Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
4-Hydroxysesamin, a Modified Natural Compound, Attenuates Neuronal Apoptosis After Ischemic Stroke via Inhibiting MAPK Pathway
Authors Wang L, Qu Z, Sun Q, Mao Z, Si P, Wang W
Received 30 October 2023
Accepted for publication 28 February 2024
Published 7 March 2024 Volume 2024:20 Pages 523—533
DOI https://doi.org/10.2147/NDT.S444760
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Lina Wang, Zhenzhen Qu, Qian Sun, Zhuofeng Mao, Peipei Si, Weiping Wang
Internal Medicine-Neurology, the Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China
Correspondence: Weiping Wang, Internal Medicine-Neurology, the Second Hospital of Hebei Medical University, No. 215 Heping Road, Xinhua District, Shijiazhuang, 050004, People’s Republic of China, Tel +86-13613316623, Email [email protected]
Background: The 4-hydroxysesamin (4-HS, a di-tetrahydrofuran lignin) is a modified sesamin that was prepared in the laboratory. This preclinical study was designed to preliminarily investigate the neuroprotective properties of 4-HS.
Methods: In vitro, neuronal injury and inflammation were simulated by oxygen-glucose deprivation and lipopolysaccharide (LPS) exposure in mouse hippocampal neuronal HT22 cell line, and treated with 4-HS and/or metformin (MET, MAPK pathway activator for exploring mechanism). CCK-8, flow cytometry, and enzyme-linked immunosorbent assay were performed to evaluate cell viability, apoptosis, and inflammation. Apoptosis- and pathway-related proteins were detected by Western blotting. Middle cerebral artery occlusion (MCAO) was constructed as a stroke model and treated with 4-HS for in vivo confirmation. Histological staining was used for in vivo evaluation of 4-HS properties.
Results: The 4-HS showed similar anti-inflammatory activity to sesamin but did not affect the cell viability of HT22 cells. In vitro, 4-HS improved the cell viability, ameliorated neuronal apoptosis, along with the reversion of apoptotic proteins (Bax, cleaved-caspase 3/9, Bcl-2) expression and inflammatory cytokines (IL-6, TNF-α, IL-10) in LPS-treated HT22 cells. The 4-HS suppressed the phosphorylation of ERK, JNK, and p38 but the addition of MET reversed 4-HS-induced changes of phenotype and protein expression in LPS-treated cells. In vivo, 4-HS showed apparent improvement in cerebral infarction, brain tissue morphology, neuronal architecture, apoptosis, and inflammation of MCAO mice, and also showed inhibiting effects on the phosphorylation of ERK, JNK, and p38, confirming in vivo results.
Conclusion: In this first pre-clinical study on 4-HS, we preliminarily demonstrated the neuroprotective properties of 4-HS both in cell and animal models, and proposed that the underlying mechanism might be associated with the MAPK pathway.
Keywords: sesamin, 4-hydroxysesamin, middle cerebral artery occlusion, ischemic stroke, neuronal apoptosis
A Letter to the Editor has been published for this article.
Introduction
Stroke is the leading cause of long-term disability and affects about 15 million people each year, being a huge and increasing global health challenge.1,2 Globally, ischemic stroke represents ~87% of total strokes3 and currently has been the main concern in stroke research.4 More critically, the global stroke burden continues to increase globally, especially in developing countries.5,6 Despite the past decade has seen advances in treatment options, the available treatments for ischemic stroke are extremely limited.7 Therefore, any alternative therapy likely to improve the outcome of stroke would be a valuable asset.
Experimental findings have defined the pathogenic mechanisms involved in stroke injury, promoting the development of various agents that target the related injury pathways.8 From the perspective of pathogenic mechanism, a complex series of pathophysiological events including apoptosis, oxidative stress, and inflammation contribute to cerebral injury after ischemic stroke.9,10 Among them, apoptosis and inflammation, which occur in the penumbra, is currently one main targeting pathogenic process of most neuroprotective agents commonly used or in development.8,11 Nevertheless, most neuroprotective agents failed to translate in clinical settings.12
Sesamin, a furofuran lignan isolated from Sesamum indicum L. seeds., is known to possess anti-oxidant, anti-inflammatory, and neuroprotective properties.13,14 As a potential natural therapeutic lignan, several in vitro and in vivo studies have illustrated its pharmacological activity in various diseases, such as ischemic brain stroke,15 rheumatoid arthritis,16 and breast cancer.17 Recent clinical results have also demonstrated the anti-inflammatory properties of sesamin supplementation in rheumatoid arthritis,18 type 2 diabetes.19 However, until now, its application is still limited in clinical practice maybe due to low bioavailability and the limited level of activity. The 4-hydroxysesamin (4-HS, a di-tetrahydrofuran lignin) is a modified sesamin that was prepared in the laboratory. The pharmacological activity of 4-HS has not been explored widely.
