Back to Journals » Research Reports in Clinical Cardiology » Volume 5
Who will benefit from anticoagulant therapy? Use of the CHADS2 score and its variants
Authors Singla A, Mittal M, Flaker G
Received 24 December 2013
Accepted for publication 10 February 2014
Published 25 April 2014 Volume 2014:5 Pages 83—92
DOI https://doi.org/10.2147/RRCC.S39091
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Atul Singla, Mayank K Mittal, Greg C Flaker
Division of Cardiovascular Medicine, Department of Internal Medicine, University of Missouri, Columbia, MO, USA
Abstract: Stroke prevention is a crucial step in the management of atrial fibrillation (AF). The assessment of stroke risk associated with AF varies, depending on the presence of various clinical risk factors (older age, congestive heart failure, diabetes mellitus, hypertension, history of stroke or transient ischemic attack, female sex, or peripheral vascular disease) incorporated in risk stratification models such as CHADS2 and CHA2DS2-VASc. Although these models have modest predictive ability in individual patients, current guidelines advocate the use of a CHA2DS2-VASc risk score to identify very low risk patients who can avoid antithrombotic therapy, as well as all others who can benefit from such therapy. More recently biomarkers and imaging has improved our knowledge of pathophysiology of AF and may further improve risk stratification for thromboprophylaxis in AF patients. These new markers combined with clinical risk scores may enable the development of novel tools to improve clinical risk assessment in AF. In this article, we summarize the recent developments in risk stratification for stroke prevention in AF, including the various schemes and new biomarkers that may lead to improved patient outcomes.
Keywords: atrial fibrillation, anticoagulant therapy, CHADS2, risk stratification, CHA2DS2-VASc, biomarkers
Introduction
Atrial fibrillation (AF) is associated with increased stroke risk. After the publication of clinical trials demonstrating the reduction of stroke with anticoagulation therapy in AF patients, it became apparent that certain patients were at higher risk for stroke than others. An accurate estimation of stroke risk would enable clinicians to choose antithrombotic therapy judiciously. Multiple risk stratification models, using clinical and echocardiographic parameters, have been published, and more continue to be suggested.1,2 In this article, we summarize the recent developments in risk stratification for stroke prevention in AF, including the various schemes and new biomarkers that may lead to improved patient outcomes.
CHADS2 score
The CHADS2 model is the most widely adopted risk stratification tool. It assigns 1 point each for age 75 years or older, congestive heart failure, diabetes mellitus, or a history of hypertension and 2 points for a history of stroke or transient ischemic attack. Derived from the Stroke Prevention and Atrial Fibrillation participants who were treated with aspirin at the time of hospital discharge, CHADS2 was validated using data from the National Registry of Atrial Fibrillation, consisting of 1,733 Medicare beneficiaries with nonvalvular AF between the ages of 65 and 95 years.3 Patients with a score of 0 had a stroke risk of 1.2% per year. Patients with a score of 1 had a risk for stroke of 2.8% per year, and patients with a score higher than 1 had a stroke risk of more than 3.6% per year. Recognizing that the bleeding risk of warfarin was approximately 2% per year,4 early recommendations from the American College of Chest Physicians stated that for patients with an absolute risk for stroke of about 1% a year, roughly equivalent to a CHADS2 score of 0, the risk for anticoagulation may outweigh the risk for stroke, and aspirin was recommended. Patients with an absolute risk for stroke of 2.5% a year, roughly equivalent to a CHADS2 score of 1, were considered to be intermediate risk, and therapy with either aspirin or warfarin was recommended. In those with a higher risk for stroke, identified by a CHADS2 score of 2 or higher, warfarin was suggested.5
Although simple to use, there were shortcomings in the CHADS2 model. First, most patients were classified as intermediate risk. In these patients, the clinician was offered a choice of therapy (warfarin or aspirin), which is an unusual recommendation for guidelines. Second, age was an independent risk factor for stroke that was associated with an incremental risk of 1.5 per decade. By using an absolute age cut-off of 75 years, many patients who were 60 or 70 years old may be at increased risk for stroke but would not receive appropriate therapy until their 75th birthday.6,7 Third, the definitions of hypertension and heart failure were not standardized. A patient with a history of well-controlled hypertension had a different stroke risk than a person with uncontrolled hypertension (systolic blood pressure, ≥140 mmHg),8,9 yet each patient received a single point for risk stratification. In early retrospective studies, congestive heart failure was identified by International Classification of Diseases, Ninth Revision, Clinical Modification, code or symptoms of heart failure in the proceeding 100 days.10 