Back to Journals » International Journal of Women's Health » Volume 16
Yolk Sac Tumor of the Ovary in Mosaic 46XX Turner Syndrome
Authors Suryawan AZ , Tjahyadi D, Hermawan M, Aprialdi D
Received 13 February 2024
Accepted for publication 9 April 2024
Published 16 April 2024 Volume 2024:16 Pages 629—635
DOI https://doi.org/10.2147/IJWH.S462375
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Everett Magann
Alfonsus Zeus Suryawan, Dian Tjahyadi, Martin Hermawan, Doni Aprialdi
Department of Obstetrics and Gynecology; Universitas Padjadjaran, Bandung, Indonesia
Correspondence: Alfonsus Zeus Suryawan, Department of Obstetrics and Gynecology, Universitas Padjadjaran, Hasan Sadikin General Hospital, Jalan Pasteur No. 38, Bandung, 40161, Indonesia, Email [email protected]
Introduction: Correlation of Turner syndrome (TS) with germ cell malignancy is acknowledge in TS patient with Y chromosome material but not otherwise. This case report wishes to highlight yolk sac tumor occurrence in patients with TS 46XX karyotype mosaicism.
Case Report: A 23-year-old nulligravid woman was admitted with abdominal mass and vaginal bleeding. She had primary amenorrhea and had already been diagnosed with TS. Her karyotype was 46XX with 5% X mosaicism. Ultrasonography revealed a solid mass measuring 14.05 x 10.99 cm based on the International Ovarian Tumor Analysis (IOTA) simple rule, M1 and M2. During surgery, a solid mass originates from her left ovary measuring 20 x 15 x 15 cm with adhesion to omentum, ileum, and caecum was found. Pathology examination reveals it’s an endodermal sinus tumors (EST).
Discussion: TS with Y cells are closely linked with germ cell malignancy but not otherwise. It’s still unclear what causes the malignancy in such cases.
Conclusion: The present report illustrates a rare case of EST occurred in a TS patient with 46XX mosaicism.
Keywords: Turner syndrome, endodermal sinus tumor, yolk sac malignancy, mosaicism, 46XX karyotype
Introduction
Turner syndrome (TS) is a complex developmental disorder which possess a 45X cell line with or without mosaicism.1 It’s extremely rare that this condition accompanied by female reproduction tract malignancy. Only female with the presence of Y chromosome material in TS possess risk of developing gonadoblastoma which is a precursor to germ cell malignancies.1,2 Few cases of gonadoblastoma occurs in woman with normal 46XX karyotype with no dysgenesis are reported.3–8 In this paper we wish to report the occurrence of a yolk sac tumor in a woman with 46XX karyotype mosaicism Turner syndrome.
Case Report
A 23 year old female was referred to our policlinic due to vaginal bleeding because of suspected uterine fibroid and Turner syndrome. Before she came to our facility, she was diagnosed with primary amenorrhea due to TS in 2016 in her previous medical facility and given hormone therapy for 6 years with Cyclo-Progynova. Patient then decided to stop taking the hormone therapy in early 2022. Three months before admission, she noticed an enlargement of her abdomen the size of a tennis ball, accompanied by vaginal bleeding. At presentation, patient appeared with developed breasts and pubic hair. Abdominal examination revealed a solid mass measuring 15 x 15 x 10 cm. Her external genitalia were within normal limits (Figure 1).
 |
Figure 1 Patient’s physical stature. (A) Patient has normal stature with height 170 cm; (B) Breast development shows Tanner 5; (C) Pubic hair development shows Tanner 4. |
Ultrasonography revealed a solid mass measuring 14.05 x 10.99 cm above the uterus, with a colour doppler score of +1 (Figure 2). Free fluid was also identified between the liver and kidneys (Morisson pouch). Ultrasonography revealed a solid mass measuring 14.05 x 10.99 cm based on the International Ovarian Tumor Analysis (IOTA) simple rule M1 and M2, and a malignant ovarian tumor with TS was suspected. Hormonal level examination had already been performed in 2016, which confirmed TS. The markers were Anti-Mullerian hormone (AMH) at 0.02 ng/mL, follicle stimulating hormone (FSH) at 116,8 mIU/mL, luteinizing hormone (LH) at 59.9 mIU/mL, and estradiol (E2) at 5.0 pg/mL. These results confirmed that the patient had primary ovarian insufficiency, which is consistent with TS.
