Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Would Combination Be Better: Swimming Exercise and Intermittent Fasting Improve High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease in Obese Rats via the miR-122-5p/SREBP-1c/CPT1A Pathway
Authors Yang K , Liu C, Shao J, Guo L, Wang Q, Meng Z, Jin X, Chen X
Received 17 November 2023
Accepted for publication 12 March 2024
Published 12 April 2024 Volume 2024:17 Pages 1675—1686
DOI https://doi.org/10.2147/DMSO.S448165
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Kang Yang,1,* Chengye Liu,1,* Jun Shao,2 Lingxiang Guo,2 Qing Wang,3 Zhaoxiang Meng,1,* Xing Jin,1,* Xianghe Chen4,*
1Rehabilitation Medicine Department, Northern Jiangsu People’s Hospital Affiliated to Yangzhou University, Yangzhou city, Jiangsu Province, People’s Republic of China; 2Cardiovascular Disease Center, Northern Jiangsu People’s Hospital Affiliated to Yangzhou University, Yangzhou city, Jiangsu Province, People’s Republic of China; 3Respiratory Department, Northern Jiangsu People’s Hospital Affiliated to Yangzhou University, Yangzhou city, Jiangsu Province, People’s Republic of China; 4College of Physical Education, Yangzhou University, Yangzhou city, Jiangsu Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xianghe Chen, College of Physical Education, Yangzhou University, Huayang West Road 196, Yangzhou city, Jiangsu Province, People’s Republic of China, Email [email protected] Xing Jin, Rehabilitation Medicine Department, Northern Jiangsu People’s Hospital Affiliated to Yangzhou University, Nantong West Road 98, Yangzhou city, Jiangsu Province, People’s Republic of China, Email [email protected]
Background: Swimming and intermittent fasting can both improve obesity-induced NAFLD, but which of the two is more effective and whether the combination of the two has a superimposed effect is inconclusive.
Methods: The model of NAFLD in obese rats was established by a high-fat diet and performed swimming, intermittent fasting, and a combination of both interventions for 8 weeks. Serum lipids and enzyme activity were measured by an automatic biochemical analyzer. Liver morphostructural analysis was observed by transmission electron microscopy, and morphology was observed by HE staining. RT‒PCR was used to detect the mRNA level.
Results: Morphology and microstructure of the liver of model rats were impaired, with the upregulation of miR-122-5p, SREBP-1c, FASN and ACC1. Eight weeks of swimming exercise, intermittent fasting and the combination of both attenuate these effects, manifested by the downregulation of miR-122-5p and upregulation of CPT1A mRNA levels. There was no significant stacking effect of the combination of the swimming and intermittent fasting interventions.
Conclusion: NAFLD leads to pathology in model rats. Eight weeks of swimming exercise, intermittent fasting and the combination of both can inhibit miR-122-5p and improve hepatic lipid metabolism, while no significant additive effects of combining the interventions were found.
Keywords: obesity, NAFLD, swimming, intermittent fasting, miR-122-5p
Introduction
Since the onset of the 21st century, the lifestyles of individuals in Western and Eastern societies have been characterized by elevated consumption of carbon, fat, and sugar, coupled with low levels of physical activity. Consequently, there has been a yearly rise in the prevalence of obesity.1,2 Obesity, characterized by abnormal fat distribution and excessive accumulation, is a prominent contributor to chronic ailments including cardiovascular disease, metabolic syndrome, and nonalcoholic fatty liver disease (NAFLD).3 In the process, the pancreatic β-cells undergo damage due to prolonged excessive production of insulin, resulting in decreased secretion of insulin and the emergence of insulin resistance (IR).4 Consequently, the accumulation of fatty acids (FAs) and triglycerides (TGs) is further exacerbated.5 The surplus FAs and TGs that cannot be metabolized are subsequently deposited in the liver, leading to disturbances in liver lipid metabolism and the development of NAFLD.6 There are four primary origins of these challenging-to-remove FAs and TGs in the context of NAFLD: heightened lipid uptake, diminished fatty acid oxidation, impaired lipoprotein synthesis or secretion, and amplified fatty acid synthesis.7–10 The latter source encompasses peroxisome proliferator-activated receptor γ (PPARγ), carbohydrate response element-binding protein (ChREBP), miR-122-5p, and sterol regulatory element-binding protein-1c (SREBP-1c), which act as positive regulators of hepatic TG levels and expedite the pace of fatty acid synthesis in NAFLD patients by modulating crucial lipid synthesis genes, such as fatty acid synthase (FASN), acetyl coa carboxylase 1 (ACC1) and carnitine palmitoyltransferase 1A (CPT1A).11–13