Later, echocardiographic criteria suggested that measures of left ventricular dysfunction (fractional shortening <25% and global left ventricular dysfunction) were predictive of stroke events.11 Cardiologists had difficulty defining heart failure by echo criteria, and the merging of the term heart failure with an echo-derived left ventricular ejection fraction was difficult. There also was confusion as to what to do with patients with heart failure but with preserved ejection fraction. In addition, the role of heart failure was challenged as an independent predictor for the risk for thromboembolism in AF patients.6,12 Finally, the CHADS2 score failed to recognize a number of important variables, including female sex13 and vascular disease (peripheral arterial disease, myocardial infarction, complex aortic plaques, or renal disease), as important risk factors for stroke.7,14
Various risk-stratification schemes
Other risk-stratification schemes have been proposed, including those by the Atrial Fibrillation Investigators,15 the American College of Chest Physicians,16 the Stroke Prevention in Atrial Fibrillation Investigators,12,17 the Framingham Heart Study,18 and the American College of Cardiology/American Heart Association/European Society of Cardiology.19 Most of these organizations categorize stroke risk into low-, moderate-, or high-risk groups. Despite substantial effort, the predictive ability of these scores was modest and highly variable.20 The discriminating ability of multiple risk schemes, as measured by c statistics, ranges from 0.56 to 0.62, where a value of 1.0 represents perfect discrimination. The proportion of patients categorized as low risk ranges from 11.7% to 37.1% across schemes, and the proportion who were high risk ranged from 16.4% to 80.4%.20 In a comparative study of risk stratification scores, it was noted that when various schemes were applied to the same patient cohort, the number of patients classified as low risk ranged from 9% to 49%.1 These studies highlighted the need for more robust risk stratification schemes, which could allow consistent recommendations for anticoagulant therapy in AF patients at risk for thromboembolism.
CHA2DS2-VASc score
The CHA2DS2-VASc model expanded on the CHADS2 score, taking into account two additional risk factors: female sex and vascular disease (Table 1).4 Vascular disease was defined as a history of a myocardial infarction, peripheral arterial disease, or complex aortic plaque. The presence of any of these features was worth 1 point. In addition, it assigned a point for female sex or age 65–74 years. Age greater than 75 years was awarded 2 points. With this strategy, a score of 0 was deemed low risk, a score of 1 was considered intermediate risk, and a score of 2 or higher was classified as high risk (Table 2).21 This scheme was validated in 1,084 subjects in the EuroHeart survey who had nonvalvular AF, who did not receive anticoagulation, and who were followed-up for 1 year. In this analysis, the CHA2DS2-VASc score had only a slightly better predictive ability (c statistic, 0.61 versus 0.56) compared with CHADS2 score.4 However, the CHA2DS2-VASc scheme categorized only 15.1% of patients as intermediate risk compared with 61.9% with the CHADS2 scheme. It also classified 75.7% patients as high risk compared with 44.7% in CHADS2.
  | Table 2 Adjusted stroke and thromboembolism rate according to CHA2DS2-VASc score |
The reclassification of intermediate-risk patients was also illustrated in an analysis of patients with a CHADS2 score of one from the Apixaban versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) and Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE) trials. Of 4,670 patients with a baseline score of CHADS2=1, 26% were classified as a CHA2DS2-VASc score of 1 (26%), with a low annual risk for systemic embolism of 1%, for whom anticoagulant therapy could be withheld.22 In another study of 47,576 anticoagulation-naive AF patients who were considered to be low to intermediate risk (CHADS2=1), 84.2% were reclassified as a CHA2DS2-VASc score 1 or higher, with stroke/thromboembolism rate per 100 person-years ranging from 1.79 to 8.18 at 1-year follow-up.23 This demonstrates that patients classified as low risk by CHADS2 scheme are not truly “low risk” and that the risk for stroke is highly variable, ranging from 1.79% to 8.18% in 1 year. The additional risk factors included in CHA2DS2-VASc significantly improve the predictive value of the CHADS2 score.
This ability to identify a truly low-risk group of patients was validated in additional studies. A Danish cohort study of 73,538 patients who did not receive anticoagulation and were hospitalized with nonvalvular AF confirmed a very low rate of stroke (0.78%/year) in the CHA2DS2-VASc low-risk group (0 points) compared with 1.67% in the CHADS2 low risk group (0 points).24 Similar findings were noted in the United Kingdom General Practice Research Database study, involving 79,884 AF patients followed-up for an average of 4 years. Low-risk subjects (CHA2DS2-VASc score, 0) were truly low risk, with annual stroke events lower than 0.5%.25 In short, patients with CHA2DS2-VASc scores of 0 are truly at low risk, and no therapy would be warranted.