To date, numerous studies have demonstrated the vital role of mitogen-activated protein kinases (MAPKs) (including ERK, JNK and p38) in the pathogenesis and development of ischemic stroke.20 The p38, as a main sub-group of MAPK superfamily, serves as a nexus for signal transduction, involving in a wide variety of biological processes, such as inflammation, proliferation, and apoptosis.21 Previous studies have indicated that the p38 MAPK inhibition is beneficial for treatment of neurological disorders.22,23 Furthermore, the natural lignans have been widely accepted to provide neuroprotection and anti-inflammatory by inhibiting p38 MAPK pathway.14,24 Therefore, this preclinical study was designed to investigate neuroprotective properties of 4-HS in the middle cerebral artery occlusion (MCAO) model and to explore the molecular signaling pathways underlying these effects.
Materials and Methods
Cell Line and Agents
The mouse hippocampal neuronal HT22 cell line was obtained from Procell Life Science & Technology Co., Ltd. (Wuhan, China). The cell line was not cross-contaminated, as identified by polymorphic short tandem repeat (STR) authentication. Sesamin was purchased from MedChemExpress (MCE) LLC (New Jersey, USA). The experimental agent 4-HS was provided by Professor S. Zhang (State Key Laboratory of Bioactive Substances and Functions of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing). Both sesamin and 4-HS were prepared in dimethyl sulfoxide (DMSO, MCE LLC, USA). Lipopolysaccharide (LPS, used for in vitro simulation of neuronal inflammation) and metformin hydrochloride (MET, as MAPK pathway activator) were purchased from Sigma-Aldrich® Merck KGaA, USA.
Cell Culture, Oxygen-Glucose Deprivation and Treatments
HT22 cell line was cultured in Dulbecco’s modified Eagle’s medium (DMEM) medium, supplementing with 10% fetal bovine serum and 1% penicillin/streptomycin. The culture condition was a temperature of 37 °C and an air atmosphere of 5% CO2/95%. The resuscitated cells were used for following in vitro experiments after twice subculturing. To mimic ischemic stroke condition in vitro, oxygen-glucose deprivation (OGD) model was established in HT22 cells by following procedure. Briefly, cells were cultured in the glucose-free DMEM medium and incubated in a hypoxic incubator that maintained a low oxygen tension (5% CO2, 0.1% O2 and 95% N2) at 37°C for 2 hours. Then, cultures were allowed to re-oxygenate under normal conditions (37°C, 5% CO2, 95% air) for reperfusion. The following in vitro experiments were conducted in OGD-induced HT22 cells.
To investigate the neuroprotective properties of 4-HS, OGD-induced HT22 cells were pre-treated with 4-HS (at a concentration of 2.5 to 10 μM) for 1 hour. Then, cells were exposed in LPS (1 μg/mL) to mimic the neuronal inflammation in vitro. OGD-induced HT22 cells that pre-treated with DMSO and LPS was set as negative control (NC). HT22 cells that pre-treated with 10% DMSO was set as blank control. In addition, to explore the effects of 4-HS on the MAPK pathway, cells were also simultaneously treated with 4-HS, LPS, and MET (1 mmol/L). After 24-hour cultivation, cells were collected for the following assays: cell viability, flow cytometry, enzyme-linked immunosorbent assay (ELISA), and Western blotting.
Cell Viability Assay
Cell viability was examined using the Cell Counting Kit-8 (CCK-8) method, using a commercialized kit (Beyotime, China). As per the manufacturer’s introduction, treated cell lines were seeded in the 96-well plate containing the DMEM culture medium at a density of 2×105 cells/well and were incubated at 37°C for 24 hours in a CO2 incubator. After the cultivation, CCK-8 reagent (10 μL) was added into each plate well of corresponding groups and thoroughly mixed, followed by another 1-hour incubation. The absorbance was finally examined at 450 nm using a microplate reader (µQuant MQX200, Bio-Tek Instruments, USA).
Flow Cytometry Assay (FCA)
Cell apoptosis was detected using the flow cytometric Annexin V-FITC/PI double staining with the commercialized Annexin-VFITC/PI Apoptosis Detection Kit (BD Biosciences, USA) on a FACSAria™ Fusion Flow Cytometers (BD Biosciences, USA), following the manufacturer’s instruction. Briefly, all treated cell lines were first seeded in the 96-well plate and incubated overnight. After that, samples were incubated with FITC-Annexin V (5 μL) and propidium iodide (0.1 μg) for 15 min at room temperature, respectively. Finally, apoptotic rates were profiled through the flow cytometer.