Based on the aforementioned sources, exercise interventions targeting increased caloric expenditure or intermittent fasting to decrease energy intake have demonstrated efficacy in enhancing high-fat diet-induced NAFLD.14 Among various aerobic exercises, swimming is highly recommended due to its accessibility, knee-friendly nature, and ability to engage multiple muscle groups, which relatively sustainably and efficiently accelerates the oxidative breakdown of FAs into ATP, ADP, water and lactic acid, reducing FAs synthesis directly in the body.15 Research has indicated that swimming exercise effectively mitigates lipid accumulation and ameliorates pathological alterations in the livers of zebrafish subjected to a high-fat diet.16 Intermittent fasting (IF) is a method of controlling diet for a period of time, consuming as few/no calories as possible on fasting days and then eating a normal diet on nonfasting days, which minimizing lipid intake directly at the source.17 Studies have demonstrated that this method can be effective in controlling body weight, improving insulin sensitivity and protecting cardiovascular health.18–20 A clinical Trial has proved that Intermittent fasting has beneficial effects on the lipid profile, and associated with weight loss and a modification of the distribution of abdominal fat in people with obesity and type 2 diabetes as well as an improvement in the control of glycemic levels.21 In summary, obesity triggers abnormal glucose metabolism in the organism, leading to impaired triggering of pancreatic β-cells, which further leads to accumulation of gluconeogenic products FAs and TGs in the liver.22 Swimming expedites the oxidative breakdown of FAs and TGs and reduces the synthesis of FAs; IF decreases lipid and sugar uptake. However, can swimming combined with intermittent fasting have an additional impact on obesity-related NAFLD? And how does the miR-122-5p/SREBP-1c/CPT1A pathway play a role in this process? These inquiries remain unanswered as there is a lack of research on the subject.
In the present study, we used a high-fat diet-induced NAFLD model with swimming exercise and intermittent fasting to evaluate the effects of exercise, dietary control and their combination on the miR-122/SREBP-1c/CPT1A pathway in the livers of NAFLD rats. We expected to find a better approach to promote liver recovery in NAFLD rats and hope that this study can provide theoretical guidance for the treatment and prevention of NAFLD.
Materials and Methods
Animals and Experimental Design
Seventy-five 4-week-old male Sprague‒Dawley rats (code: SCXK: Su 2017–0007) were purchased from Yangzhou University (Yangzhou, China). Rats were housed in individual cages, 4–5 per cage, under standard conditions with a 12-hour light/dark cycle, temperature of 23±2°C, and free access to food and water. All animal experiments were approved by the Animal Ethics Committee of Yangzhou University (approval number: YZU-TYXY-0031). The experimental procedures were in agreement with the “Regulations for the Administration of Affairs Concerning Experimental Animals of China”, for welfare of experimental animals under “Laws of the People’s Republic of China”.
All animals were adaptively fed for 1 week and then randomly divided into a normal control group (N, n=15) and an obesity model (n=60), respectively fed a normal chow diet23 and a high-fat diet24 (formulation in Table S1; purchased from Jiangsuxietong Pharmaceutical Bioengineering Co., Ltd. Nanjing, China) for 10 weeks. A body weight ≥20% of the average weight of the normal group was used as the criterion for obesity. Fifty-eight obese model rats were successfully established and randomly divided into the obesity control group (O, n=14), obesity+swimming exercise group (S, n=14), obesity+intermittent fasting group (I, n=15) and obesity+swimming combined with intermittent fasting group (C, n=15).
