Back to Journals » International Medical Case Reports Journal » Volume 17
Whole-Exome Sequencing Identifies DYNC2H1 Mutations as a Cause of Jeune Asphyxiating Thoracic Dystrophy Without Extra‐Skeletal Organ Involvement
Authors Asseri AA , Alzoani AA, Almahdi M, Almahdi H, Almushayt N, Alyazidi NS, Al Mufarrih BM
Received 31 October 2023
Accepted for publication 18 March 2024
Published 23 March 2024 Volume 2024:17 Pages 209—214
DOI https://doi.org/10.2147/IMCRJ.S447466
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Vinay Kumar
Ali Alsuheel Asseri,1 Ahmad A Alzoani,2 Mohammed Almahdi,3 Hussein Almahdi,4 Nouf Almushayt,3 Noha Saad Alyazidi,3 Basmah Mohammed Al Mufarrih5
1Department of Child Health, King Khalid University, Abha, 61421, Saudi Arabia; 2Department of Neonatology, Abha Maternity and Children Hospital, Ministry of Health, Abha, 62521, Saudi Arabia; 3Department of Pediatrics, Abha Maternity and Children Hospital, Ministry of Health, Abha, 62521, Saudi Arabia; 4College of Medicine, King Khalid University, Abha, 61421, Saudi Arabia; 5Department of Obstetrics and Gynecology, Abha Maternity and Children Hospital, Ministry of Health, Abha, 62521, Saudi Arabia
Correspondence: Ali Alsuheel Asseri, Department of Child Health, College of Medicine, King Khalid University, Abha, Saudi Arabia, Tel +966500186013, Fax +9662418139, Email [email protected]
Abstract: Jeune syndrome, or asphyxiating thoracic dystrophy (JATD), is a rare autosomal recessive skeletal dysplasia with heterogeneous genetic and clinical phenotypes, which primarily affects cartilage and bone development. Herein, we report a patient with a lethal form of SRTD3 without polydactyly (JATD), which led to severe restrictive lung disease and fatal respiratory failure. A full-term boy was born to a 30-year-old mother who was known to have hypothyroidism and was on thyroxine. The parents were first-degree cousins and had one healthy older son. Fetal ultrasound showed a cephalic fetus, normal amniotic fluid and a fundal placenta. All long bones and ribs were below the 1% percentile. The femur was bowed with no fractures or signs of significant demineralization at time of imaging. Head and abdominal circumference were within normal range. An echocardiogram on the 2nd day of life showed severe pulmonary hypertension (PHTN). Nitric oxide was started due to the presence of persistent hypoxia and severe PHTN. The patient continued to require high cardiorespiratory support, but the medical condition worsened, and respiratory failure persisted. The patient died of severe respiratory failure at 16 days of life due to respiratory insufficiency secondary to a severely restricted thoracic cage. Whole-exome sequencing (WES) revealed a homozygous mutation in the DYNC2H1 (NM_001377.3) gene, namely, the c.9041G>T NP_001368.2: p.(Arg3014Ile) missense variant, which results in the substitution of the arginine codon at amino acid position 3014 with an isoleucine codon. The phenotyping of the patient’s JATD and the detection of a homozygous variant in the DYNC2H1 gene confirmed the diagnosis of short-rib thoracic dysplasia-3 without polydactyly. In summary, the patient had isolated skeletal anomalies without polydactyly or other organ involvement. Additionally, the infant had severe PHTN on top of the respiratory failure, which eventually caused death. Considerably more work will need to be done to determine the clinical spectrum of JATD and understand its genetic heterogeneity.
Keywords: SRTD3, Jeune syndrome, asphyxiating thoracic dystrophy, DYNC2H1
Introduction
Jeune syndrome, or asphyxiating thoracic dystrophy (JATD [MIM 208500]), is a rare autosomal recessive skeletal dysplasia with heterogeneous genetic and clinical phenotypes, which primarily affects cartilage and bone development.1 Although the exact prevalence of JATD is unknown, reports estimate an incidence of 1 per 100,000 to 130,000 live births.2,3 In Saudi Arabia, few cases have been reported with genetic heterogeneity and clinical variability.4,5 Until recently, JATD was deemed a separate clinical syndrome; however, since 2006, JATD has been considered part of the subclass of short-rib thoracic dysplasia3 (SRTD3; OMIM #613091) with or without polydactyly.1,3 As of now, there are 21 categories of short-rib thoracic dysplasia with or without polydactyly type 1–21 (SRTD1-21).3 SRTD3 subtype is caused by a mutation of the DYNC2H1 gene (dynein, cytoplasmic 2, heavy chain 1; OMIM #603297), which has more than 200 reported variants with variable pathogenicity.3
Although JATD has a distinctive clinical and radiological phenotype, it is considered one of the skeletal ciliopathy disorders that share many clinical and radiological manifestations. The clinical characteristics of JATD include short stature, a narrow chest, micromelia, limb shortening, a small and narrow thorax, and short ribs.6 Moreover, anomalies of major organs such as the brain, eyes, heart, kidneys, liver, pancreas, intestines, and genitalia can also occur.6 A small and narrow thorax leads to thoracic cage compression on the lungs and subsequent pulmonary hypoplasia. The degree of postnatal respiratory insufficiency correlates with the severity of thoracic bone abnormality. Due to the limitations of conducting an objective physiological assessment in neonates, clinical assessment and the need for invasive ventilation are the parameters of assessing the severity of disease. Herein, we describe a patient with a lethal form of SRTD3 without polydactyly, which led to severe restrictive lung disease and fatal respiratory failure.
