Back to Journals » Blood and Lymphatic Cancer: Targets and Therapy » Volume 13
Zanubrutinib in Mantle Cell Lymphoma Management: A Comprehensive Review
Authors Alsuhebany N , Pan C, Holovac E, Do B, McBride A
Received 15 July 2023
Accepted for publication 10 November 2023
Published 24 November 2023 Volume 2023:13 Pages 67—76
DOI https://doi.org/10.2147/BLCTT.S426588
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Wilson Gonsalves
Nada Alsuhebany,1– 3 Congshan Pan,4 Eileen Holovac,5 Brian Do,6 Ali McBride7
1College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Kingdom of Saudi Arabia; 2King Abdulaziz Medical City, National Guard Health Affairs, Riyadh, Kingdom of Saudi Arabia; 3King Abdullah International Medical Research Center, Riyadh, Kingdom of Saudi Arabia; 4Department of Oncology Pharmacy, University of Arizona Cancer Center, Tucson, AZ, USA; 5Department of Oncology Pharmacy, VA Loma Linda Healthcare System, Loma Linda, CA, USA; 6Department of Oncology Pharmacy, Southern Arizona VA Hlth Care, Tucson, AZ, USA; 7WW HEOR Markets, Bristol-Myers Squibb, New York City, NY, USA
Correspondence: Nada Alsuhebany, Department of Clinical Pharmacy, College of Pharmacy, King Saud Bin Abdulaziz University for Health Sciences, PO Box 3660, Riyadh, 11481, Kingdom of Saudi Arabia, Tel +966500828585, Email [email protected]
Purpose: The pharmacology, pharmacokinetics, pharmacodynamics, clinical efficacy, and safety of zanbrutinib are described.
Summary: Mantle cell lymphoma (MCL) is a mature B-cell lymphoma that is typically associated with unfavorable outcomes, and virtually all patients with MCL have refractory or relapsed disease despite aggressive treatment. The treatment paradigm for MCL has transformed dramatically over the past decade owing to rapid advancements in immunotherapy and molecular-targeted therapies. Zanubrutinib, a second-generation Bruton’s tyrosine kinase inhibitor (BTKI) designated for mature B-cell non-Hodgkin’s lymphoma (NHL), has drastically improved the survival outcomes in relapsed/refractory (R/R) MCL patients. This selective BTKI is a small molecule that functions by forming a covalent bond in the active site of BTK. The inhibition of BTK activity is essential for the signaling of B-cell antigen receptor (BCR) and cytokine receptor pathways. In a preclinical study, zanubrutinib inhibited malignant B-cell proliferation and reduced tumor growth. Zanubrutinib was granted FDA-accelerated approval based on the results of Phase I and II trials. The investigator-assessed overall response rate was 83.7%, of which 78% of patients achieved complete response. The median duration of response was 19.5 months, and the median progression-free survival was 22.1 months. The most common (≥ 20%) all-grade adverse events were low neutrophil count (46.5%), upper respiratory tract infection (38.4%), rash (36.0%), low white blood cell count (33.7%), and low platelet count (32.6%).
Conclusion: Zanubrutinib is a selective, next-generation, orally active, irreversible BTK inhibitor. The selectivity of zanubrutinib and its superior efficacy, with a well-tolerated safety profile, have proven to be attractive options for other malignancies.
Keywords: zanubrutinib, mantle cell lymphoma, Bruton’s tyrosine kinase inhibitor
Background
Mantle cell lymphoma (MCL) is a rare B-cell lymphoma typically associated with unfavorable outcomes. It is a subtype of non-Hodgkin’s lymphoma (NHL), which accounts for 5–10% of all NHL cases in the United States.1 It is twice as common in non-Hispanic whites as in blacks, and is estimated to be 2–7 fold more common in men than in women.2 The annual incidence of MCL is about one case per 200,000 people and is rising.3,4 It has an aggressive clinical course, and approximately 70% of the patients do not present any symptoms until the advanced stage.5 MCL typically affects the older population with an average age of diagnosis of 68 years.