Intermittent Fasting and Swimming Exercise Protocol
The specific intervention protocol was adapted from previous research and slightly modified to suit the current situation.25,26 Rats in groups I and C were fasted for 2 nonconsecutive days/8-day cycles and 7 cycles for 8 weeks. Groups S and C underwent weightless swimming training at 18:00 on each training day, 3 training days/8-day cycle, and 7 cycles for 8 weeks.27,28 Of these, the protocol for group C was designed to allow at least one day of rest between fasting days and training days to ensure animal welfare. During the first week of acclimatization training, the swimming time ranged from 15 min to 60 min within this week, and the swimming pool was a 120 cm×80 cm×70 cm rectangular swimming bucket with a water depth of 50 cm and a water temperature of 31±2°C. During the training process, the condition of the rats was constantly monitored to prevent drowning and death, and feces were fished out in time to keep the water clean and hygienic. The specific experimental procedure is shown in Figure 1.
Tissue Preparation
Samples were collected 12 h after the last exercise session (8 h fasting prior to sampling). The next morning, the final fasted body weight was weighed, and all rats were anesthetized with an intraperitoneal injection of 20% urethane at a dose of 0.5 mL/kg before blood collection by aortic exsanguination. The blood was then rested for 30 min at 4°C before centrifugation at 4000 rpm×10 min at 4°C, and the serum was aspirated and stored at −20°C for analysis of serum lipids and enzyme activity. Liver tissues were collected for later HE staining with 10% formaldehyde fixative, transmission electron microscopy by 2% glutaraldehyde fixative at 4°C and mRNA determination. All aspects of the experiment were conducted according to the guidelines provided by the ethical committee of experimental animal care at Yangzhou University.
Assays of Serum Lipids and Enzyme Activity
Serum levels of alanine aminotransferase (ALT), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and fasting blood glucose (FBG) were measured by a Hitachi automatic biochemical analyzer (Hitachi, Tokyo, Japan). Fasting blood insulin (FBI) was assessed by an insulin ELISA kit (Solarbio, Beijing, China). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated by the formula “HOMA-IR=FBI×FBG/22.5”.
Morphostructural Analysis of Liver Tissue
Rat liver right lobe tissue was cleaned with 0.01 M PBS, fixed in 10% formaldehyde for 48 hours and washed again with 0.01 M PBS. The cleaned liver tissues were cut into 4 mm thin slices, dehydrated through graded alcohol and then placed in transparent xylene solution to displace the alcohol. Then, according to the standard procedure for HE staining, the liver tissues were sequentially embedded in immersion wax, sectioned, deparaffinized, stained, dehydrated, replaced and embedded in neutral resin. Pathological changes in the rat liver tissues were observed under a microscope.
Microstructural Analysis of Liver Tissue
The rat liver tissues were cut into 1 mm slices, immediately added to 2% glutaraldehyde fixative and fixed at 4°C for 2 h. The tissues were washed with 0.1 M PBS for 2 h in the same 4°C environment, with 3–5 liquid changes in between, fixed with 1% osmic acid for 2 h, and then further washed with 0.1 M PBS for 2 h, with 3–5 liquid changes in between. Then, according to the standard procedure of transmission electron microscopy observation, the liver tissues were sequentially dehydrated, infiltrated, embedded, ultrathin sectioned, electron stained and then placed under the electron microscope to observe the change in microstructure.
Determination of mRNA Levels in the Liver
According to the reagent manual, total RNA was extracted from livers with TRIzol (TianGen, Beijing, China). The quality of total RNA was checked by Agarose gel electrophoresis, and the purity and concentration of mRNA were detected by an ultraviolet spectrophotometer. The PrimeScriptTM RT Reverse Reagent Kit was then used with gDNA Eraser (Takara, Shiga, Japan) to convert mRNA into cDNA. The MiRcute Plus miRNA First-Strand cDNA Kit (TianGen, Beijing, China) was used for miR-122-5p. PCR amplification was performed using TB Green® Premix Ex TaqTMII (Takara, Shiga, Japan). Primers were designed using Primer Premier primer design software and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). U6 and β-actin were used as internal references respectively to detected the mRNA expression of miR-122-5p and SREBP-1c, DGAT2, FASN, ACC1, CPT1A. Reaction conditions were predenaturation at 95 °C for 2 min, and the PCR reaction (94 °C, 10s, 60 °C, 40s) for 40 cycles. The 2−ΔΔCt method was used to calculate the mRNA expression of each gene. The primer sequences are listed in Table 1.
 |
Table 1 List of Primer Sequences |
Statistical Analysis
SPSS 20.0 was used for statistics, and GraphPad Prism 5.0 was used for image generation. Data are expressed as the mean±SD. N group and O group were subjected to independent sample T tests. One-way ANOVA analysis of variance was used for O group, S group, I group and C group. Tukey’s test was used to analyze significant interaction effects. Significance was set at 0.05.