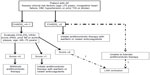
In summary, the major strength of the CHA2DS2-VASc scheme lies in predicting patients who are at truly low risk for stroke and in avoiding anticoagulation therapy. The ability to characterize low-risk AF patients with confidence allows clinicians to identify patients who can safely be treated with aspirin or possibly no therapy, sparing them the risk for bleeding, the cost, and the inconvenience of anticoagulant therapy. In addition, the CHA2DS2-VASc points out that certain low-risk patients (CHADS2=0-1) have a stroke risk that is highly variable. For example, anticoagulation is warranted in a person with a CHADS2 score of 2, 3, or 4, as the stroke risk is high and the addition of a CHA2DS2-VASc score is not needed. It is reclassification of the CHADS2=0-1 patients that is crucial (Figure 1). Further, the CHA2DS2-VASc scheme identifies more patients in the at-risk population, allowing earlier initiation of anticoagulation therapy for primary prevention for stroke and thromboembolism. Many patients, particularly older women, are redistributed from the low- to the higher-risk categories.26
The ability to identify a truly high-risk group of patients might also be an advantage. Exclusion of left atrial appendage, either surgically or by implantation of intravascular devices, might be warranted in a patient who has exceptionally high risk for stroke, such as patients with a CHADS2 score of 2 and higher or CHA2DS2-VASc scores of 4 or higher, particularly those who are unable to tolerate anticoagulation (Figure 1).27 In the ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology (ASAP) study, 150 AF patients with a mean CHADS2 score and CHA2DS2-VASc score of 2.8±1.2 and 4.4±1.7, respectively, who were ineligible for anticoagulant therapy underwent left atrial appendage closure with the Watchman™ (Boston Scientific, Natick, MA, USA) device. On the basis of the mean CHADS2 score being 2.8, the annualized ischemic stroke rate was expected to be 7.3% if treated with aspirin alone; however, the observed rate was only 1.7%, representing 77% fewer events than expected.27 This study emphasizes the need to identify truly high-risk patients with risk stratification schemes more robust than the CHADS2 score. More data are also required to confirm the safety and efficacy of closure of the left atrial appendage, using percutaneous devices and surgical techniques in patients at various levels of risk. Nonetheless, new European AF guidelines discourage the low-/moderate-/high-risk strata and emphasize a risk factor-based approach, preferably using the CHA2DS2-VASc score, given that stroke risk accumulates as the number of risk factors increases.28
Role of echocardiography in risk stratification of AF
Current guidelines recommend obtaining a baseline transthoracic echocardiogram (TTE) for all newly diagnosed patients with AF. TTE helps in the documentation of left ventricular dimensions, left atrial dimension, and left ventricular mass. The role of TTE in identifying patients at risk of developing AF has been well documented,29,30 but data supporting its role in identifying patients at risk for thromboembolic complications from AF are limited.11 In the Cardiovascular Health Study, of all the echocardiographic variables assessed, only left atrial size and left ventricular posterior wall thickness were significant predictors of occurrence of AF, but not thromboembolic events.29 The stroke associated with AF is most commonly a result of emboli originating in the left atrium, and more precisely, the left atrial appendage, a structure that is often not adequately visualized on TTE. A comprehensive evaluation of the left atrial appendage often involves a more invasive approach using transesophageal echocardiography.31 New techniques in the field of echocardiography such as three-dimensional echocardiography, tissue Doppler imaging, or speckle tracking may in the future add additional information.
Role of biomarkers in risk-stratifying AF patients
A range of biomarkers that reflect underlying pathophysiological processes of atrial fibrillation and stroke has been associated with clinical events and, conceptually, may help refine further stroke risk assessment in AF patients. These include estimates of renal function, myocardial necrosis, and markers of inflammation (C-reactive protein; CRP).
Glomerular filtration rate
Renal dysfunction has emerged as an important risk factor for stroke in AF patients.32,33 A meta-analysis of 33 prospective studies, including 280,000 patients experiencing 8,000 stroke events, found that patients with a baseline estimated glomerular filtration rate of less than 60 mL/minute per 1.73 m2 had a 43% higher risk for future stroke than those with a normal glomerular filtration rate.34 The potential underlying mechanism of increased risk is unclear but likely includes accelerated atherosclerosis, increased blood pressure, and more advanced and recalcitrant AF. In a recent analysis of AF patients treated with warfarin or rivaroxaban, a reduced creatinine clearance was a strong independent predictor of stroke, second only to prior transient ischemic attack or stroke.35 One may speculate that the incorporation of renal dysfunction may improve the predictive performance of existing risk stratification schemes. One such model is the R2CHADS2 score, developed from the Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) and validated in the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study, an independent AF patient cohort.35 R2CHADS2 resulted in a 12% (95% confidence interval, 6%–19.5%) net reclassification improvement in patients receiving warfarin and a 22.6% (95% confidence interval, 14.5%–30.7%) net reclassification improvement in patients not receiving warfarin when compared with the CHADS2 index. However, the performance of the R2CHADS2 score for stroke prediction remains modest, with a C-statistic of 0.587, similar to a CHADS2 or CHA2DS2-VASc score, with a C-statistic of 0.575 and 0.578, respectively.35 Similar results were noted in another study of 5,912 AF patients, where the addition of renal impairment to CHADS2 or CHA2DS2-VASc scores did not improve their predictive value at 1-year follow up.36 Interestingly, although renal dysfunction was associated with higher rates of stroke, after adjustment of the CHADS2 risk factors, renal impairment did not significantly increase the risk for stroke.