Case Description
A full-term boy was born to a 30-year-old mother who was known to have hypothyroidism and was on thyroxine. The parents were first-degree cousins and had one healthy older son. The mother had regular follow-ups during pregnancy, experienced normal fetal movements, and no concern was expressed. She was referred at 33 weeks’ gestation to the maternal–fetal medicine clinic at Abha Maternity and Children Hospital after a routine antenatal ultrasound found that the baby had a short femur. Fetal ultrasound showed a cephalic fetus, normal amniotic fluid, and a fundal placenta. The fetal femur to foot length ratio was 0.59 (normal ratio ≥1) and the chest to abdomen ratio was 0.72 (normal ratio ≥0.89). All long bones and ribs were below the 1% percentile. The femur was bowed with no fractures or signs of significant demineralization at time of imaging. The femur length/abdominal circumference ratio was 0.14 (it is normally between 0.20 and 0.24). A ratio of less than 0.16 in a population at risk is consistent with severe skeletal dysplasia.7–9 The head and abdominal circumferences were within normal ranges. The cardiac axis was shifted likely due to the narrow chest of the fetus (Figures 1A–D).8,9
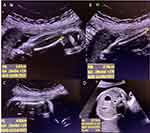 |
Figure 1 Antenatal ultrasound showing (A) short tibia, (B) short fibula, (C) short femur, and (D) abdominal circumference. |
The baby was delivered cephalically, was vigorous, had clear amniotic fluid, and required only initial steps of newborn resuscitation. The APGAR score was 7.8 at 1 and 5 minutes, respectively, with an acceptable cord blood gas value. General appearance of the neonate showed an apparently small and narrow chest in comparison with his abdomen (Figures 2A and B), coarse facial features, a large head, a narrow anterior fontanel, and no abnormality in the shape of the head, eyes, ears or neck. There were normal first and second heart sounds, with no added sounds; the abdomen was soft and not distended; there was no organomegaly, abnormal tone or poor reflexes; and he had normal male genitalia. The lower limbs were apparently short bilaterally, but there was no discrepancy in their size or shape; the feet had a normal upper extremity and no abnormal digits. Chest and upper extremity radiograph revealed short horizontal ribs, a long and narrow thorax, severely shortened bones with round metaphyseal ends with lateral spikes, high clavicles, and severe pulmonary hypoplasia (Figures 3A and B). Laboratory work-up included a renal function test and electrolyte screening: creatinine: 0.5 mg/dl (reference range [RR] 0.30–1.00 mg/dL), urea: 16 mg/dl (RR: 8.0–25 mg/dL), sodium: 140 mmol/L (RR: 135–145 mmol/L), potassium: 4.3 mmol/L (RR: 3.5–5.0 mmol/L), magnesium: 1.8 mg/dl (RR: 1.7–2.1 mg/dl), calcium: 9 mg/dl (RR: 8.8–10.8 mg/dl); and a liver function test: alanine transaminase: 16 IU/L (RR:10–55 IU/L) and aspartate aminotransferase: 57 IU/L (RR: 9.0–32 IU/L). All laboratory results were within normal ranges. Chest ultrasound was done and revealed bilateral pleural effusion that was more evident on the left-hand side and showed signs of consolidation. Abdominal ultrasound was done but was unremarkable, and cranial ultrasound showed prominent ventricles but was otherwise unremarkable. Detailed ophthalmology examination produced normal results.