Results
Effects of Swimming and Intermittent Fasting on Weight and Glucose Metabolism Index
Figure 2 shows the weight, FBG, FBI and HOMA-IR of rats in each group at the 19th week. Compared with the N group, the O group manifested a significant increase in weight (P<0.01), FBG (P<0.05), FBI (P<0.05) and HOMA-IR (P<0.05), indicating that the obese model rats induced by a high-fat diet developed glucose metabolism disorders and weight gain.
However, compared to the O group, the S group, I group and C group all exhibited significantly lower weight (P<0.05), lower FBG (P<0.05), lower FBI (P<0.05), and HOMA-IR (P<0.05), suggesting that 8 weeks of swimming exercise, intermittent fasting and these combinations alleviated the above indicators. Additionally, Tukey’s test results indicated that the combination of swimming exercise and intermittent fasting had no significant superimposed effect on weight, FBG, FBI or HOMA-IR.
Effects of Swimming and Intermittent Fasting on the Morphostructure of Liver Tissue
As shown in Figure 3 of liver tissue, the N group showed a clear structure of liver lobules, no lipid droplet vacuoles, neatly arranged hepatic cords and normal hepatic sinusoids. Group O showed varying degrees of fatty vacuole degeneration, with recognizable structures of liver lobules, disorganized hepatic cords and constricted hepatic sinusoids. Group S had fewer lipid droplet vacuoles in the hepatocytes, and the disorganization of the hepatic cords had become milder. Group I had a significantly lower degree of steatosis, and the structure of the hepatocytes tended to be normalized. Group C showed more obvious improvements in the morphology and structure of the liver tissues than groups S and I. However, a small number of fat droplet vacuoles were still observed. Group C showed a more obvious improvement in the morphology and structure of liver tissue than groups S and I. Hepatocyte sinusoids tended to be normalized, but a small number of lipid droplet vacuoles could still be seen.
Effects of Swimming and Intermittent Fasting on the Microstructure of Liver Tissue
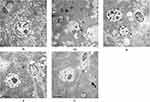
As shown in Figure 4, the nuclei of rat hepatocytes in group N were round, with clear and intact nuclear membranes, abundant rough endoplasmic reticulum in the cytoplasm, and abundant mitochondrial cristae. The cytoplasm of hepatocytes from rats in group O was full of fat vacuoles of various sizes, and even the nuclei of the rats were squeezed by fat droplets, with obvious expansion of the endoplasmic reticulum, a reduction in the number of mitochondria, and blurring of the cristae. Group I rats had round nuclei, abundant mitochondria, rough endoplasmic reticulum, clear structure and few lipid droplets. Group C rats had round nuclei, abundant mitochondria and rough endoplasmic reticulum, clear structure, abundant glycogen particles in the cytoplasm and further reduction of lipid droplets. Group C rats had round nuclei, abundant mitochondria and rough endoplasmic reticulum, clear structure and abundant glycogen particles in the cytoplasm, and further reduction of lipid droplets.
Effects of Swimming and Intermittent Fasting on Serum Lipids and Enzyme Activity
ALT is a specific enzyme in the liver that has been shown to be an important indicator of the extent of liver damage and is released from hepatocytes when they are damaged, leading to an increase in its concentration in the blood. The high concentrations of TC, TG, LDL-c and low concentrations of HDL-c in the serum indicate increased fat accumulation and further deterioration of NAFLD. As shown in Table 2, compared to group N, serum ALT (P<0. 01), TC (P<0.05), TG (P<0.05), LDL-c (P<0.05) and HDL-c (P<0.05) were significantly increased and HDL-c (P<0.05) was significantly decreased in group O, suggesting that obesity due to long-term high-fat feeding caused abnormal liver function and induced nonalcoholic steatosis in rats.