In summary, patients with atrial fibrillation and renal dysfunction are at increased risk for stroke. However, because of the lack of consistent results of renal dysfunction as an independent predictor of stroke. In addition to the usual CHADS2 risk factors, it is not of additive predictive value to the routine clinical scoring system.
Brain natriuretic peptide
Natriuretic peptides (NT proBNP, BNP) are secreted by cardiomyocytes and maintain salt and water homeostasis in normal and diseased states. BNP levels are higher in patients with AF than those with sinus rhythm,37 and they return to normal level with restoration of sinus rhythm with cardioversion,38 likely reflecting the changes in atrial stretch and function.39 Therefore, it is believed that in a patient with AF, BNP is primarily produced in the atria, whereas in a patient with heart failure, natriuretic peptide is mainly released by ventricles.40,41 Elevated levels of BNP have been correlated with left atrial appendage dysfunction,42,43 which itself is associated with increased risk for thromboembolic events in AF patients.14 The prevalence of elevated biomarkers and their association with cardiovascular events in AF patients receiving chronic anticoagulation were studied independently in two clinical trials: Apixaban for the Prevention of Stroke in Subjects with Atrial Fibrillation (ARISTOTLE) and Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY). The risk for stroke and systemic thromboembolism was significantly lower in the lowest NT-proBNP quartile, which was defined as serum NT-proBNP levels of 363 ng/L or less in the ARISTOTLE study and 387 ng/L or less in the RE-LY study. The addition of NT-proBNP to the CHA2DS2-VASc score led to only modest improvement in their risk prediction for stroke, as measured by C-statistics: 0.62–0.65 (P<0.001) for ARISTOTLE and 0.62–0.63 (P=0.2) for the RE-LY substudy.44,45 These data suggest that AF patients who belong to a particular clinical risk category can carry a wide range of thromboembolic risk based on NT-proBNP levels. Unfortunately, there is no additive or synergistic effect of using a clinical score with natriuretic peptides on thromboembolic risk prediction. This association of increased thromboembolic risk with a biomarker associated with salt and water homeostasis and atrial stretch is interesting and may help with a better understanding of the relationship between AF and stroke.
In summary, natriuretic peptides may help identify residual risk for thromboembolism in AF patients receiving anticoagulation. However unlike the clinical risk scores, they do not allow identification of patients who should be started on anticoagulation.
Troponin
Troponins are regulatory proteins that are integral to the contractile system of the cardiomyocyte. They are released during cardiac cell death or injury. The exact mechanism by which cardiac troponins are elevated in AF patients is still unclear. It could be a result of coexisting coronary artery disease or heart failure in AF patients. Some investigators believe that increased wall stress, demand–supply mismatch, or angiotensin 2-induced oxidative stress are possible explanations for troponin elevations in selected AF patients.46
The association of troponin and increased cardiovascular events was evaluated prospectively in a subgroup of the original RE-LY cohort. This substudy included 6,189 AF patients, of whom 55% had detectable troponins. The group was divided into quartiles according to their level of troponin at entry into the study. The thromboembolic risk was related to elevation in serum troponin values. Patients in the highest quartile of troponin 1 (≥0.040 μg/L) were at fivefold increased risk compared with those with troponin 1 in the lowest quartile (≤0.010 μg/L). The addition of troponin 1 to the CHADS2 score led to only modest improvement in its risk-prediction accuracy for stroke and systemic embolism, as measured by C-statistics, going from 0.61 to 0.65 (P<0.04). Similarly, the improvement in the prediction accuracy of the CHA2DS2VASc score went from 0.62 to 0.65 (P<0.049).45
Similar results were noted in the ARISTOTLE substudy, which included 14,821 AF patients, of whom 98.5% had detectable troponins. Adjusted Cox analysis demonstrated a twofold increased risk for stroke and systemic embolism in patients with high-sensitivity (hs)-troponin I ≥10.1 ng/L. Adding hs-TnI to the CHA2DS2VASc score improved C-statistics from 0.63 to 0.65 for stroke or systemic embolism.47 Thus, both the RE-LY and the ARISTOTLE substudies showed that rising levels of troponin, a marker of myocardial cell death, is independently associated with increased risk for stroke and systemic embolism but leads to only modest improvement of thromboembolic risk prediction scoring systems.