 |
Figure 2 Clinical appearance of the patient with thoracic dysplasia. Pictures show short horizontal ribs causing severe thoracic narrowing (A and B). |
The neonate developed respiratory failure requiring intubation in the first hour of life, with an escalation of invasive ventilation from conventional ventilation to high-frequency ventilation. An echocardiogram on the 2nd day of life showed severe pulmonary hypertension (PHTN). Nitric oxide was started due to the presence of persistent hypoxia and severe PHTN. The pediatric pulmonologists were consulted and suspected that the neonate had skeletal dysplasia with thoracic hypoplasia. The team ordered whole-exome sequencing (WES), and the neonate remained on respiratory support. The WES was performed by PerkinElmer Genomics, Inc. (PerkinElmer, Waltham, MA, USA), the detailed method of which has been published previously.10 The patient continued to require high cardiorespiratory support, but his medical condition worsened, and respiratory failure persisted. The patient died of severe respiratory failure at 16 days of life due to respiratory insufficiency secondary to a severely restricted thoracic cage. Later, the WES revealed a homozygous mutation in the DYNC2H1 (NM_001377.3) gene, namely, the c.9041G>T NP_001368.2: p.(Arg3014Ile) missense variant, which results in the substitution of the arginine codon at amino acid position 3014 with an isoleucine codon; the phenotyping of the patient’ss JATD and detection of a homozygous variant in the DYNC2H1 gene confirmed the diagnosis of short-rib thoracic dysplasia-3 without polydactyly.
Discussion
Our patient was suspected antenatally to have skeletal dysplasia and immediately after delivery developed respiratory failure due to severe pulmonary hypoplasia. There was no evidence of major organ anomalies. He had no proved sepsis or airway anomalies that could explain his respiratory failure. The cause of respiratory failure in the current patient is likely multifactorial in nature. Pulmonary hypoplasia with compromised gas exchange as well as poor lung compliance secondary to a small thoracic cavity are the proposed mechanisms of the early respiratory failure.1,6 Contrary to expectations, our patient had a long and narrow thoracic cavity causing severe respiratory insufficiency, severe pulmonary hypertension, and eventually death. Most of the lethal forms of JATD involve a bell-shaped chest caused by two axes narrowing transversely and vertically, which cause severe restricted lung expansion and subsequently respiratory failure.1,6,11 It is difficult to explain the course of our patient and the presence of severe PHTN, but it might be related to pulmonary hypoplasia resulting from structural abnormalities of the pulmonary vasculature. Extrapolated findings from congenital diaphragmatic hernia associated PHTN have shown that the spectrum of structural pulmonary vasculature includes fewer pulmonary arteries per unit volume and thicker artery walls.12,13 Moreover, these changes in pulmonary vasculature will cause failure to transition from the high fetal pulmonary vascular resistance (PVR) to the lower PVR of the normal newborn lung. Additional research is needed to better explore the mechanisms of PHTN in patients with JATD and further develop therapeutic interventions.
Given the wide heterogeneity of the clinical and radiological manifestations of the autosomal recessive skeletal ciliopathies, several disorders were considered in the current patient, such as SRTD (JATD phenotype) and short-rib polydactyly syndrome (SRPS). The patient did not have polydactyly, hence the provisional diagnosis was a JATD phenotype without polydactyly. The WES report revealed a homozygous mutation in the DYNC2H1 gene c.9041G>T p.(Arg3014Ile). DYNC2H1 is located at 11q22.3 and encodes dynein, cytoplasmic 2, heavy chain 1, which is a cytoplasmic dynein involved in retrograde transport in the cilia.3,14 The dynein-2 complex is essential for transporting proteins and other molecules within the cilia, which is important for ciliogenesis, including hedgehog signaling, which is critical for human skeletal development.3,15,16 Mutation of the DYNC2H1 gene altered primary cilium function, causing a heterogeneous spectrum of multiorgan disorders affecting mainly the skeleton, renal system, and extremities.2,3 Our patient had a severe clinical phenotype, which added more complexity to the genotype–phenotype correlation of this wide variety of syndromes. A further study with more focus on DYNC2H1 genetic analysis and detailed clinical phenotyping is therefore recommended.
Our patient had pulmonary hypoplasia due to restricted fetal respiratory movement that started intrauterine, thus the therapeutic interventions were limited. Post-delivery interventions were palliative, including invasive ventilation and treatment of associated comorbidities such as PHTN. Our patient received maximum respiratory support as well as PHTN therapies, but despite that he expired due to severe persistent respiratory failure. Chest expansion surgeries were not discussed with the family because of the patient’s critical condition. Several thoracic expansion procedures were proposed, with variable outcomes.11 Due to the rarity and short lifespan of this disease, long-term outcomes of these techniques are unknown. An important practical implication of identifying the genetic cause of this condition is that it would help in genetic counseling and family planning. Furthermore, shared decision-making between the affected family and the clinician about the prenatal genetic screening/diagnosis is the optimal approach to managing this disease, which currently lacks any form of curative therapy.