 |
Table 2 Animal Characteristics |
 |
Table 3 Relative mRNA Expression in the miR-122-5p/SREBP-1c/CPT1A Pathway |
Compared with group O, the serum ALT (P<0.01), TC (P<0.05), TG (P<0.05) and LDL-c (P<0.05) of rats in groups S, I and C were significantly decreased, and the difference in HDL-c was not significant, although it showed an increasing trend, indicating that 8 weeks of swimming exercise, intermittent fasting and a combination of both reversed the pathology of obesity-induced liver parenchymal damage and nonalcoholic steatosis. In addition, Tukey’s test results showed that the combination of swimming exercise and intermittent fasting had no significant superimposed effect on ALT, TC, TG, LDL-c and HDL-c.
Effects of swimming and intermittent fasting on relative mRNA expression in the miR-122-5p/SREBP-1c/CPT1A pathway in obese rats.
As shown in Figure 5 and Table 3, the mRNA expression levels of miR-122-5p (P<0.05), SREBP-1c (P<0.05), DGAT2 (P<0.05), FASN (P<0.05) and ACC1 (P<0.05) were significantly increased in the liver of the O group compared with the N group, while CPT1A (P<0.05) was significantly decreased, indicating that obesity inhibited NAFLD via the miR-122-5p/SREBP-1c/CPT1A pathway.
Compared with the O group, the expression levels of miR-122-5p (P<0.05), SREBP-1c (P<0.05), FASN (P<0.05) and ACC1 (P<0.05) were significantly decreased in the S, I and C groups, while CPT1A (P<0.05) was significantly increased. Although DGAT2 showed a decreasing trend, the difference was not significant. These results indicate that swimming, intermittent fasting and their combination can reverse the negative influence of obesity-induced NAFLD in rats to some extent. Unfortunately, Tukey’s test results showed that the combination of swimming exercise and intermittent fasting still had no significant superimposed effect.
Discussion
In the present study, we observed the effects of prolonged (8 weeks) swimming exercise, intermittent fasting, and the combination of the two on nonalcoholic fatty liver disease in rats with a high-fat diet (HFD)-induced obesity model. We found that 8 weeks of swimming exercise, intermittent fasting, and the combination of both attenuated lipid metabolism in obese rats. Exercise/intermittent fasting/these combination-induced improvements in these effects were associated with inhibition of the miR-122-5p/SREBP-1c/CPT1A signaling pathway in the liver tissue of obesity model rats. The upregulation of the hepatic lipid metabolism marker gene miR-122-5p played an important role in this process, and its high expression predicted overloaded TC and TG accumulation. We will discuss this point later.
In this study, we found that peripheral blood glucose levels, peripheral insulin levels and body weight increased in HFD-induced obese model rats, whereas swimming exercise, intermittent fasting and a combination of both significantly improved insulin levels, blood glucose levels, body weight and insulin resistance status in obese rats. These results are in line with previous studies in our group. One point that needs to be clarified is why we made an extra effort to test glucose metabolic indices in obese rats. Our previous studies have shown that excessive fat accumulation leads to damage to pancreatic β-cells, which have to overproduce insulin for a long time, resulting in insulin resistance in the organism.29 In this paper, the abnormal increase in glucose metabolic indices, especially HOMA-IR, in group O rats supports this point. Our obese rats are in the process of developing T2DM, which is an important reason why many basic studies and clinical cases often find that NAFLD caused by abnormalities in hepatic lipid metabolism due to obesity is often accompanied by T2DM.30–32 Additionally, these results were also reflected in the changes in serum ALT, TC, TG, LDL-c and HDL-c, further confirming that long-term swimming exercise, intermittent fasting and the combination of both can ameliorate the pathology of serum lipid metabolism abnormalities in obese rats.