The vast majority of newly diagnosed patients present with reciprocal chromosomal translocation of t(11;14)9q13;q32), a mutation of the proto-oncogene CCND1 that upregulates the production of cyclin D1.1 Cyclin D1 is a protein that controls the progression from G1 to S phase in many different cell types, including mature B cells.6 This translocation is a hallmark of MCL and is expressed in virtually all MCL patients. 10–28% of MCL patients present with missense mutations in the TP53 gene that damage the DNA repair mechanism and apoptosis.7,8 Other common point mutations include overexpression of the BCL-2 gene, which enhances cell survival, and deletion of the ATM tumor suppressor gene.
The clinical presentation of MCL varies depending on the disease origin. Conventional MCL arises from naïve B cells that express SOX 11, a biomarker that represses the tumor suppressor gene PRDM1 for cell termination during plasma cell differentiation.9 Patients typically have nodal/extranodal involvement, lymphadenopathy, splenomegaly, or even bone marrow involvement. Only 4% of patients present with GI symptoms; however, GI infiltration is found in up to 88% of patients.10 Up to one-third of these patients present with classic B symptoms including night sweats, unexplained weight loss, and fever. A considerable percentage of patients also present with peripheral blood involvement.11 On the contrary, a small group of patients remains largely asymptomatic and can be observed for a long time without any therapeutic interventions. These patients usually have normal LDH and B2M levels, low MIPI scores, and no B symptoms and are generally classified as smoldering MCL.12
Treatment of MCL is largely based on patient age, stage, tumor burden, genetic features, and other established risk factors, owing to its diverse presentation. The most widely adapted tool for risk stratification, prognosis, and treatment guidance for advanced MCL is the mantle cell international prognostic index (MIPI). It utilizes four independent prognostic factors, including performance status, age, LDH, and leukocyte count, to stratify patients into three risk groups and predict five-year survival.13 The International Prognostic Index (IPI) was developed to aid in prognostic in NHL; due to its inaccuracy in MCL, it is now mainly used for low-risk MCL patients.14 Other factors for adapting treatments include CNS involvement, blastoid cytomorphology, and ki-67%.
Because of its virulent course and the nature of the disease, virtually all patients with MCL have refractory or relapsed disease despite aggressive treatment.4 Prior to the introduction of Bruton’s tyrosine kinase inhibitors (BTKIs), the treatment landscape for relapsed/refractory (R/R) MCL was challenging and limited.15 The standard-of-care treatments available before the advent of BTK inhibitors often resulted in suboptimal responses and a significant proportion of patients exhibited rapid disease progression. Data from earlier studies indicated that the overall response rate for these treatments was considerably low, with a median progression-free survival and overall survival of only a few months.16,17 Furthermore, these treatments were often accompanied by adverse events, further complicating the management of R/R MCL.
The treatment paradigm for MCL has transformed dramatically over the past decade owing to rapid advancements in immunotherapy and molecular-targeted therapies. BTKIs, which are a breakthrough agents for mature B-cell NHL, have drastically improved survival outcomes in relapsed/refractory settings. BTK functions as an integral regulator in B cell proliferation, survival, and differentiation.18,19 The inhibition of BTK reduces the proliferation of malignant B-cells and eliminates malignant B-cells from tissues and lymphoid organs.20 Ibrutinib and acalabrutinib are earlier generations of BTK inhibitors that show promising results in MCL; however, the risk for bleeding and atrial fibrillation is of concern.
Zanubrutinib (BGB-3111) is the newest BTK inhibitor used for the treatment of MCL. It was approved by the FDA on November 14, 2019, for adult patients with MCL who have received at least one prior therapy.21 It is a potent, highly selective, and irreversible BTK inhibitor that is better tolerated than earlier generation BTK inhibitors. The National Comprehensive Cancer Network (NCCN) guidelines currently recommend the use of zanubrutinib (category 2A) as second-line therapy because of its extended response duration.22,23 This article discusses the pharmacology, safety, efficacy, current evidence, and future direction of zanubrutinib in MCL.