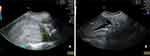 |
Figure 2 Patient’s abdominal ultrasonography. (A) Suspected solid ovarian mass with colour Doppler +1; (B) Free fluid present on Morrison pouch. |
We confirmed that her karyotype was 46XX, with 5% X mosaicism, which had been identified by fluorescence in situ hybridization (FISH) analysis in 2016. FISH analysis included 300 cells using X and Y probes, with 15 cells containing only one X chromosome and 285 cells containing two X chromosomes. There was no Y chromosome found by FISH analysis (Figure 3).
 |
Figure 3 Patient’s chromosomal analysis. (A) Patient’s chromosome was 46XX; (B) FISH analysis reveals X mosaicism with no Y chromosome (X probe: red; Y probe: green). |
It was intended that the patient would then have a joint exploratory laparotomy with Gynecology Oncology department. Alpha-feto protein (AFP), βHCG and lactic dehydrogenase (LDH) examination were planned for her next visit before the operation. However, before her next visit, she was admitted to the emergency department because of severe abdominal pain and increased bowel sound. We performed abdominal roentgen and found high intestinal obstruction. Emergency laparotomy was performed in the Digestive surgery and Gynecology Oncology department. Suboptimal debulking was performed (total abdominal hysterectomy; bilateral salpingo-oophorectomy; peritoneal sampling; omental sampling) after the identification of a solid mass measuring 20 x 15 x 15 cm on her left ovary, with adhesion to the omentum, ileum, and caecum (Figure 4). After total abdominal hysterectomy and bilateral salpingo-oophorectomy was performed, a residual mass measuring 5 x 3 x 2 cm was found on her ileum. Removal was not an available option because the patient’s family refused to accept the possibility of a colostomy. After further evaluation of her bowel, it was returned to normal and the operation was concluded. Her post-operational diagnosis became suspected ovarian carcinoma stage IIIC, Turner syndrome, and obstructive ileus due to an ovarian mass.
Pathology results revealed that the mass was an ovarian yolk sac tumor metastasized to the left and right of the peritoneum and omentum. The uterus and right adnexa were free from cancer cells. The patient was then treated with a three-weekly BEP (Bleomycin, Etoposide, and Cisplatin) regimen. Currently, the patient has finished 4 cycles of this regimen and has been evaluated in the department of oncology and gynecology policlinic. There’s no residual mass present on ultrasound examination and no abnormalities on tumor markers (AFP, bHCG and LDH). The patient was placed on hormone replacement therapy after evaluation.
Discussion
Yolk sac tumors or endodermal sinus tumors (EST) are derived from the primitive yolk sac.9,10 Most ESTs tend to be unilateral and rapidly growing cancers. ESTs are also the third most frequent malignant germ cell tumors of the ovary.10 Epithelial cell ovarian cancers make up the majority of cases, around 90%, and encompass several histologic types, each characterized by distinct molecular alterations, clinical characteristics, and responses to treatment. Conversely, non-epithelial ovarian cancers, constituting the remaining 10%, primarily include germ cell tumors, sex cord-stromal tumors, and exceptionally rare types like small cell carcinomas. Among these, germ cell tumors are predominant in women under 30 years old, with a majority diagnosed at early stages (60–70%).11 This patient’s mass developed relatively quickly in three months, which is consistent with the rapid growth of germ cell malignancy and usually present with acute pelvic pain related to capsular distention, hemorrhage, or necrosis.10
TS carries a risk of ovarian malignancy if Y chromosome material is present.10,12 Although gonadoblastomas are benign tumors, they can be precursors to germ cell malignancies, such as dysgerminomas (most commonly), teratomas, embryonal carcinomas, or endodermal sinus tumors.13 Around 5% of ovarian tumors that occur during pregnancies are malignant. Presently, surgical intervention is recommended for ovarian masses exceeding 6 cm in diameter or when symptoms are present.14 The Y chromosome is strongly linked with germ cell malignancy due to the presence of the gonadoblastoma-Y (GBY) region. It is postulated to serve a normal function in the testis, but could exert oncogenic effects in dysgenetic gonads.15