ELISA
The concentrations of interleukin-6 (IL-6), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α) in cell culture supernatant and serum were detected in the 96-well plate using the commercialized ELISA kits, including Mouse IL-6 ELISA Kit (ab222503, Abcam), Mouse IL-10 ELISA Kit (ab255729, Abcam), Mouse TNF alpha ELISA Kit (ab208348, Abcam). The ELISA procedure was conducted as per the manufacturer’s protocol (Abcam, USA).
Western Blotting
Western blotting for targeting proteins was conducted using the standard procedure. After extracting total proteins from cells or tissues with the RIPA lysis buffer solution (Beyotime, China), the concentration of proteins was quantified by the bicinchoninic acid method using a commercialized kit (Beyotime, China). Thereafter, followed by the electrophoretic separation and the PVDF membrane transfer, Western blotting proceeded using primary antibodies against caspase 9 (Cat. 40100, 1:1000 dilution, HuaBio-Antibodies), cleaved-caspase 9 (Cat. 60008, 1:1000 dilution, HuaBio-Antibodies), caspase 3 (Cat. HJ0829, 1:500 dilution, HuaBio-Antibodies), cleaved-caspase 3 (Cat. 3253u12, 1:500 dilution, HuaBio-Antibodies), Bcl-2 (Cat. 101717, 1:1000 dilution, HuaBio-Antibodies), Bax (Cat. HO1224, 1:1000 dilution, HuaBio-Antibodies), ERK (Cat. HN0810; 1:1000 dilution, HuaBio-Antibodies), p-ERK (Cat. HN1019; 1:1000 dilution, HuaBio-Antibodies), JNK (Cat. HO0705; 1:1000 dilution, HuaBio-Antibodies), p-JNK (Cat. HN0525; 1:1000 dilution, HuaBio-Antibodies), p38 (Cat. HO0201; 1:1000 dilution, HuaBio-Antibodies), p-p38 (Cat. 80927; 1:1000 dilution, HuaBio-Antibodies), and GAPDH (Cat. HO1224, 1:2000 dilution, HuaBio-Antibodies; as loading control), respectively. Subsequently, the PVDF membrane was probed with corresponding HRP-linked anti-IgG antibodies as secondary antibodies. Protein bands were finally visualized with an enhanced chemiluminescence method and quantified after normalization with the density of loading control GAPDH. The band intensity was analyzed with the ImageJ software (National Institutes of Health, USA).
Animals and Treatments
A total of 18 healthy C57BL/6 mice (male; aged 6 weeks; weight 18–20 g) were purchased from the Shanghai Model Organisms Center, Inc. (Shanghai, China) and were acclimatized to the laboratory environment for 2 weeks before experiments. All mice were kept in standard environmental conditions under a 12-hour light/dark cycle, at a temperature of 21–23 °C and a relative humidity of 50–60%. All mice had free access to food and water. Ethical approval for animals was obtained from the Ethics Committee of The Second Hospital of Hebei Medical University (Approval No. 2020-AE006) before starting the protocol-specified procedure. Any procedures for animals were conducted according to the Guide for the Care and Use of Laboratory Animals.
In this study, the MCAO modeling was established as per the standard Zea-Longa method, as previously described.25 In brief, mice were first anesthetized with pentobarbital sodium (40 mg/kg) and fixed in the supine position. To expose the main arteries, a 1-cm skin incision was cut in the midpoint between the left orbit and the external auditory canal. The 4–0 nylon intraluminal suture was slowly inserted into the internal carotid artery and advanced intracranially to block blood flow into the middle cerebral artery. At 24 hours after MCAO modeling, each mouse underwent the neurological function assessment to confirm the successful modeling.
For in vivo experiments, a least 5 repeats were necessary, and meanwhile, considering the mortality rate (10%–20%) of mice during MCAO modeling, a total of 6 mice were required in each group. Thus, a total of 18 mice were randomly assigned into 3 groups, including the sham-operated (blank control), MCAO-negative control (NC), and 4-HS treated groups. Mice in the 4-HS treated group underwent MCAO modeling and were intraperitoneally injected with 4-HS (15 mg/kg dissolved in 0.1% DMSO) for 4 weeks. The administration route and dose of 4-HS were selected according to Saif Ahmad et al.26 Mice in the sham group underwent the same surgical procedures for the MCAO modeling, without the occlusion of the middle cerebral artery and intraperitoneally injected with equivalent 0.1% DMSO for 4 weeks. Mice in the MCAO-NC group underwent MCAO modeling and were intraperitoneally injected with equivalent 0.1% DMSO for 4 weeks.