One difference between these two studies was that a high-sensitivity troponin assay (measured in nanograms per liter) was used in the ARISTOTLE study compared with the RE-LY study, which used traditional troponin assay (measured in micrograms per liter). The use of hs-TnI assays in the ARISTOTLE substudy allowed investigators to detect even lower levels of circulating troponins, which could explain why 98.5% of patients in the ARISTOTLE study had detectable troponin compared with just 57% in the RE-LY study. The proportions of patients with elevated troponins, defined as the 99th percentile of the normal, healthy population, was 9.2% in ARISTOTLE and 8% in the RE-LY study. This improved sensitivity for identifying patients who had “subclinical” evidence of myocardial cell death may lead to earlier or more accurate risk prediction of clinical events. Because these studies used different assays, an absolute number for troponin elevation cannot be established to guide clinical decision making, such as that available for myocardial infarction.45–48
In another study, patients with elevated levels of hs-troponin T were 2.2 times more likely to have adverse cardiovascular events and were at 1.8-fold increased risk for all-cause mortality compared with patients with normal hs-TnT, even after adjusting for CHADS2 score.49 These results were derived from real-world AF patients who are receiving chronic (>6 months) stable anticoagulation with warfarin (time in therapeutic range, >70%) and are complementary to the previously discussed results of the RE-LY and ARISTOTLE clinical trials.
In summary, the use of biomarkers of cardiac necrosis can help subclassify thromboembolic risk of individuals within a particular clinical risk group who are receiving long-term anticoagulation. It is interesting that even tiny markers of cell death such as hs-TnT are associated with a central nervous system event. The association of increased thromboembolic risk with a biomarker associated with myocardial death and injury remains elusive. The mechanisms that have been proposed so far include changes in microvascular blood flow, atrial calcium overload, oxidative stress and oxygen demand–supply mismatch. It is possible that alteration in the Virchow’s triad leads to pathogenesis of AF induced thrombi via a complex cascade of events that include pro-inflammatory state, endothelial dysfunction, and platelet activation in a dilated poorly contractile left atrium, which allows stagnation of blood.48–50 Which one of these individual or combined mechanisms leads to troponin elevation in AF patients with stroke needs to be identified. However, troponin measurement cannot replace clinical risk scores when deciding who should receive anticoagulation.
CRP
One of the acute phase reactants secreted by the liver in response to inflammation is CRP. CRP is also believed to be an opsonin that can help clear apoptotic myocytes.51 It is believed that initiation of rapid atrial firing could lead to cellular calcium overload in the atrial myocytes and may promote their apoptosis.52 This phenomenon supports the hypothesis that AF may have direct inflammatory effects on atrial myocytes and could potentially explain why CRP, an inflammatory marker, would be elevated in AF. CRP levels were found to be elevated in patients with persistent AF compared with patients with paroxysmal AF. Similarly, compared with patients in normal sinus rhythm, AF patients were noted to have higher CRP levels.53 In a large, population-based study, elevated CRP levels were associated with the presence of AF at the beginning of the study and predicted the future development of AF.54 Elevated baseline CRP levels were also associated with a higher failure rate of electric cardioversion.55 A change in highly sensitive CRP levels was associated with failure of pulmonary vein isolation ablation procedure.56 Most interestingly, restoration of sinus rhythm returned CRP levels to normal, thereby providing indirect evidence that AF could be inflammatory in origin. It still remains controversial whether inflammation is a consequence or a cause of AF. Nonetheless, there are no convincing reports illustrating the role of CRP in risk-stratifying patients with AF.
Although clinical risk stratification has improved dramatically over the last decade because of the use of improved scoring systems, the search for an ideal biomarker continues. There are multiple novel biomarkers on the horizon, such as D-dimer, cystatin C, E-selectin, P-selectin, CD40 ligand, interleukin 6, von Willebrand factor, plasminogen activator inhibitor 1, and tissue factor, that are currently being investigated for their role in the management of patients with AF.48,50
Role of magnetic resonance imaging in risk stratification
Magnetic resonance imaging (MRI) has emerged as an important noninvasive tool for the evaluation of patients with AF. In the setting of AF, MRI of the brain and heart are two distinct entities, which warrant independent discussion.