Conclusion
This study summarizes a case of an infant born with skeletal dysplasia and severe respiratory insufficiency. Genetic analysis confirmed the diagnosis of SRTD3 (JATD phenotype) by revealing a mutation in the DYNC2H1 gene. The patient had isolated skeletal anomalies without polydactyly or other organ involvement. Additionally, the infant had severe PHTN on top of the respiratory failure that eventually caused his death. Considerably more work will need to be done to determine the clinical spectrum of JATD as well as understand its genetic heterogeneity.
Consent for Publication
Written informed consent was obtained from the parents for both case report publication and inclusion of all images. Institutional approval was not necessary for the publication of this case.
Acknowledgments
The authors would like to thank the neonate’s family.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Poyner SE, Bradshaw WT. Jeune syndrome: considerations for management of asphyxiating thoracic dystrophy. Neonatal Network. 2013;32(5):342–352. doi:10.1891/0730-0832.32.5.342
2. Chen LS, Shi SJ, Zou PS, Ma M, Chen XH, Cao DH. Identification of novel DYNC2H1 mutations associated with short rib-polydactyly syndrome type III using next-generation panel sequencing. Genet Mol Res. 2016;15(2). doi:10.4238/gmr.15028134
3. Chen W, Li Y, Zhang J, et al. Genetic variations in the DYNC2H1 gene causing SRTD3 (short-rib thoracic dysplasia 3 with or without polydactyly). Front Genet. 2023; 2023:14.
4. Shaheen R, Schmidts M, Faqeih E, et al. A founder CEP120 mutation in Jeune asphyxiating thoracic dystrophy expands the role of centriolar proteins in skeletal ciliopathies. Hum Mol Genet. 2015;24(5):1410–1419. doi:10.1093/hmg/ddu555
5. Tuz K, Bachmann-Gagescu R, O’Day DR, et al. Mutations in CSPP1 cause primary cilia abnormalities and Joubert syndrome with or without Jeune asphyxiating thoracic dystrophy. Am J Hum Genet. 2014;94(1):62–72. doi:10.1016/j.ajhg.2013.11.019
6. Keppler-Noreuil KM, Adam MP, Welch J, Muilenburg A, Willing MC. Clinical insights gained from eight new cases and review of reported cases with Jeune syndrome (asphyxiating thoracic dystrophy). Am J Med Genet A. 2011;155(5):1021–1032. doi:10.1002/ajmg.a.33892
7. Rahemtullah A, Mcgillivray B, Wilson RD. Suspected skeletal dysplasias: femur length to abdominal circumference ratio can be used in ultrasonographic prediction of fetal outcome. America J Obs Gyne. 1997;177(4):864–869. doi:10.1016/S0002-9378(97)70284-9
8. Krakow D, Lachman RS, Rimoin DL. Guidelines for the prenatal diagnosis of fetal skeletal dysplasias. Genet Med. 2009;11(2):127–133. doi:10.1097/GIM.0b013e3181971ccb
9. Milks KS, Hill LM, Hosseinzadeh K. Evaluating Skeletal Dysplasias on Prenatal Ultrasound: An Emphasis on Predicting Lethality. Pediatric Radiology. Springer Verlag; 2017:134–145.
10. Asseri AA, Alzoani A, Almazkary AM, et al. Mucopolysaccharidosis type i presenting with persistent neonatal respiratory distress: a case report. Diseases. 2023;11(2):67. doi:10.3390/diseases11020067
11. Blanco F, Elliott S, Sandler A. Management of Congenital Chest Wall Deformities. Semin Plast Surg. 2011;25(01):107–116. doi:10.1055/s-0031-1275177
12. O’toole SJ, Irish MS, Holm BA, Glick PL Congenital diaphragmatic hernia pulmonary vascular abnormalities in congenital diaphragmatic hernia; 2024.
13. Fike CD, Aschner JL. Looking Beyond PPHN: The Unmet Challenge of Chronic Progressive Pulmonary Hypertension in the Newborn. Pulmonary Circulation. Taylor and Francis Inc.; 2013:454–466.
14. Dagoneau N, Goulet M, Geneviève D, et al. DYNC2H1 mutations cause asphyxiating thoracic dystrophy and short rib-polydactyly syndrome, type III. Am J Hum Genet. 2009;84(5):706–711. doi:10.1016/j.ajhg.2009.04.016
15. Feng Q, Gicking A, Hancock WO, et al. Platform: cytoskeletal motors 853-plat dynactin p150 promotes processive motility of ddb complexes by mini-mizing diffusional behavior of dynein; 2024.
16. Markova TV, Vm K, Melchenko EV, et al. Сlinical and genetic characteristics of skeletal cyliopathies – short-rib thoracic dysplasia. Pediatric Trauma Ortho Recons Surg. 2022;10(1):43–56. doi:10.17816/PTORS91116
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