Preparation of Brain Tissue and Slices
Five mice per group were euthanized with an intraperitoneal injection of pentobarbital sodium (200 mg/kg). Brain tissues were harvested, and parts of brain tissues were post-fixed in 4% paraformaldehyde for 24 hours, paraffin-embedded, and sliced into 2-mm thick coronal sections for 2, 3, 5-Triphenyltetrazolium chloride (TTC) staining, hematoxylin and eosin (H&E) staining, and Nissl staining. The remaining brain tissues were frozen and stored at −80°C until the Western blotting assay of targeted proteins.
TTC Staining
The paraffin sections were stained with 2% TTC solution at 37°C for 30 min and kept in dark. Thereafter, the stained sections were photographed with a digital camera and the infarct area was analyzed with the ImageJ software (National Institutes of Health, USA). The area of infarct (white-stained area) and non-infarct area (red-stained area) was calculated and expressed as the percentage (%) of infarct volume in total cerebral tissue.
H&E Staining and Nissl Staining
The paraffin-embedded brain sections were firstly deparaffinized and rehydrated, then they were stained with hematoxylin and eosin (Beyotime, China) for H&E staining, or Nissl staining solution (Beyotime, China) for Nissl staining. After dehydration in a series of alcohol solutions with increasing concentrations, sections were treated with mounting medium. Finally, the stained sections were examined under a microscope (OLYMPUS, Japan).
TUNEL Staining
TUNEL staining was performed on paraffin-embedded sections by using the One Step TUNEL Apoptosis Assay Kit (Beyotime, China). According to manufacturer’s protocol, after deparaffinization and rehydration, sections were exposed in TUNEL solution for 30 min, followed by DAPI staining. TUNEL-positive cells were examined under a fluorescence microscope (OLYMPUS, Japan).
Statistical Analysis
Data were expressed as the mean and the standard error of the mean (SEM) of at least three independent repeats. All in vitro experiments were repeated in triplicate and in vivo experiments were repeated on 5 mice in each group. The between-group comparisons were performed by one-way analysis of variance followed by Tukey’s multiple-comparison test. All statistical analyses were conducted using the GraphPad Prism software package v8.0 (GraphPad Software, San Diego, CA, USA). The p-values less than 0.05 were considered statistically significant.
Results
4-HS Attenuates the Neuronal Apoptosis and Inflammation in LPS-Treated Hippocampal Neuronal Cells
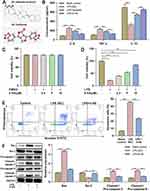
The 2D chemical structure and 3D conformer of 4-HS (PubChem CID: 5318999) that were retrieved from the PubChem (https://pubchem.ncbi.nlm.nih.gov) is displayed in Figure 1A. In the pre-experiments, we first compared the anti-inflammatory activity of 4-HS with that of sesamin in OGD-induced cells, which demonstrated the non-inferiority of 4-HS to sesamin, as evidenced by the similar effects on IL-6, TNF-α, and IL-10 (Figure 1B). Specifically, 4-HS decreased the levels of pro-inflammatory cytokines (IL-6 and TNF-α) and increased the levels of anti-inflammatory cytokine IL-10 in LPS-treated cells (Figure 1B). Meanwhile, a modified natural compound, 4-HS alone was confirmed to not affect the cell viability of HT22 cells (Figure 1C), supporting further investigation. As shown in Figure 1D, 4-HS significantly improved the cell viability in the OGD/LPS-treated hippocampal neuronal cells in a dose-dependent manner. Accordingly, the relatively high concentration of 4-HS (10 μM) was selected for the subsequent in vitro experiments.
FCA showed that LPS induced significant neuronal apoptosis in OGD-induced cells compared to the control group, but that was ameliorated by 4-HS (Figure 1E). Western blotting for apoptosis-related proteins also revealed that 4-HS inhibited the expression of pro-apoptotic protein Bax and the activation of cleaved-caspase 3/9, while increased the expression of the anti-apoptotic protein Bcl-2 (Figure 1F). Collectively, these results demonstrated that 4-HS might have neuroprotective properties by preventing neuronal apoptosis and inflammation.