Brain MRI
The existing clinical scoring system defines stroke according to the clinical symptoms of stroke, but it does not account for subclinical strokes, which patients may suffer without overt neurological findings and which are incidentally picked up on brain imaging. MRI of the brain assists with identification of these subclinical strokes and may have a role in risk-stratifying AF patients. Two studies are currently underway. In the AVEROESS MRI substudy, brain MRIs were performed at baseline and during follow-up for patients who were receiving either aspirin or apixaban for stroke prophylaxis. Prospective Urban Rural Epidemiological Study-MRI (PURE-MRI), a cross-sectional study, is also currently underway to investigate the prevalence, risk factors, and effect of covert cerebral ischemia in the Canadian population. The investigators plan to extend it to a multinational PURE cohort if useful information is derived from this preliminary investigation.57 A recent cross-sectional analysis of 270 cardiovascular clinic patients found that silent cerebral ischemia was twice as common in AF patients as in controls (90% versus 46%). According to the MRI results, the researchers hypothesize that most of these ischemias were embolic in nature.58
The Mesh Ablator Versus Cryoballoon Pulmonary Vein Ablation of Symptomatic Paroxysmal Atrial Fibrillation (MACPAF) investigators conducted serial brain MRIs on patients (n=37) with paroxysmal AF who underwent pulmonary vein isolation and identified silent ischemia on postprocedural MRI in 41% of patients. In this study, 70% of patients had a CHADS2 score of 0 or 1.59 Brain MRI can also identify cerebral microbleeds, which may be a predictor of future intracranial hemorrhages.60 Fisher has proposed an algorithm on using screening brain MRI for intermediate-risk AF patients to determine the need for anticoagulation.61 Screening MRIs may improve the predictive performance of a clinical risk scoring system. The presence of microbleed or silent ischemia on brain MRI may allow physicians to counsel their patients and help them make well-informed decisions regarding the risks and benefits of oral anticoagulation.
Cardiac MRI
Late gadolinium enhancement on cardiovascular MRI (LGE-MRI) can identify and quantify left atrial fibrosis, a variable of structural atrial remodeling that induces and maintains AF.62,63 Daccarett et al studied clinical, AF, and CHADS2 score characteristics by clinical examination and systematic chart review of 387 patients who underwent LGE-cardiac MRI before pulmonary vein isolation.64 A history of previous stroke was present in 36 (9.3%) patients. It was noted that patients with previous strokes and a higher CHADS2 score had a significantly higher percentage of left atrial fibrosis. A logistic regression analysis of all variables except strokes demonstrated that left atrial fibrosis independently predicted cerebrovascular events (P=0.002) and significantly increased the predictive performance of the clinical risk score (area under the curve, 0.77). Although this study had several limitations, it did provide a plausible correlation between left atrial structural remodeling and ischemic strokes.64 Cardiac MRI has the potential to provide mechanistic understanding behind an increased risk for stroke with AF. LGE-MRI has also been shown to be useful in localizing and quantifying scar formation in the left atrium and identifying predictors of successful radiofrequency ablation.65 The role of MRI in the evaluation of AF patients is early in its development and warrants further investigation before routine use in clinical practice.
Conclusion
The decision to start a patient with AF on anticoagulation is still based on clinical risk scores alone. Because biomarker studies were performed on patients already receiving anticoagulation, their role seems mostly to lie in mechanistic understanding of stroke, such as accelerated atherosclerosis, inflammation, endothelial dysfunction, myocardial stretch, or injury, rather than being incorporated in clinically derived risk factors (which were developed in nonanticoagulated patients). They may be able to identify patients at particularly high risk for stroke who may benefit from surgical or catheter-based procedures that eliminate or isolate the left atrial appendage, a residual risk for stroke and thromboembolism in patients receiving anticoagulation.