The unusual thing in this case is that there’s no Y chromosome present, which means the patient should not have any risk of developing germ cell malignancy. Even though the Y chromosome is an important factor in terms of malignancy risk, Scully et al reported that gonadoblastomas also occur in 45X/46XX mosaicism and 45XO.16 Even after publication, Scully et al reported two cases with the normal 46XX karyotype.16 Chandrapattan et al also reviewed 12 similar cases of germ cell malignancy, in which all of the women had the 46XX karyotype.3–8,17 The main difference between those cases and our case is that our patient had TS with mosaic 46XX, with only 5% of 45XO. Canto et al’s study found a risk of 33% for the development of gonadoblastoma in a TS patient with prophylactic gonadectomy, which is very high.18 Due to an elevated risk of developing cancer, experts advise that prophylactic gonadectomy be performed as early as possible if Y chromosome material is present.19 In a study by Mittal et al involving 18 cases of TS with Y chromosome material, tumors were identified in 6 patients (33.3%); 4 (22.2%) with dysgerminoma and 2 (11.1%) with gonadoblastoma.20 This translates into slightly more than 50% of women with TS with Y chromosome material having a malignancy.
In contrast, a review of 114 TS cases revealed that the occurrence of gonadoblastoma among Y-positive patients seemed to be low (7–10%), and thus the risk may have been overestimated in previous studies.21 We suspected that our patient had already developed gonadoblastoma, which slowly progressed to germ cell malignancy, which in this case turned out to be EST.
The classic phenotype of a person with TS is short stature (with nearly all patients being <5 feet tall) and delayed puberty.1 However, in mosaic cases (46X, i(Xq); 46XX; 47XXX; 46X, del(Xp)) normal puberty and spontaneous menarche may occur in 70% of patients.22 Due to the patient’s 46XX karyotype mosaicism, she had normal secondary sexual development. Mosaicism with 46XX occurs in 15–25% of TS cases.19 This type of mosaicism ismostly associated with a milder phenotype, including less risk of severe congenital heart disease and lymphatic abnormalities.19 Yolk sac malignancies are relatively inert in hormonal profile, and rarely associated with androgen and estrogen secretion.16,23
Following surgery the patient received a three-weekly BEP (Bleomycin, Etoposide, and Cisplatin) regimen. This regimen is highly recommended for EST. A cumulative high-dose BEP regimen results in excellent overall survival and does not seem to impair ovarian function.24 A study comparing a BEP regimen and a non-BEP regimen in yolk sac malignancy showed that the latter was independently and significantly associated with poor overall survival.25
In terms of preserving fertility, conservative surgery could be performed in children or adolescents with early-stage germ cell tumors.26 The National Comprehensive Cancer Network (NCCN), in its latest guidelines, stated that fertility-sparing surgery should be performed if the patient desired children; if not, complete surgical staging should be performed.27 Chan et al, in their review of the Surveillance, Epidemiology, and End Results (SEER) database from 1988–2001, stated that there is no significant difference between complete surgical staging and fertility-sparing surgery in EST.28 Considering the risks and benefits of fertility-sparing surgery is mandatory to accommodate patient decision making. We summarize similar cases of patients having the 46XX karyotype and germ cell tumours and their therapy of choice in Table 1.
 |
Table 1 Literature Review of 46XX Karyotype Patients with Germ Cell Malignancy |
In this case we could not perform fertility-sparing surgical staging because the mass was at an advanced stage and fertility was not desired by the patient. This case proved that we cannot diminish the possibility of precancerous ovarian lesions in women with Turner syndrome even when no Y chromosome material is present.
Conclusion
Germ cell premalignancy and malignancy in TS patient with no Y chromosome material is very rare but should not be ruled out. Exclusion of a Y cell line, the presence of which increases the risk of gonadoblastomas and subsequent gonadal germ cell tumors, is necessary. Further analysis should be carried out to identify which genetic aberration in TS patients without the Y cell line contributes to germ cell malignancy.