In addition, numerous studies have shown that the liver is an important organ in obesity-induced injury, often characterized by nonalcoholic fatty liver disease and nonalcoholic hepatitis, among others.33–35 Wilson et al found that the onset of obesity often involves the liver parenchyma.26 In our study, we found that the morphostructure of the liver of model rats was impaired with varying degrees of fatty vacuole degeneration, with recognizable structures of the liver lobules, disorganized hepatic cords and constricted hepatic sinusoids. Transmission electron microscopy observations showed that obesity resulted in the rat liver cytoplasm being filled with many fat vacuoles of varying sizes and even nuclei being extruded by lipid droplets, with obvious dilation of the endoplasmic reticulum, a reduced number of mitochondria and blurred cristae. Long-term (8 weeks) swimming exercise, intermittent fasting and the combination of the two can significantly improve the morphology of nonalcoholic fat in obese rats. These results suggest that long-term swimming exercise, intermittent fasting and the combination of the two can improve the morphostructure and microstructure of the liver of obese rats.
Interestingly, we found that 8 weeks of swimming exercise, intermittent fasting and the combination of both inhibited miR-122-5p and activated CPT1A to improve hepatic lipid metabolism, which decreased SREBP-1c, DGAT2, FASN and ACC1 mRNA levels. Our results are consistent with previous findings that exercise improves obesity-related NAFLD.36–38 In addition, Wilson et al25 found that intermittent fasting was superior to exercise in improving NAFLD, whereas in our study, although intermittent fasting was superior to swimming exercise in many measures, the difference was not significant. Another interesting question about the current study is why the combination of the two interventions did not have a significant stacking effect, which seems to be inconsistent with common sense. This may be related to the length of our intervention and the frequency of the intervention. We also tried to look for answers in other studies. Byrne et al39 demonstrated greater weight loss efficiency when intermittent energy restriction was interrupted with periods of energy balance ‘rest periods’ to potentially reduce any compensatory metabolic responses that can occur to return the body to its original weight. Such compensatory mechanisms goes some way to explain the modest additive effect observed. Keenan et al40 found that the 4-day-per-week fasting group improved total cholesterol, LDL cholesterol, and HDL cholesterol better than the 2-day-per-week fasting group on the basis of both resistance exercise, which validated our earlier conjecture that the stacking effect was inconspicuous because of the frequency and timing of interventions from another perspective. We will also further break down the swimming exercise and intermittent fasting frequency and intervention duration at a later stage to test our hypothesis and better explain the non-significant stacking effect in this study.
In summary, our study found that swimming exercise, intermittent fasting and the combination of both inhibited miR-122-5p, which in turn downregulated the expression of downstream SREBP-1c, FASN and ACC1 and activated CPT1A to regulate the oxidative synthesis of fatty acids in the liver, ultimately ameliorating NAFLD. There was no significant stacking effect of the combination of the swimming and intermittent fasting interventions.
There are many limitations in present study as well, one of which is the failure to knockdown or overexpress miR-122-5p in in vitro experiments. This means that our study lacks a certain degree of persuasiveness in exploring the mechanisms by which swimming exercise, intermittent fasting, and a combination of the two, ameliorate NAFLD in obese rats. Our research team will address this issue in future cellular experiments.Western Blot and oil red o staining was not performed is another weak point, which we will address and improve in later studies.
Conclusion
We investigated the protective role of exercise swimming, intermittent fasting and the combination of both in high-fat diet-induced NAFLD in obese rats. We provided evidence that obesity-induced NAFLD is associated with increased miR-122-5p activity and that swimming exercise, intermittent fasting and the combination of both attenuate these effects, which may be influenced by downregulation of the miR-122-5p/SREBP-1c/DGAT2/FASN/ACC1/CPT1A pathway (summarized in Figure 6).
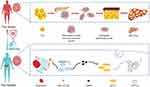 |
Figure 6 Mechanism by which swimming exercise combined with intermittent fasting ameliorates high-fat diet-induced nonalcoholic fatty liver in obese rats via the miR-122-5p/SREBP-1c/CPT1A pathway. |
Acknowledgments
We are grateful for the language editing service by Qian Zhang and expert technical assistance by Zhimei Liu.
Ethics
All animal experiments were approved by the Experimental animal ethics committee of Yangzhou University (approval number: YZU-TYXY-0031) and carried out in accordance with National Institute of Health’s Guide for the Care and Use of Laboratory Animals.