4-HS Presents Neuroprotective Properties by Inhibiting the p38 MAPK Pathway
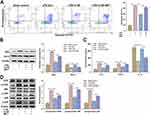
As the p38 MAPK pathway appears to play a major role in apoptosis and has been causally implicated in neurological disorders, it here was selected as a targeted downstream signal. As expected, after adding the MAPK pathway activator MET, the apoptosis rate was significantly increased compared to 4-HS treatment (Figure 2A). Meanwhile, the MET addition reversed the 4-HS-induced downregulation of Bax and upregulation of Bcl-2 (Figure 2B). Regarding the inflammatory cytokines, a similar tendency was observed that MET increased the levels of IL-6 and TNF-α, while decreased the level of IL-10 compared to 4-HS treatment (Figure 2C). Further, as shown in Figure 2D, 4-HS suppressed the phosphorylation of ERK, JNK, and p38 in OGD/LPS-treated cells, which were expectedly reversed by MET. In summary, the data suggested that 4-HS alleviated neuronal apoptosis and inflammation by inhibiting the p38 MAPK pathway.
In vivo Confirmation for the Neuroprotective Properties of 4-HS in MCAO Model
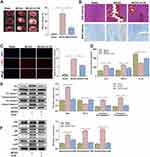
To confirm the in vivo neuroprotective properties of 4-HS, MCAO mice were constructed as stroke models and intraperitoneally administrated with 4-HS. As shown in Figure 3A, an obvious cerebral infarction in the TTC staining was seen in the MCAO mice, indicating the successful construction of the stroke model. After the 4-HS treatment, we observed an apparent improvement in cerebral infarction, manifesting as a significant decrease in infarct volume in MCAO mice (Figure 3A). Meanwhile, the HE staining revealed that 4-HS treatment improved the neuronal injury in MCAO rats, as evidenced by a more organized cellular arrangement and an overall improvement in tissue structure in the 4-HS-treated MCAO rats (Figure 3B). Nissl staining showed that the disintegrated and disappeared Nissl bodies in MCAO rats reappeared and increased after 4-HS treatment (Figure 3B). Further, TUNEL staining revealed that 4-HS treatment significantly decreased the TUNEL-positive cells in MCAO rats, indicating the improvement of apoptosis (Figure 3C). The ELISA also verified the anti-inflammatory property of 4-HS since 4-HS treatment decreased the levels of IL-6 and TNF-α but increased the level of IL-10 in MCAO mice (Figure 3D). By Western blotting for apoptosis-related proteins, the inhibition of pro-apoptotic protein Bax expression and cleaved-caspase 3/9 activation, as well as the increase of anti-apoptotic protein Bcl-2 expression were found by the 4-HS treatment in MCAO mice (Figure 3E). Meanwhile, Figure 3E confirms the inhibitory effects of 4-HS on the p38 MAPK pathway, as evidenced by the decreased phosphorylation of ERK, JNK, and p38. Overall, the in vivo finding suggested that 4-HS demonstrated neuroprotective properties of 4-HS by alleviating neuronal apoptosis and inflammation via the p38 MAPK pathway in the stroke model.
Discussion
Despite the natural phytochemical compound sesamin has been generally recognized as a therapeutic lignan in various diseases based on the robust preclinical and clinical evidence,13,27 the poor water solubility of sesamin has considerably limited its release efficiency and bioavailability.28 Thus, numerous efforts have been devoted to enhancing their solubility and permeability. The 4-HS was modified by a laboratory by introducing more hydroxyls, which structurally contribute to higher solubility in water. Here, in this first pre-clinical study on 4-HS, we preliminary demonstrated the neuroprotective properties of 4-HS in the stroke model and revealed its possible mechanisms that 4-HS suppressed neuronal apoptosis and inflammation by inhibiting the p38 MAPK pathway.