Pharmacology
Zanubrutinib is a second-generation BTK inhibitor approved in November 2019.24,25 Zanubrutinib is a small molecule inhibitor of BTK. It functions by forming a covalent bond with a cysteine residue in the active site of BTK. This binding leads to inhibition of BTK activity, which is essential for the signaling of B-cell antigen receptor (BCR) and cytokine receptor pathways. BTK signaling activates pathways necessary for B-cell proliferation, trafficking, chemotaxis, and adhesion (Figure 1). In a preclinical study, zanubrutinib inhibited malignant B-cell proliferation and reduced tumor growth.26
In both preclinical and clinical studies, zanubrutinib has demonstrated an anti-tumor effect in chronic lymphocytic leukemia (CLL), with an inhibitory effect on interleukin‐2 inducible tyrosine kinase (ITK) and epidermal growth factor receptor (EGFR) in vitro.25,27,28 It can also regulate the immune microenvironment by suppressing the response of checkpoint molecules on suppressor cells and adhesion receptors on B cells by restoring T-cell exhaustion. At higher doses, zanubrutinib impairs rituximab‐dependent cytotoxicity mediated by NK cells. Compared to ibrutinib, zanubrutinib demonstrated better BTK selectivity and fewer off-target effects in several cell-based and in vitro enzymatic assays.26,27
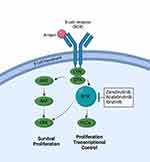 |
Figure 1 BTK Inhibitors Mechanism of Action. |
Mechanism of Resistance
Ibrutinib, a first-generation BTK inhibitor, has documented secondary resistance mechanisms in CLL, in which mutations in the BTK active site at Cys481 result in diminished drug binding to zanubrutinib.27,29 Furthermore, the enzymatic activity of the BTK Leu528Trp mutation has shown a remarkable loss of activity of zanubrutinib compared to wild-type BTK and BTK Cys481.29
One study identified a unique resistance to zanubrutinib in four CLL patients with BTK Leu528Trp mutation and detectable Cys481 mutation at a lower variant allele frequency (VAF), in which all patients progressed while on zanubrutinib.29 The modeled crystal structure suggested BTK Leu528Trp mutation causes steric clashes at the drug-binding site, which hinders zanubrutinib binding and leads to loss of activity. In MCL, comparable BTK mutations, like BTKC481S, have been identified.30,31 Recent studies have also explored resistance pathways including the nuclear factor kappa‐B, epigenetic modifiers like EP300, CREBBP, and WHSCI, as well as the epidermal growth factor receptor pathway.32,33 Another study showed that double mutations in BTK gatekeeper residues, such as T474I/C481S, T474M/C481T, and T474M/C481S, cause strong resistance to ibrutinib, acalabrutinib, and zanubrutinib.34 Hence, these agent-specific mutations may require the addition of mutations in the genetic screening for BTK inhibitor use and resistance in real-world practice.
Pharmacokinetics and Pharmacodynamics
The maximum plasma concentration (Cmax) and area under the plasma drug concentration-time curve (AUC) increased in a dose-dependent manner over a dose range of 40–320 mg in patients with B-cell malignancies.27,35 Based on in vivo kinetic data, oral zanubrutinib was rapidly absorbed with a median time to Cmax of approximately 2 hours. Food intake did not affect zanubrutinib absorption in healthy subjects. Its volume of distribution at steady state is 881 liters with 94% plasma protein-bound.35,36 A single oral dose of 160 mg or 320 mg of zanubrutinib has an average half-life of approximately 2–4 hours and oral clearance of 182 liters/hour. The excretion of a single dose of zanubrutinib 320 mg was 87% in feces (38% unchanged) and 8% in urine (<1% unchanged).
The recommended dose of zanubrutinib in MCL is either 160 mg twice daily or 320 mg once daily. The dosing was based two studies, a Phase 2 study in which patients received a 160-mg dose of zanubrutinib twice daily and a Phase 1/2 study where response rates were observed to be similar between 160 mg BID and 320 mg once daily dosing regimens.37 Despite the limited patient count in the once-daily dose group, population pharmacokinetics and exposure-response analyses were employed to bridge the data between these two regimens. The analyses confirmed that both dosing regimens achieved comparable plasma exposure and BTK inhibition. Additionally, the variations in trough concentration and maximum plasma concentration between once-daily and twice-daily dosing were determined to be insignificant in terms of impacting both efficacy and safety endpoints.