Funding
This work was supported by the Scientific Research Fund of Northern Jiangsu People’s Hospital (SBKY21010), Yangzhou Science and Technology Program (YZ2022099), The China Postdoctoral Science Foundation (2019M661957), The Special grants from China Postdoctoral Science Foundation (2021T140580), 2020 Yangzhou University ‘Highend Talent Support Program’ and 2021 Yangzhou University ‘Qinglan Project’.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Jensen T, Abdelmalek MF, Sullivan S, et al. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018;68(5):1063–1075.
2. Yustisia I, Tandiari D, Cangara MH, et al. A high-fat, high-fructose diet induced hepatic steatosis, renal lesions, dyslipidemia, and hyperuricemia in non-obese rats. Heliyon. 2022;8(10):e10896.
3. Deprince A, Haas JT, Staels B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol Metabol. 2020;42:101092.
4. Hong SH, Choi KM. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int J Mol Sci. 2020;21(2):494.
5. Yan T, Luo Y, Yan N, et al. Intestinal peroxisome proliferator-activated receptor α-fatty acid-binding protein 1 axis modulates nonalcoholic steatohepatitis. Hepatology. 2023;77(1):239–255.
6. Govaere O, Petersen SK, Martinez-Lopez N, et al. Macrophage scavenger receptor 1 mediates lipid-induced inflammation in non-alcoholic fatty liver disease. J Hepatol. 2022;76(5):1001–1012.
7. Badmus OO, Hillhouse SA, Anderson CD, et al. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): functional analysis of lipid metabolism pathways. Clin Sci. 2022;136(18):1347–1366.
8. Zhang Y, Lin S, Peng J, et al. Amelioration of hepatic steatosis by dietary essential amino acid-induced ubiquitination. Molecular Cell. 2022;82(8):1528–42.e10.
9. Lee KC, Wu PS, Lin HC. Pathogenesis and treatment of non-alcoholic steatohepatitis and its fibrosis. Clin molecular hepatol. 2023;29(1):77–98.
10. Lee E, Korf H, Vidal-Puig A. An adipocentric perspective on the development and progression of non-alcoholic fatty liver disease. J Hepatol. 2023;78(5):1048–1062.
11. Inomata Y, Oh JW, Taniguchi K, et al. Downregulation of miR-122-5p Activates Glycolysis via PKM2 in Kupffer Cells of Rat and Mouse Models of Non-Alcoholic Steatohepatitis. Int J Mol Sci. 2022;23(9):567.
12. Hu Y, Peng X, Du G, et al. MicroRNA-122-5p Inhibition Improves Inflammation and Oxidative Stress Damage in Dietary-Induced Non-alcoholic Fatty Liver Disease Through Targeting FOXO3. Front Physiol. 2022;13:803445.
13. Monraz-Méndez CA, Escutia-Gutiérrez R, Rodriguez-Sanabria JS, et al. Moringa oleifera Improves MAFLD by Inducing Epigenetic Modifications. Nutrients. 2022;14(20):4225.
14. Ezpeleta M, Gabel K, Cienfuegos S, et al. Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: a randomized controlled trial. Cell Metab. 2023;35(1):56–70.e3.
15. Pi H, Liu M, Xi Y, et al. Long-term exercise prevents hepatic steatosis: a novel role of FABP1 in regulation of autophagy-lysosomal machinery. FASEB j. 2019;33(11):11870–11883.
16. Zou Y, Chen Z, Sun C, et al. Exercise Intervention Mitigates Pathological Liver Changes in NAFLD Zebrafish by Activating SIRT1/AMPK/NRF2 Signaling. Int J Mol Sci. 2021;22(20):10940.
17. Lavallee CM, Bruno A, Ma C, et al. The Role of Intermittent Fasting in the Management of Nonalcoholic Fatty Liver Disease: a Narrative Review. Nutrients. 2022;14(21):4655.
18. Dong TA, Sandesara PB, Dhindsa DS, et al. Intermittent Fasting: a Heart Healthy Dietary Pattern? Am j Med. 2020;133(8):901–907.
19. Freire R. Scientific evidence of diets for weight loss: different macronutrient composition, intermittent fasting, and popular diets. Nutrition. 2020;69:110549.
20. Varady KA, Cienfuegos S, Ezpeleta M, et al. Cardiometabolic Benefits of Intermittent Fasting. Annu. Rev. Nutr. 2021;41:333–361.