Disclosure
Dr Flaker has received clinical research support from Boehringer Ingelheim. He also has received consultative fees from Bristol Myers Squibb, Pfizer, Janssen, and Daiichi Sankyo Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
Stroke Risk in Atrial Fibrillation Working Group. Comparison of 12 risk stratification schemes to predict stroke in patients with nonvalvular atrial fibrillation. Stroke. 2008;39(6):1901–1910. | |
Singer DE, Chang Y, Borowsky LH, et al. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc. 2013;2(3):e000250. | |
Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–2870. | |
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137(2):263–272. | |
Albers GW, Dalen JE, Laupacis A, Manning WJ, Petersen P, Singer DE. Antithrombotic therapy in atrial fibrillation. Chest. 2001;119(Suppl 1):194S–206S. | |
Stroke Risk in Atrial Fibrillation Working Group. Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology. 2007;69(6):546–554. | |
Hughes M, Lip GY; Guideline Development Group, National Clinical Guideline for Management of Atrial Fibrillation in Primary and Secondary Care, National Institute for Health and Clinical Excellence. Stroke and thromboembolism in atrial fibrillation: a systematic review of stroke risk factors, risk stratification schema and cost effectiveness data. Thromb Haemost. 2008;99(2):295–304. | |
D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25(1):40–43. | |
Lip GY, Frison L, Grind M; SPORTIF Invetigators. Effect of hypertension on anticoagulated patients with atrial fibrillation. Eur Heart J. 2007;28(6):752–759. | |
[No authors listed]. Predictors of thromboembolism in atrial fibrillation: I. Clinical features of patients at risk. The Stroke Prevention in Atrial Fibrillation Investigators. Ann Intern Med. 1992;116(1):1–5. | |
[No authors listed]. Predictors of thromboembolism in atrial fibrillation: II. Echocardiographic features of patients at risk. The Stroke Prevention in Atrial Fibrillation Investigators. Ann Intern Med. 1992;116(1):6–12. | |
Hart RG, Pearce LA, McBride R, Rothbart RM, Asinger RW. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: analysis of 2012 participants in the SPAF I-III clinical trials. The Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Stroke. 1999;30(6):1223–1229. | |
Poli D, Antonucci E, Grifoni E, Abbate R, Gensini GF, Prisco D. Gender differences in stroke risk of atrial fibrillation patients on oral anticoagulant treatment. Thromb Haemost. 2009;101(5):938–942. | |
[No authors listed]. Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography. Ann Intern Med. 1998;128(8):639–647. | |
[No authors listed]. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154(13):1449–1457. | |
You JJ, Singer DE, Howard PA, et al; American College of Chest Physicians. Antithrombotic Therapy for Atrial Fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(Suppl 2):e531S–e575S. | |
Johnson KG, Johnson DC. Obstructive sleep apnea is a risk factor for stroke and atrial fibrillation. Chest. 2010;138(1):239; author reply 239–240. | |
Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290(8):1049–1056. | |
Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57(11):e101–e198. | |
Fang MC, Go AS, Chang Y, Borowsky L, Pomernacki NK, Singer DE; ATRIA Study Group. Comparison of risk stratification schemes to predict thromboembolism in people with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2008;51(8):810–815. | |
Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41(12):2731–2378. | |
Coppens M, Eikelboom JW, Hart RG, et al. The CHA2DS2-VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur Heart J. 2013;34(3):170–176. | |
Olesen JB, Torp-Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0–1: a nationwide cohort study. Thromb Haemost. 2012;107(6):1172–1179. | |
Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. | |
Van Staa TP, Setakis E, Di Tanna GL, Lane DA, Lip GY. A comparison of risk stratification schemes for stroke in 79,884 atrial fibrillation patients in general practice. J Thromb Haemost. 2011;9(1):39–48. | |
Mason PK, Lake DE, DiMarco JP, et al. Impact of the CHA2DS2-VASc score on anticoagulation recommendations for atrial fibrillation. Am J Med. 2012;125(6):603.e1–e6. | |
Reddy VY, MÖbius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol. 2013;61(25):2551–2556. | |
Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719–2747. | |
Patton KK, Ellinor PT, Heckbert SR, et al. N-terminal pro-B-type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation. 2009;120(18):1768–1774. | |
Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373(9665):739–745. | |
Wheeler R, Masani ND. The role of echocardiography in the management of atrial fibrillation. Eur J Echocardiogr. 2011;12(10):i33–i38. | |
Seliger SL, Gillen DL, Longstreth WT Jr, Kestenbaum B, Stehman-Breen CO. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64(2):603–609. | |
Go AS, Fang MC, Udaltsova N, et al; ATRIA Study Investigators. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009;119(10):1363–1369. | |
Lee M, Saver JL, Chang KH, Liao HW, Chang SC, Ovbiagele B. Low glomerular filtration rate and risk of stroke: meta-analysis. BMJ. 2010;341:c4249. | |
Piccini JP, Stevens SR, Chang Y, et al; ROCKET AF Steering Committee and Investigators. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation. 2013;127(2):224–232. | |