No adverse effects were observed in patients with mild or moderate renal impairment who were treated with zanubrutinib. However, it has not been studied in patients with severe renal impairment (creatinine clearance <30 mL/min) and end-stage renal disease on dialysis.24,35,38 Participants with mild (Child-Pugh class A), moderate (Child-Pugh class B), and severe liver (Child-Pugh class C) impairment experienced total AUC elevations of 11%, 21%, and 60%, respectively, compared to participants with normal hepatic function.24,35,38 The recommended dose of zanubrutinib in severe liver disease is 80 mg twice daily.
Drug Interaction and Dose Modification
The metabolism of zanubrutinib occurs mainly via cytochrome P450(CYP)3A4.24,27,35 Hence, the co-administration of zanubrutinib with CYP3A4 inducers or inhibitors can decrease or increase its level of exposure (Table 1). The recommended dose reduction of zanubrutinib is 80 mg once daily when administered concurrently with strong inhibitors and 80 mg twice daily when administered concurrently with moderate CYP3A inhibitors. However, the co-administration of either moderate or strong CYP3A inducers with zanubrutinib should be avoided. Zanubrutinib is not a substrate of the organic anion transporter (OAT) but is likely a substrate of p-glycoprotein.24
 |
Table 1 Dose Modifications for Use with CYP3A Inhibitors or Inducers |
Pharmacodynamic studies in patients with B-cell malignancies showed that at a total daily dose of 320 mg of zanubrutinib, the median steady-state BTK occupancy in peripheral blood was maintained at 100% over 24 h.24,27,35 In an in vitro study, zanubrutinib demonstrated comparable potency to ibrutinib at inhibiting BTK while twenty-fold less potent than ibrutinib at inhibiting ITK.38 This illustrates the selectivity of zanubrutinib for BTK and its less off-target kinase inhibition effect than ibrutinib.
Clinical Efficacy
Phase I
Tam et al evaluated zanubrutinib in patients with different B-cell malignancies including relapsed/refractory MCL in phase 1/2 study (BGB-3111-AU-003).39 The study was a dose-escalation, global, multicenter, single-arm trial that included 32 previously treated MCL patients. Zanubrutinib was administered at 160 mg twice daily (n = 14) or 320 mg once daily (n = 18). The median follow-up was 18.8 months. The results showed that overall response rate (ORR) was 84%, with 25% achieving complete response (CR). The median response (DOR) was 18.5 months. The median progression-free survival (PFS) was 21.1 months. Eighteen patients discontinued treatment, 10 because of progressive disease, and 8 because of adverse events (AEs).
Phase II
The FDA’s accelerated approval was based on the results of two trials: BGB-3111-AU-003 and BGB-3111-206 (Table 2). Song et al (BGB-3111-206) conducted a single-arm, multicenter, Phase II trial assessing the use of zanubrutinib 160 mg twice daily in patients with R/R MCL.40 The trial enrolled a total of 86 patients at 13 centers in China. The investigator-assessed ORR was 83.7%, and 78% (n = 67) of patients achieved CR. The median response was 19.5 months. The 12-month and 15-month event free survival rates were 81.8% (95% CI 70.7–89.0) and 72.1% (95% CI 61.0–80.5), respectively. The median PFS was 22.1 months; 12- and 15-month PFS were 74.6% (95% CI 63.7–82.6) and 72.1% (95% CI 61.0–80.5), respectively. Subgroup analysis showed that for response rates, there were no clear differences across the subgroups analyzed, including the poor prognosis subgroup. The median DOR and PFS were 30.2 months and 25.0 months in patients with blastoid histology. Nevertheless, prolonged DOR and PFS were observed in patients with low-or intermediate-risk combined MCL International Prognostic Index score (MIPI-b), lower Ki67 index (≤30%), fewer prior lines of therapy, and TP53 wild-type (WT). In patients with mutated and WT TP53, median PFS was 14.7 months (95% CI, 2.9-NE) in mutated TP53 and not reached (95% CI, 19.4-NE) in WT TP53 patients, respectively.