Abbreviations
AMH, Anti-Mullerian hormone; AFP, Alpha-feto protein; BEP, Bleomycin, Etoposide, and Cisplatin; EST, Endodermal sinus tumors; E2, Estradiol; FISH, Fluorescence in situ hybridization; FSH, Follicle-stimulating hormone; LDH, Lactic Dehydrogenase; LH, Luteinizing hormone; IOTA, International Ovarian Tumor Analysis; TS, Turner syndrome.
Take-Home Messages
- Ovarian malignancy in all TS patients should be ruled out at first before suspecting other causes of abdominal enlargement.
- Screening for gonadoblastoma should be performed in all TS cases since it is an inherited risk for germ cell malignancy. Prophylactic gonadectomy should be performed if the patient bears a high risk.
- Prophylactic gonadectomy could be performed as early as possible in all female individuals with Y chromosome material identified on standard karyotyping.
Registration of Research Studies
Registration of research is not applicable in our case.
Ethical Approval
This study is exempt from ethical approval, as determined by the institutional and departmental review board.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of their written consent is available for review by the Editor-in-Chief of this journal.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study did not receive external funding.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Zhong Q, Layman LC. Genetic considerations in the patient with Turner syndrome--45,X with or without mosaicism. Fertil Steril. 2012;98(4):775–779. doi:10.1016/j.fertnstert.2012.08.021
2. Taubel DA, Rebar RW. Puberty. In: Berek JS, editor. Berek & Novak’s Gynecology. Philadelphia: Wolters Kluwer; 2020.
3. Obata NH, Nakashima N, Kawai M, Kikkawa F, Mamba S, Tomoda Y. Gonadoblastoma with dysgerminoma in one ovary and gonadoblastoma with dysgerminoma and yolk sac tumor in the contralateral ovary in a girl with 46XX karyotype. Gynecol Oncol. 1995;58(1):124–128. doi:10.1006/gyno.1995.1195
4. Zhao S, Kato N, Endoh Y, Jin Z, Ajioka Y, Motoyama T. Ovarian gonadoblastoma with mixed germ cell tumor in a woman with 46, XX karyotype and successful pregnancies. Pathol Int. 2000;50(4):332–335. doi:10.1046/j.1440-1827.2000.01041.x
5. Erhan Y, Toprak AS, Ozdemir N, Tiras B. Gonadoblastoma and fertility. J Clin Pathol. 1992;45(9):828–829. doi:10.1136/jcp.45.9.828
6. Erdemoglu E, Ozen S. Ovarian gonodoblastoma with yolk sac tumor in a young 46, XX female: case report. Eur J Gynaecol Oncol. 2007;28(6):516–518.
7. Raafey MA, Abdulwaasey M, Fatima SS, Uddin Z, Tariq MU. Bilateral gonadoblastoma with dysgerminoma in a phenotypically normal female with 46XX karyotype: report of a rare case and literature review. Cureus. 2020;12(7):e8990. doi:10.7759/cureus.8990
8. Gorosito M, Pancera B, Sarancone S, Nocito AL. Gonadoblastoma: an unusual ovarian tumor. Ann Diagn Pathol. 2010;14(4):247–250. doi:10.1016/j.anndiagpath.2010.03.006
9. Young RH, Clement PB, Scully RE. Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament: atlas of tumor pathology; 1999.
10. Berek JS, English DP, Longacre TA, Friedlander M. Ovarian, fallopian tube, and peritoneal cancer. In: Berek JS, editor. Berek & Novaks Gynecology.