21. Morales-Suarez-Varela M, Collado Sánchez E, Peraita-Costa I, et al. Intermittent Fasting and the Possible Benefits in Obesity, Diabetes, and Multiple Sclerosis: a Systematic Review of Randomized Clinical Trials. Nutrients. 2021;13(9):3179.
22. Alnami A, Bima A, Alamoudi A, et al. Modulation of Dyslipidemia Markers Apo B/Apo A and Triglycerides/HDL-Cholesterol Ratios by Low-Carbohydrate High-Fat Diet in a Rat Model of Metabolic Syndrome. Nutrients. 2022;14(9):1903.
23. Chen X, Yang K, Jin X, et al. Bone Autophagy: a Potential Way of Exercise-Mediated Meg3/P62/Runx2 Pathway to Regulate Bone Formation in T2DM Mice. Diabetes Metabolic Syndrome Obesity. 2021;14:2753–2764.
24. Lang X, Zhao N, He Q, et al. Treadmill exercise mitigates neuroinflammation and increases BDNF via activation of SIRT1 signaling in a mouse model of T2DM. Brain Res Bull. 2020;165:30–39.
25. Wilson RA, Deasy W, Stathis CG, et al. Intermittent Fasting with or without Exercise Prevents Weight Gain and Improves Lipids in Diet-Induced Obese Mice. Nutrients. 2018;10(3):546.
26. Wilson RA, Stathis CG, Hayes A, et al. Intermittent Fasting and High-Intensity Exercise Elicit Sexual-Dimorphic and Tissue-Specific Adaptations in Diet-Induced Obese Mice. Nutrients. 2020;12(6):1764.
27. Al-Thepyani M, Algarni S, Gashlan H, et al. Evaluation of the Anti-Obesity Effect of Zeaxanthin and Exercise in HFD-Induced Obese Rats. Nutrients. 2022;14(23):4944.
28. Farag MA, Ammar NM, Kholeif TE, et al. Rats’ urinary metabolomes reveal the potential roles of functional foods and exercise in obesity management. Food Funct. 2017;8(3):985–996.
29. Chen X, Yang K, Sun P, et al. Exercise improves bone formation by upregulating the Wnt3a/β-catenin signalling pathway in type 2 diabetic mice. Diabetol Metab Syndr. 2021;13(1):116.
30. Malone JI, Hansen BC. Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite? Pediatric Diabetes. 2019;20(1):5–9.
31. Al-Sulaiti H, Diboun I, Agha MV, et al. Metabolic signature of obesity-associated insulin resistance and type 2 diabetes. J Transl Med. 2019;17(1):348.
32. Walsh JS, Vilaca T. Obesity, Type 2 Diabetes and Bone in Adults. Calcified Tissue Int. 2017;100(5):528–535.
33. Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92:82–97.
34. Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17(8):484–495.
35. Rong L, Zou J, Ran W, et al. Advancements in the treatment of non-alcoholic fatty liver disease (NAFLD). Front Endocrinol. 2022;13:1087260.
36. Farzanegi P, Dana A, Ebrahimpoor Z, et al. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): roles of oxidative stress and inflammation. Eur j Sport Sci. 2019;19(7):994–1003.
37. Sodum N, Kumar G, Bojja SL, et al. Epigenetics in NAFLD/NASH: targets and therapy. Pharmacol Res. 2021;167:105484.
38. Carbajo-Pescador S, Porras D, García-Mediavilla MV, et al. Beneficial effects of exercise on gut microbiota functionality and barrier integrity, and gut-liver crosstalk in an in vivo model of early obesity and non-alcoholic fatty liver disease. Dis Models Mech. 2019;12(5):567.
39. Byrne NM, Sainsbury A, King NA, et al. Intermittent energy restriction improves weight loss efficiency in obese men: the MATADOR study. Int J Obesity. 2018;42(2):129–138.
40. Keenan S, Cooke MB, Chen WS, et al. The Effects of Intermittent Fasting and Continuous Energy Restriction with Exercise on Cardiometabolic Biomarkers, Dietary Compliance, and Perceived Hunger and Mood: secondary Outcomes of a Randomised, Controlled Trial. Nutrients. 2022;14(15):3071.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.