 |
Table 2 Clinical Efficacy Outcomes in MCL |
Phase III
There is an ongoing Phase III study (NCT04002297) comparing the efficacy and safety of zanubrutinib plus rituximab followed by zanubrutinib monotherapy versus bendamustine plus rituximab followed by observation in transplant-ineligible patients with previously untreated mantle cell lymphoma.41 The study has not reported any interim results yet. In contrast, there are two phase III studies have been published on CLL/SLL. In a phase III clinical trial (SEQUOIA trial),42 zanubrutinib was assessed in patients with CLL/SLL with or without del17p. Patients without del17p were assigned to receive zanubrutinib (arm A) or bendamustine–rituximab (arm B). Patients with del17p were enrolled in arm C and administered zanubrutinib. At a median follow-up of 26.2 months, PFS was significantly improved in group A compared to that in group B (HR, 0.42 [95% CI 0.28 to 0.63]; two-sided p < 0.0001). The median overall survival (OS) was not achieved in either group (Arm A vs Arm B). In arm C, the median PFS was not reached (95% CI NE–NE), and 24-month OS was 93.6% (95% CI 87.1–96.9).
The phase III ALPINE trial assessed the efficacy of zanubrutinib in relapsed/refractory CLL/SLL patients who received at least one line of non-BTK inhibitor systemic therapy.43 The study also included patients with high-risk features, such as del17p and/or TP53 mutation. The ORR was significantly higher with zanubrutinib than with ibrutinib (78.3% vs 62.5%), and this benefit continued to be exhibited in patients with del17p and/or TP53 (80.5% vs 50%). The 12-month OS rates were 97% and 92.7%, respectively. Although ALPINE trial was conducted in CLL/SLL patients, the fact that this trial was a head-to-head comparison of zanubrutinib with ibrutinib, which showed that zanubrutinib had superior efficacy and favorable safety profile; which could be extrapolated to MCL population
Safety
Regarding safety, zanubrutinib was considered to be well tolerated (Table 3).24 Tam et al44 conducted a pooled safety analysis of zanubrutinib monotherapy in 6 clinical trials. Seven hundred and seventy-nine patients were included in the pooled analysis, and most (88%) had R/R B-cell malignancies. Of the 43% of patients who discontinued treatment, only 10% discontinued treatment because of adverse events. Most patients (97%) reported ≥ one adverse event, although these events were primarily low-grade. The most common toxicities (≥ 10%) included upper respiratory tract infection, diarrhea, cough, contusion, rash, urinary tract infection, hematuria, constipation, fatigue, hypertension, pneumonia, lung infection, decreased neutrophil count, anemia, decreased platelet count, decreased WBC count, and neutropenia. At least 1 grade ≥3 treatment-emergent adverse event was reported in 66% of patients, and 37% were determined to be treatment-related. The most common serious adverse events included pneumonia, lung infection, urinary tract infection, pyrexia, cellulitis, anemia, and pleural effusion. In addition, 22 patients (3%) developed atrial fibrillation, and the majority of opportunistic infections occurred within the first 6–18 months of therapy. If any grade 3 or 4 hematologic or non-hematologic complications occur, interruption or dose modification of zanubrutinib might be needed (Table 4).45
 |
Table 3 Zanubrutinib Adverse Events |
 |
Table 4 Zanubrutinib Dose Modification Based on AEs |
Pooled data of 10 clinical trials, revealed low rates of treatment discontinuation due to zanubrutinib side effects. Notably, adverse events like infections, myelosuppression, hypertension, secondary malignancies, and atrial fibrillation either remained stable or decreased over time.44 Furthermore, zanubrutinib has fewer cardiovascular adverse events compared to ibrutinib, including atrial fibrillation and ventricular arrhythmias.46 Additionally, most patients who do not tolerate ibrutinib or acalabrutinib toxicities, usually tolerate zanubrutinib as one study showed that most toxicities did not recur or recurred at a lower severity with zanubrutinib compared to other BTKis.47
Dosage and Administration
Zanubrutinib is available as 80-mg capsules for oral administration with or without food. Patients were instructed to swallow capsules with water and to not open, break, or chew capsules.45
Cost
Zanubrutinib was administered at a standard dose of 160 mg orally twice daily, or 320 mg daily. It was supplied in 80 mg capsules. The average wholesale price (AWP) of one 80 mg capsule is $139.97, and the AWP of a 30-day supply is $16,796.4.45
Cost Effectiveness
A comparative cost-effectiveness study of BTKIs based on phase I/II clinical trial in R/R MCL showed that in comparison with ibrutinib, acalabrutinib had a 3-year ICER of $90,571 while zanubrutinib had a 3-year ICER of $58,422.48 Compared with ibrutinib, zanubrutinib was associated with incremental one life-year of PFS of 0.38 and one quality-adjusted life-year of PFS of 0.30 at incremental costs of $18,484. This study indicates a further PFS benefit of second-generation BTKIs.