11. Saani I, Raj N, Sood R, et al. Clinical challenges in the management of malignant ovarian germ cell tumours. Int J Environ Res Public Health. 2023;20(12):6089. doi:10.3390/ijerph20126089
12. Ito K, Kawamata Y, Osada H, Ijichi M, Takano H, Sekiya S. Pure yolk sac tumor of the ovary with mosaic 45X/46X + mar Turner’s syndrome with a Y-chromosomal fragment. Arch Gynecol Obstet. 1998;262(1–2):87–90. doi:10.1007/s004040050233
13. Bremer GL, Land JA, Tiebosch A, van der Putten HW. Five different histological subtypes of germ cell malignancies in an XY female. Gynecol Oncol. 1993;50(2):247–248. doi:10.1006/gyno.1993.1201
14. Boussios S, Moschetta M, Tatsi K, Tsiouris AK, Pavlidis N. A review on pregnancy complicated by ovarian epithelial and non-epithelial malignant tumors: diagnostic and therapeutic perspectives. J Adv Res. 2018;12:1–9. doi:10.1016/j.jare.2018.02.006
15. Lau YF, Li Y, Kido T. Gonadoblastoma locus and the TSPY gene on the human Y chromosome. Birth Defects Res C Embryo Today. 2009;87(1):114–122. doi:10.1002/bdrc.20144
16. Scully RE. Gonadoblastoma. A review of 74 cases. Cancer. 1970;25(6):1340–1356. doi:10.1002/1097-0142(197006)25:6<1340::AID-CNCR2820250612>3.0.CO;2-N
17. Chandrapattan P, Jena A, Patnayak R, Mangaraj S, Naik S, Panda S. Gonadoblastoma with dysgerminoma presenting as virilizing disorder in a young child with 46, XX karyotype: a case report and review of the literature. Case Rep Endocrinol. 2022;2022:5666957. doi:10.1155/2022/5666957
18. Canto P, Kofman-Alfaro S, Jiménez AL, et al. Gonadoblastoma in Turner syndrome patients with nonmosaic 45,X karyotype and Y chromosome sequences. Cancer Genet Cytogenet. 2004;150(1):70–72. doi:10.1016/j.cancergencyto.2003.08.011
19. Gravholt CH, Andersen NH, Conway GS, et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol. 2017;177:3.
20. Mittal S, Weaver J, Aghababian A, et al. Deferring gonadectomy in patients with turner syndrome with a genetic Y component is not a safe practice. J Pediatr Urol. 2023;19(3):294.e1–e5. doi:10.1016/j.jpurol.2022.12.012
21. Gravholt CH, Fedder J, Naeraa RW, Müller J. Occurrence of gonadoblastoma in females with turner syndrome and Y chromosome material: a population study*. J Clin Endocrinol Metab. 2000;85(9):3199–3202.
22. Sybert VP. Phenotypic effects of mosaicism for a 47,XXX cell line in Turner syndrome. J Med Genet. 2002;39(3):217–220. doi:10.1136/jmg.39.3.217
23. Kao CS, Idrees MT, Young RH, Ulbright TM. “Dissecting gonadoblastoma” of scully: a morphologic variant that often mimics germinoma. Am J Surg Pathol. 2016;40(10):1417–1423. doi:10.1097/PAS.0000000000000704
24. Kang H, Kim TJ, Kim WY, et al. Outcome and reproductive function after cumulative high-dose combination chemotherapy with bleomycin, etoposide and cisplatin (BEP) for patients with ovarian endodermal sinus tumor. Gynecol Oncol. 2008;111(1):106–110. doi:10.1016/j.ygyno.2008.05.033
25. Satoh T, Aoki Y, Kasamatsu T, et al. Administration of standard-dose BEP regimen (bleomycin+etoposide+cisplatin) is essential for treatment of ovarian yolk sac tumour. Eur J Cancer. 2015;51(3):340–351. doi:10.1016/j.ejca.2014.12.004
26. Billmire D, Vinocur C, Rescorla F, et al. Outcome and staging evaluation in malignant germ cell tumors of the ovary in children and adolescents: an intergroup study. J Pediatr Surg. 2004;39(3):424–429. doi:10.1016/j.jpedsurg.2003.11.027
27. NCCN. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer. NCCN Clinical Practice Guidelines in Oncology; 2024.
28. Chan JK, Tewari KS, Waller S, et al. The influence of conservative surgical practices for malignant ovarian germ cell tumors. J Surg Oncol. 2008;98(2):111–116. doi:10.1002/jso.21079
29. Maidarti M, Garinasih PD, Anggraeni TD. Conservative surgical staging as a means to preserve fertility in patients with dysgerminoma: a case report. Ann Med Surg. 2023;85(3):427–430. doi:10.1097/MS9.0000000000000146
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