The most striking result of our study was the confirmation of the neuroprotective properties of the modified 4-HS in stroke models, proposing a potential therapeutic lignan. It is well known that the low cellular toxicity of natural compounds and the possibility of their long-term usage are important for various therapeutic areas, highlighting the essentiality of toxicity screening in the early stages of drug development.29 In this study, we preliminarily confirmed that the 4-HS showed no cellular toxicity, supporting the further investigation of its activity against the disease and the potency. On this basis, the CCK-8 and FCA analyses first revealed the improvement effects of 4-HS on cell viability, manifesting as the increased cell activity and decreased neuronal apoptosis after 4-HS treatment. Moreover, both the in vitro and in vivo ELISA results demonstrated that 4-HS suppressed the proinflammatory cytokines TNF-α and IL-6, but increased the anti-inflammatory cytokine IL-10, implying the anti-inflammation activity of 4-HS. These above results were generally consistent with that of sesamin.15,30,31 Meanwhile, the neuroprotective properties of 4-HS were further supported by in vivo experiments, which demonstrated a marked improvement in cerebral infarction, brain tissue morphology, neuronal architecture, and neuronal apoptosis, after 4-HS treatment in the histological staining. Additionally, in the Western blotting, the 4-HS was observed to inhibit the pro-apoptotic protein Bax and the activation of cleaved-caspase 3/9, while increased the anti-apoptotic protein Bcl-2, further confirming its inhibiting effect on the neuronal apoptosis. Collectively, the present study demonstrated the protective effects of 4-HS on stroke, which were found to be attributed to its anti-apoptosis and anti-inflammatory activities. Despite the investigation on 4-HS is relatively superficial, the extensive evidence on the neuroprotective effects of natural sesamin on various neurodegenerative disorders might support our present finding.32
Identifying the potential targets is also the pivotal step for drug discovery.33 Based on extensive pre-clinical findings, the p38 MAPK pathway plays a crucial part in apoptosis, transcriptional regulation, cytokine production, and cytoskeletal reorganization in various diseases.34 Meanwhile, the natural sesamin has also been proven to suppress the phosphorylation of the p38 MAPK signaling pathway in previous studies.35,36 Accordingly, in this study, the p38 MAPK pathway was first investigated as the downstream signaling of 4-HS. Consistent with the findings of sesamin,35–37 4-HS indeed significantly inhibited the phosphorylation of ERK, JNK, and p38, accompanied by the decrease of neuronal apoptosis, inflammation, and neural injury, suggesting the impaired activation of the p38 MAPK pathway in the 4-HS-induced neuroprotective function. Meanwhile, the addition of p38 MAPK pathway activator MET significantly reversed the effects of 4-HS, as evidenced by the aggravation in neuronal apoptosis and inflammation after MET treatment, which further supported the participation of p38 MAPK in the neuroprotective step of 4-HS. Indeed, a wide range of pre-clinical studies have also demonstrated that the inhibition of the p38 MAPK pathway provides neuroprotection in experimental cerebral ischemia since it could be involved in the apoptosis process via the regulation of mitochondrial pro-apoptotic proteins.23,38 Thus, this fact further supported the neuroprotection of 4-HS in experimental stroke. On the basis of in vitro and in vivo results, our investigation suggests a potential neuroprotective lignan for the treatment of ischemic stroke and provides a new insight into the association between 4-HS and the p38 MAPK pathway.
Despite the encouraging findings for the 4-HS, several limitations need to be recognized in this first, preliminary, pre-clinical study. Firstly, as a typical of pre-clinical study, all findings were from cells or animal models, which cannot reflect clinical results and translate to clinical practice. Additionally, the study is preliminary with a limited sample size, and the detailed mechanism has yet to be illustrated. Validating our results in more samples or other models will determine whether the 4-HS represents an effective therapeutic or dietary strategy for stroke.
Conclusion
In summary, this study represents the first verification of the neuroprotective effects of 4-HS in vitro and in vivo. These findings demonstrated that the 4-HS suppressed neuronal apoptosis and inflammation by inhibiting the p38 MAPK pathway in the MCAO model, suggesting that the 4-HS might be a potential therapeutic lignan for ischemic stroke or a dietary strategy to prevent disease progression. Given the preliminary nature of this pre-clinical investigation into 4-HS, there exist untapped potentials and more potent properties awaiting exploration for this agent. The insights gained from this study pave the way for further research avenues aimed at unraveling the full spectrum of 4-HS’s therapeutic capabilities, opening new vistas for future investigations and potential clinical applications.
Abbreviations
4-HS, 4-hydroxysesamin; MCAO, middle cerebral artery occlusion; STR, short tandem repeat; DMSO, dimethyl sulfoxide; OGD, oxygen-glucose deprivation; LPS, lipopolysaccharide; MET, metformin hydrochloride; DMEM, Dulbecco’s modified Eagle’s medium; ELISA, enzyme-linked immunosorbent assay; CCK-8, Cell Counting Kit-8; FCA, flow cytometry assay; IL-6, interleukin-6; IL-10, interleukin-10; TNF-α, tumor necrosis factor-α; NC, negative control; TTC, Triphenyltetrazolium chloride; SEM, standard error of the mean.
Data Sharing Statement
All data generated or analyzed during this study are available from the corresponding author upon request.
Ethical Approval
The protocol for this investigation in animals was approved by Ethics Committee of The Second Hospital of Hebei Medical University (Approval No. 2020-AE006). Any procedures for animals were conducted according to the Guide for the Care and Use of Laboratory Animals.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Murphy SJ, Werring DJ. Stroke: causes and clinical features. Medicine. 2020;48(9):561–566. doi:10.1016/j.mpmed.2020.06.002
2. Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383(9913):245–254. doi:10.1016/S0140-6736(13)61953-4
3. Ajoolabady A, Wang S, Kroemer G, et al. Targeting autophagy in ischemic stroke: from molecular mechanisms to clinical therapeutics. Pharmacol Therapeutics. 2021;225:107848.