Future Directions
Zanubrutinib is a selective second-generation BTKI approved for relapsed/refractory MCL. Ongoing trials are assessing the role of zanubrutinib in patients with newly diagnosed MCL and CLL/SLL. Several ongoing studies are investigating zanubrutinib in MCL patients (Table 5). Some of these trials are investigating the role of zanubrutinib in the upfront line and in combination with other regimens. Future studies comparing all BTK inhibitors with zanubrutinib are essential to guide treatment selection and characterize the toxicity profile of each BTK inhibitor. Other ongoing trials on zanubrutinib include marginal zone lymphoma, diffuse large B-cell lymphoma, Waldenström macroglobulinemia, and mature B-cell malignancies.
 |
Table 5 Ongoing Studies of Zanubrutinib in MCL |
Conclusions
The treatment landscape for MCL is rapidly evolving, with new and more effective therapies emerging all the time. Zanubrutinib is a new selective BTK inhibitor with a single approved indication for R/R MCL in patients who have received at least one prior therapy. The mechanism of action and well-tolerated safety profile make zanubrutinib an attractive option to be evaluated in other malignancies. Several studies are underway to assess the role of zanubrutinib in upfront therapy and its efficacy in combination with other drug regimens for MCL. Moving forward, understanding the resistance pathways and exploring combination therapies may further optimize the management and survival of MCL.
Disclosure
Ali McBride is employed by Bristol-Myers Squibb. The authors report no other conflicts of interest in this work.
References
1. Vijaya B, Roberto V, Julie V. In: Chapter 81 - Mantle Cell Lymphoma. Hoffman R, Benz EJ, Silberstein LE, et al., editors. Syed Ali Abutalib, Hematology.
2. Wang Y, Ma S. Racial differences in mantle cell lymphoma in the United States. BMC Cancer. 2014;14:764. doi:10.1186/1471-2407-14-764
3. Zhou Y, Wang H, Fang W, et al. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer. 2008;113(4):791–798. doi:10.1002/cncr.23608
4. Lynch DT, Acharya U. Mantle cell lymphoma. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536985/.
5. Ye H, Desai A, Zeng D, et al. Smoldering mantle cell lymphoma. J Exp Clin Cancer Res. 2017;36(1):185. doi:10.1186/s13046-017-0652-8
6. Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6(1):24. doi:10.1186/1476-4598-6-24
7. Ott G, Kalla J, Ott MM, et al. Blastoid variants of mantle cell lymphoma: frequent bcl-1 rearrangements at the major translocation cluster region and tetraploid chromosome clones. Blood. 1997;89(4):1421–1429. doi:10.1182/blood.V89.4.1421
8. Greiner TC, Moynihan MJ, Chan WC, et al. p53 mutations in mantle cell lymphoma are associated with variant cytology and predict a poor prognosis. Blood. 1996;87(10):4302–4310. doi:10.1182/blood.V87.10.4302.bloodjournal87104302
9. Shaffer AL, Lin KI, Kuo TC, et al. Blimp −1 orchestrates plasma cell differentiation by extinguishing the mature B cel gl gene expression program. Immunity. 2002;17(1):51–62. doi:10.1016/S1074-7613(02)00335-7
10. Romaguera JE, Medeiros LJ, Hagemeister FB, et al. Frequency of gastrointestinal involvement and its clinical significance in mantle cell lymphoma [published correction appears in Cancer. 2003 Jun 15;97(12):3131]. Cancer. 2003;97(3):586–591. doi:10.1002/cncr.11096
11. Ferrer A, Salaverria I, Bosch F, et al. Leukemic involvement is a common feature in mantle cell lymphoma. Cancer. 2007;109(12):2473–2480. doi:10.1002/cncr.22715
12. Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16(8):2780–2795. doi:10.1200/JCO.1998.16.8.2780
13. Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma [published correction appears in Blood. 2008 Jun 15;111(12):5761]. Blood. 2008;111(2):558–565. doi:10.1182/blood-2007-06-095331
14. Geisler CH, Kolstad A, Laurell A, et al. The Mantle Cell Lymphoma International Prognostic Index (MIPI) is superior to the International Prognostic Index (IPI) in predicting survival following intensive first-line immunochemotherapy and autologous stem cell transplantation (ASCT). Blood. 2010;115(8):1530–1533. doi:10.1182/blood-2009-08-236570
15. Bond DA, Martin P, Maddocks KJ. Relapsed mantle cell lymphoma: current management, recent progress, and future directions. J Clin Med. 2021;10(6):1207. doi:10.3390/jcm10061207
16. Kumar A, Sha F, Toure A, et al. Patterns of survival in patients with recurrent mantle cell lymphoma in the modern era: progressive shortening in response duration and survival after each relapse. Blood Cancer J. 2019;9(6):50. doi:10.1038/s41408-019-0209-5
17. Visco C, Di Rocco A, Evangelista A, et al. Outcomes in first relapsed-refractory younger patients with mantle cell lymphoma: results from the MANTLE-FIRST study [published correction appears in Leukemia. 2021 Jan 22;:]. Leukemia. 2021;35(3):787–795. doi:10.1038/s41375-020-01013-3
18. Corneth OBJ, Klein Wolterink RGJ, Hendriks RW. BTK Signaling in B Cell Differentiation and Autoimmunity. Curr Top Microbiol Immunol. 2016;393:67–105. doi:10.1007/82_2015_478
19. Kim HO. Development of BTK inhibitors for the treatment of B-cell malignancies. Arch Pharm Res. 2019;42(2):171–181. doi:10.1007/s12272-019-01124-1
20. Chang BY, Francesco M, De Rooij MF, et al. Egress of CD19(+)CD5(+) cells into peripheral blood following treatment with the Bruton tyrosine kinase inhibitor ibrutinib in mantle cell lymphoma patients. Blood. 2013;122(14):2412–2424. doi:10.1182/blood-2013-02-482125
21. Food and Drug Administration. Food and Drug Administration grants have accelerated the approval of zanubrutinib for mantle cell lymphoma. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-zanubrutinib-mantle-cell-lymphoma.
22. BRUKINSA (Zanubrutinib) [prescribing information]. San Mateo, CA: BeiGene USA, Inc.; 2019.
23. National Comprehensive Cancer Network (NCCN). B-cell lymphomas. Version 6; 2023. Available from: www.nccn.org.
24. BRUKINSA (zanubrutinib) [package insert]. San Mateo, CA: BeiGene, Ltd; 2019.
25. Zou Y, Zhu H, Li X, et al. The impacts of zanubrutinib on immune cells in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Hematol Oncol. 2019;37(4):392–400. doi:10.1002/hon.2667
26. Tam C, Grigg AP, Opat S, et al. The BTK inhibitor, Bgb-3111, is safe, tolerable, and highly active in patients with relapsed/ refractory B-cell malignancies: initial report of a phase 1 first-in-human trial [abstract]. Blood. 2015;126(23):Abstract 832. doi:10.1182/blood.V126.23.832.832
27. Tam C, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851–859. doi:10.1182/blood.2019001160
28. Wu J, Liu C, Tsui ST, Liu D. Second‐generation inhibitors of Bruton tyrosine kinase. J Hematol Oncol. 2016;9(1):80. doi:10.1186/s13045-016-0313-y
29. Handunnetti SM, Tang CPS, Nguyen T, et al. BTK Leu528Trp - a potential secondary resistance mechanism specific for patients with chronic lymphocytic leukemia treated with the next generation BTK inhibitor zanubrutinib. Blood. 2019;134(Supplement_1):170. doi:10.1182/blood-2019-125488
30. Cheah CY, Chihara D, Romaguera JE, et al. Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann Oncol. 2015;26(6):1175–1179. doi:10.1093/annonc/mdv111
31. Rahal R, Frick M, Romero R, et al. Pharmacological and genomic profiling identifies NF‐κB‐targeted treatment strategies for mantle cell lymphoma. Nat Med. 2014;20(1):87–92. doi:10.1038/nm.3435