Banerjee A, Fauchier L, Vourc’h P, et al. Renal impairment and ischemic stroke risk assessment in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. J Am Coll Cardiol. 2013;61(20):2079–2087. | |
Silvet H, Young-Xu Y, Walleigh D, Ravid S. Brain natriuretic peptide is elevated in outpatients with atrial fibrillation. Am J Cardiol. 2003;92(9):1124–1127. | |
Wozakowska-Kapłon B. Effect of sinus rhythm restoration on plasma brain natriuretic peptide in patients with atrial fibrillation. Am J Cardiol. 2004;93(12):1555–1558. | |
Albåge A, Kenneb łck G, van der Linden J, Berglund H. Improved neurohormonal markers of ventricular function after restoring sinus rhythm by the Maze procedure. Ann Thorac Surg. 2003;75(3):790–795. | |
Inoue S, Murakami Y, Sano K, Katoh H, Shimada T. Atrium as a source of brain natriuretic polypeptide in patients with atrial fibrillation. J Card Fail. 2000;6(2):92–96. | |
Goetze JP, Friis-Hansen L, Rehfeld JF, Nilsson B, Svendsen JH. Atrial secretion of B-type natriuretic peptide. Eur Heart J. 2006;27(14):1648–1650. | |
Igarashi Y, Kashimura K, Makiyama Y, Sato T, Ojima K, Aizawa Y. Left atrial appendage dysfunction in chronic nonvalvular atrial fibrillation is significantly associated with an elevated level of brain natriuretic peptide and a prothrombotic state. Jpn Circ J. 2001;65(9):788–792. | |
Tamura H, Watanabe T, Nishiyama S, et al. Elevated plasma brain natriuretic peptide levels predict left atrial appendage dysfunction in patients with acute ischemic stroke. J Cardiol. 2012;60(2):126–132. | |
Hijazi Z, Wallentin L, Siegbahn A, et al. N-terminal pro-B-type natriuretic peptide for risk assessment in patients with atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation). J Am Coll Cardiol. 2013;61(22):2274–2284. | |
Hijazi Z, Oldgren J, Andersson U, et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation. 2012;125(13):1605–1616. | |
van den Bos EJ, Constantinescu AA, van Domburg RT, Akin S, Jordaens LJ, Kofflard MJ. Minor elevations in troponin I are associated with mortality and adverse cardiac events in patients with atrial fibrillation. Eur Heart J. 2011;32(5):611–617. | |
Hijazi Z, Siegbahn A, Andersson U, et al; ARISTOTLE Investigators. High-Sensitivity Troponin I for Risk Assessment in Patients With Atrial Fibrillation: Insights From the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) Trial. Circulation. 2014;129(6):625–634. | |
Hijazi Z, Oldgren J, Siegbahn A, Granger CB, Wallentin L. Biomarkers in atrial fibrillation: a clinical review. Eur Heart J. 2013;34(20):1475–1480. | |
Roldán V, Marín F, Díaz J, et al. High sensitivity cardiac troponin T and interleukin-6 predict adverse cardiovascular events and mortality in anticoagulated patients with atrial fibrillation. J Thromb Haemost. 2012;10(8):1500–1507. | |
Kornej J, Apostolakis S, Bollmann A, Lip GY. The emerging role of biomarkers in atrial fibrillation. Can J Cardiol. 2013;29(10):1181–1193. | |
Mevorach D. Opsonization of apoptotic cells. Implications for uptake and autoimmunity. Ann N Y Acad Sci. 2000;926:226–235. | |
Aimé-Sempé C, Folliguet T, Rücker-Martin C, et al. Myocardial cell death in fibrillating and dilated human right atria. J Am Coll Cardiol. 1999;34(5):1577–1586. | |
Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104(24):2886–2891. | |
Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108(24):3006–3010. | |
Malouf JF, Kanagala R, Al Atawi FO, et al. High sensitivity C-reactive protein: a novel predictor for recurrence of atrial fibrillation after successful cardioversion. J Am Coll Cardiol. 2005;46(7):1284–1287. | |
Kornej J, Reinhardt C, Kosiuk J, et al. Response of high-sensitive C-reactive protein to catheter ablation of atrial fibrillation and its relation with rhythm outcome. PLoS One. 2012;7(8):e44165. | |
Smith E, O’Donnell M. PURE-MRI – a proposal to determine the prevalence and consequences of covert ischemia in Canada; 2013. Available from: http://www.hsf.ca/research/en/pure-mri-proposal-determine-prevalence-and-consequences-covert-ischemia-canada. Accessed November 16, 2013. | |
Gaita F, Corsinovi L, Anselmino M, et al. Prevalence of silent cerebral ischemia in paroxysmal and persistent atrial fibrillation and correlation with cognitive function. J Am Coll Cardiol. 2013;62(21):1990–1997. | |
Haeusler KG, Koch L, Herm J, et al. 3 Tesla MRI-detected brain lesions after pulmonary vein isolation for atrial fibrillation: results of the MACPAF study. J Cardiovasc Electrophysiol. 2013;24(1):14–21. | |
Flaker GC, Eikelboom JW, Shestakovska O, et al. Bleeding during treatment with aspirin versus apixaban in patients with atrial fibrillation unsuitable for warfarin: the apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment (AVERROES) trial. Stroke. 2012;43(12):3291–3297. | |
Fisher M. MRI screening for chronic anticoagulation in atrial fibrillation. Front Neurol. 2013;4:137. | |
Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119(13):1758–1767. | |
Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol. 1997;20(2 Pt 2):397–413. | |
Daccarett M, Badger TJ, Akoum N, et al. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol. 2011;57(7):831–838. | |
Akoum N, Daccarett M, McGann C, et al. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a DE-MRI guided approach. J Cardiovasc Electrophysiol. 2011;22(1):16–22. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.