4. Saini V, Guada L, Yavagal DR. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology. 2021;97(20 Suppl 2):S6–S16. doi:10.1212/WNL.0000000000012781
5. Feigin VL, Krishnamurthi RV, Parmar P, et al. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990-2013: the GBD 2013 Study. Neuroepidemiology. 2015;45(3):161–176. doi:10.1159/000441085
6. Katan M, Luft A. Global Burden of Stroke. Semin Neurol. 2018;38(2):208–211. doi:10.1055/s-0038-1649503
7. Barthels D, Das H. Current advances in ischemic stroke research and therapies. Biochim Biophys Acta Mol Basis Dis. 2020;1866(4):165260. doi:10.1016/j.bbadis.2018.09.012
8. Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF. Pathogenic mechanisms following ischemic stroke. Neurol Sci. 2017;38(7):1167–1186. doi:10.1007/s10072-017-2938-1
9. Radak D, Katsiki N, Resanovic I, et al. Apoptosis and Acute Brain Ischemia in Ischemic Stroke. Curr Vasc Pharmacol. 2017;15(2):115–122. doi:10.2174/1570161115666161104095522
10. Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4(6):461–470. doi:10.1111/j.1747-4949.2009.00387.x
11. Luo Y, Tang H, Li H, Zhao R, Huang Q, Liu J. Recent advances in the development of neuroprotective agents and therapeutic targets in the treatment of cerebral ischemia. Eur J Med Chem. 2019;162:132–146. doi:10.1016/j.ejmech.2018.11.014
12. Chen RL, Balami JS, Esiri MM, Chen LK, Buchan AM. Ischemic stroke in the elderly: an overview of evidence. Nat Rev Neurol. 2010;6(5):256–265. doi:10.1038/nrneurol.2010.36
13. Dalibalta S, Majdalawieh AF, Manjikian H. Health benefits of sesamin on cardiovascular disease and its associated risk factors. Saudi Pharm J. 2020;28(10):1276–1289. doi:10.1016/j.jsps.2020.08.018
14. Rosalina R, Weerapreeyakul N. An Insight into Sesamolin: physicochemical Properties, Pharmacological Activities, and Future Research Prospects. Molecules. 2021;26(19):5849. doi:10.3390/molecules26195849
15. Ahmad S, Elsherbiny NM, Haque R, et al. Sesamin attenuates neurotoxicity in mouse model of ischemic brain stroke. Neurotoxicology. 2014;45:100–110. doi:10.1016/j.neuro.2014.10.002
16. Khansai M, Phitak T, Klangjorhor J, et al. Effects of sesamin on primary human synovial fibroblasts and SW982 cell line induced by tumor necrosis factor-alpha as a synovitis-like model. BMC Complement Altern Med. 2017;17(1):532. doi:10.1186/s12906-017-2035-2
17. Lee CC, Liu KJ, Wu YC, Lin SJ, Chang CC, Huang TS. Sesamin inhibits macrophage-induced vascular endothelial growth factor and matrix metalloproteinase-9 expression and proangiogenic activity in breast cancer cells. Inflammation. 2011;34(3):209–221. doi:10.1007/s10753-010-9226-z
18. Helli B, Shahi MM, Mowla K, Jalali MT, Haghighian HK. A randomized, triple‐blind, placebo‐controlled clinical trial, evaluating the sesamin supplement effects on proteolytic enzymes, inflammatory markers, and clinical indices in women with rheumatoid arthritis. Phytother Res. 2019;33(9):2421–2428. doi:10.1002/ptr.6433
19. Mohammad Shahi M, Zakerzadeh M, Zakerkish M, Zarei M, Saki A. Effect of Sesamin Supplementation on Glycemic Status, Inflammatory Markers, and Adiponectin Levels in Patients with Type 2 Diabetes Mellitus. J Diet Suppl. 2017;14(1):65–75. doi:10.1080/19390211.2016.1204404
20. Zheng Y, Han Z, Zhao H, Luo Y. MAPK: a Key Player in the Development and Progression of Stroke. CNS Neurological Disorders. 2020;19(4):248–256. doi:10.2174/1871527319666200613223018
21. Zhang J, Shen B, Lin A. Novel strategies for inhibition of the p38 MAPK pathway. Trends in Pharmacological Sciences. 2007;28(6):286–295. doi:10.1016/j.tips.2007.04.008
22. Lee JK, Kim N-J. Recent Advances in the Inhibition of p38 MAPK as a Potential Strategy for the Treatment of Alzheimer’s Disease. Molecules. 2017;22(8):1287. doi:10.3390/molecules22081287
