggested that both spouses undergo chromosome testing and screening for carriers of monogenic diseases. Unfortunately, the couple refused. Therefore, the final diagnosis of this case is unclear. Although they received a healthy baby when they gave birth again, there was a certain level of risk involved. Therefore, the screening and diagnostic awareness of genetic diseases still need to be popularized and strengthened in China. Women with a history of poor pregnancy and childbirth should be made aware of the importance and necessity of genetic testing.
Conclusion
MLA is a rare case of autosomal recessive inheritance, and it has been discovered that it is caused by biallelic disease-causing variants of the SMOC1 gene or by PORCN variants. This case was diagnosed with MLA based solely on the phenotype of the fetus, as a comprehensive exon analysis was not conducted. It is suggested that cases satisfying the requirements for a pathological diagnosis should undergo a pathological examination and molecular diagnosis to establish a definitive diagnosis.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of Shanghai First Maternity and Infant Hospital. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from the participant.
Consent to Publish
Consent for the publication of the case was obtained from the patient.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
No external funding received to conduct this study.
Disclosure
The authors declare that they have no competing interests.
References
1. Garavelli L, Pedori S, Dal Zotto R, et al. Anophthalmos with limb anomalies (Waardenburg opththalmo (sic)-acromelic syndrome): report of a new Italian case with renal anomaly and review. Genet Couns. 2006;17(4):449–455.
2. Mancini C, Zonta A, Botta G, et al. A fetal case of microphthalmia and limb anomalies with abnormal neuronal migration associated with SMOC1 biallelic variants. Eur J Med Genet. 2019;62(11):103578.
3. Rainger J, van Beusekom E, Ramsay JK, et al. Correction: loss of the BMP antagonist, SMOC-1, causes ophthalmo-acromelic (waardenburg anophthalmia) syndrome in humans and mice. PLoS Genet. 2018;14(12):e1007866. doi:10.1371/journal.pgen.1007866
4. Waardenburg PJ. Autosomal-Recessive Anophthalmia with Malformations of the Hands and Feet. Vol. I. Waardenburg AFaDK PJ, editor. Assen, The Netherlands: Royal Van Gorcum; 1961:773.
5. Al Gazali LI, Sabarinathan DK, Khidir A. Microphthalmia and distal limb abnormalities in a child of consanguineous parents. Clin Dysmorphol. 1994;3:258–262. doi:10.1097/00019605-199407000-00013
6. Kondo Y, Koshimizu E, Megarbane A, et al. Whole-exome sequencing identified a homozygous FNBP4 mutation in a family with a condition similar to microphthalmia with limb anomalies. Am J Med Genet A. 2013;161A(7):1543–1546. doi:10.1002/ajmg.a.35983
7. Zhou F, Leder P, Zuniga A, et al. Formin1 disruption confers oligodactylism and alters Bmp signaling. Hum Mol Genet. 2009;18(13):2472–2482. doi:10.1093/hmg/ddp185
8. Suzuki S, Marazita ML, Cooper ME, et al. Mutations in BMP4 are associated with subepithelial, microform, and overt cleft lip. Am J Hum Genet. 2009;84:406–411. doi:10.1016/j.ajhg.2009.02.002
9. Okada I, Hamanoue H, Terada K, et al. SMOC1 is essential for ocular and limb development in humans and mice. Am J Hum Genet. 2011;88(1):30–41. doi:10.1016/j.ajhg.2010.11.012
10. Fukahori K, Yamoto K, Saitsu H, et al. PORCN -related microphthalmia with limb anomalies: case report and literature review. Am J Med Genet A. 2023;191(2):636–639. doi:10.1002/ajmg.a.63048
11. Lack EE. Extragonadal germ cell-tumours of the head and neck region: review of 16 cases. Hum Pathol. 1985;16:56–64. doi:10.1016/S0046-8177(85)80214-8
12. Tapper D, Lack EE. Teratomas in infancy and childhood. A 54-year experience at the children’s hospital medical center. Ann Surg. 1983;198:398–410. doi:10.1097/00000658-198309000-00016
13. Monticelli M, De Marco R, Garbossa D. Lenz microphthalmia syndrome in neurosurgical practice: a case report and review of the literature. Childs Nerv Syst. 2021;37(8):2713–2718. doi:10.1007/s00381-020-05035-1
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.