32. Balasubramanian S, Schaffer M, Deraedt W, et al. Mutational analysis of patients with primary resistance to single‐agent ibrutinib in relapsed or refractory mantle cell lymphoma (MCL). Blood. 2014;124(21):78. doi:10.1182/blood.V124.21.78.78
33. Lenz G, Balasubramanian S, Goldberg J, et al. Sequence variants in patients with primary and acquired resistance to ibrutinib in the Phase 3 MCL 3001 (RAY) trial. J Clin Oncol. 2016;34(suppl):abstr 7570. doi:10.1200/JCO.2016.34.15_suppl.7570
34. Estupinan HY, Wang Q, Berglof A, et al. BTK gatekeeper residue variation combined with cysteine 481 substitution causes super-resistance to irreversible inhibitors acalabrutinib, ibrutinib and zanubrutinib. Leukemia. 2021;35(5):1317–1329. doi:10.1038/s41375-021-01123-6
35. Sayed YY. Zanubrutinib: first Approval. Drugs. 2020;80(1):91–97. doi:10.1007/s40265-019-01252-4
36. Weaver AN, Jimeno A. Zanubrutinib: a new BTK inhibitor for treatment of relapsed/refractory mantle cell lymphoma. Drugs Today. 2020;56(8):531–539. doi:10.1358/dot.2020.56.8.3158047
37. Ou YC, Tang Z, Novotny W, et al. Rationale for once-daily or twice-daily dosing of zanubrutinib in patients with mantle cell lymphoma. Leuk Lymphoma. 2021;62(11):2612–2624. doi:10.1080/10428194.2021.1929961
38. Flinsenberg TWH, Tromedjo CC, Hu N, et al. Differential effects of BTK inhibitors ibrutinib and zanubrutinib on NK-cell effector function in patients with mantle cell lymphoma. Haematologica. 2020;105(2):e76–e79. doi:10.3324/haematol.2019.220590
39. Tam CS, Opat S, Simpson D, et al. Zanubrutinib for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv. 2021;5(12):2577–2585. doi:10.1182/bloodadvances.2020004074
40. Song Y, Zhou K, Zou D, et al. Zanubrutinib in relapsed/refractory mantle cell lymphoma: long-term efficacy and safety results from a phase 2 study. Blood. 2022;139(21):3148–3158. doi:10.1182/blood.2021014162
41. Dreyling M, Tam CS, Wang M, et al. A Phase III study of zanubrutinib plus rituximab versus bendamustine plus rituximab in transplant-ineligible, untreated mantle cell lymphoma. Future Oncol. 2021;17(3):255–262. doi:10.2217/fon-2020-0794
42. Tam CS, Brown JR, Kahl BS, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. 2022;23(8):1031–1043. doi:10.1016/S1470-2045(22)00293-5
43. Hillmen P, Eichhorst B, Brown JR, et al. First interim analysis of ALPINE study: results of a phase 3 randomized study of zanubrutinib vs ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma.
44. Tam CS, Dimopoulos M, Garcia-Sanz R, et al. Pooled safety analysis of zanubrutinib monotherapy in patients with B-cell malignancies. Blood Adv. 2022;6(4):1296–1308. doi:10.1182/bloodadvances.2021005621
45. Lexicomp Online [online database]. Hudson, OH: Lexi-Comp; 2022.
46. Tam CS, Wallis N, Zhang M, et al. Rate of atrial fibrillation in patients with B-cell malignancies who undergo treatment with zanubrutinib. Am J Hematol. 2022;97:525–526. doi:10.1002/ajh.26736
47. Shadman M, Flinn IW, Levy MY, et al. Zanubrutinib in patients with previously treated B-cell malignancies intolerant of previous Bruton tyrosine kinase inhibitors in the USA: a phase 2, open-label, single-arm study. Lancet Haematol. 2023;10(1):e35–e45. doi:10.1016/S2352-3026(22)00320-9
48. Alrawashdh N, McBride A, Slack M, Persky D, Andritsos L, Abraham I. Cost-effectiveness and value of information analyses of Bruton’s tyrosine kinase inhibitors in the treatment of relapsed or refractory mantle cell lymphoma in the United States. J Manag Care Spec Pharm. 2022;28(4):390–400. doi:10.18553/JMCP.2022.28.4.390/ASSET/IMAGES/SMALL/FIG2.GIF
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