23. Barone FC, Irving EA, Ray AM, et al. Inhibition of p38 mitogen-activated protein kinase provides neuroprotection in cerebral focal ischemia. Medicinal Research Reviews. 2001;21(2):129–145. doi:10.1002/1098-1128(200103)21:2<129::AID-MED1003>3.0.CO;2-H
24. Thanh Le T, Tuan Ha M, Han K-H, Kim Y-B, Ah Kim J, Sun Min B. Anti-Inflammatory Lignans from the Roots of Asarum heterotropoides var. mandshuricum and Their Mechanism of Action. Chemistry & Biodiversity. 2022;19(6):e202100986. doi:10.1002/cbdv.202100986
25. Liu W, Shao C, Zang C, Sun J, Xu M, Wang Y. Protective effects of dexmedetomidine on cerebral ischemia/reperfusion injury via the microRNA-214/ROCK1/NF-kappaB axis. BMC Anesthesiol. 2021;21(1):203. doi:10.1186/s12871-021-01423-5
26. Ahmad S, ElSherbiny NM, Jamal MS, et al. Anti-inflammatory role of sesamin in STZ induced mice model of diabetic retinopathy. J Neuroimmunol. 2016;295-296:47–53. doi:10.1016/j.jneuroim.2016.04.002
27. Majdalawieh AF, Yousef SM, Abu-Yousef IA, Nasrallah GK. Immunomodulatory and anti-inflammatory effects of sesamin: mechanisms of action and future directions. Crit Rev Food Sci Nutr. 2022;62(18):5081–5112. doi:10.1080/10408398.2021.1881438
28. Wang CY, Yen CC, Hsu MC, Wu YT. Self-Nanoemulsifying drug delivery systems for enhancing solubility, permeability, and bioavailability of sesamin. Molecules. 2020;25(14):3119. doi:10.3390/molecules25143119
29. Barba-Ostria C, Carrera-Pacheco SE, Gonzalez-Pastor R, et al. Evaluation of Biological Activity of Natural Compounds: current Trends and Methods. Molecules. 2022;27(14):4490. doi:10.3390/molecules27144490
30. Cheng FC, Jinn TR, Hou RC, Tzen JT. Neuroprotective effects of sesamin and sesamolin on gerbil brain in cerebral ischemia. Int J Biomed Sci. 2006;2(3):284–288. doi:10.59566/IJBS.2006.2284
31. Baluchnejadmojarad T, Mansouri M, Ghalami J, Mokhtari Z, Roghani M. Sesamin imparts neuroprotection against intrastriatal 6-hydroxydopamine toxicity by inhibition of astroglial activation, apoptosis, and oxidative stress. Biomed Pharmacother. 2017;88:754–761. doi:10.1016/j.biopha.2017.01.123
32. Ghaderi MA, Emami SA, Beirak Olia MD, Javadi B. The Role of Sesamin in Targeting Neurodegenerative Disorders: a Systematic Review. Mini Rev Med Chem. 2023;23(6):756–770. doi:10.2174/1389557522666220523112027
33. Muroi M, Osada H. Proteomics-based target identification of natural products affecting cancer metabolism. J Antibiot (Tokyo). 2021;74(10):639–650. doi:10.1038/s41429-021-00437-y
34. Obata T, Brown GE, Yaffe MB. MAP kinase pathways activated by stress: the p38 MAPK pathway. Critical Care Medicine. 2000;28(4):N67–N77. doi:10.1097/00003246-200004001-00008
35. Xu P, Cai F, Liu X, Guo L. Sesamin inhibits lipopolysaccharide-induced proliferation and invasion through the p38-MAPK and NF-kappaB signaling pathways in prostate cancer cells. Oncol Rep. 2015;33(6):3117–3123. doi:10.3892/or.2015.3888
36. Jeng KC, Hou RC, Wang JC, Ping LI. Sesamin inhibits lipopolysaccharide-induced cytokine production by suppression of p38 mitogen-activated protein kinase and nuclear factor-kappaB. Immunol Lett. 2005;97(1):101–106. doi:10.1016/j.imlet.2004.10.004
37. Freise C, Sommer K, Querfeld U. Protective effects of the polyphenols (+)-episesamin and sesamin against PDGF-BB-induced activation of vascular smooth muscle cells are mediated by induction of haem oxygenase-1 and inhibition of mitogenic signalling. Journal of Functional Foods. 2015;18:586–597. doi:10.1016/j.jff.2015.08.021
38. Zhang XM, Zhang L, Wang G, et al. Suppression of mitochondrial fission in experimental cerebral ischemia: the potential neuroprotective target of p38 MAPK inhibition. Neurochem Int. 2015;90:1–8. doi:10.1016/j.neuint.2015.06.010
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.