Back to Journals » Journal of Inflammation Research » Volume 17
Valsartan Mitigates the Progression of Methotrexate-Induced Acute Kidney Injury in Rats via the Attenuation of Renal Inflammation and Oxidative Stress
Authors Kutbi D, Almalki RS
Received 25 January 2024
Accepted for publication 4 April 2024
Published 11 April 2024 Volume 2024:17 Pages 2233—2243
DOI https://doi.org/10.2147/JIR.S456610
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Dina Kutbi,1,2 Riyadh S Almalki3
1Department of Pharmacy, King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia; 2Department of Pharmacology, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia; 3Department of Pharmacology and Toxicology, Faculty of Pharmacy, Umm AL-Qura University, Makkah, Saudi Arabia
Correspondence: Dina Kutbi, Department of Pharmacy, King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia, Email [email protected]
Background: Methotrexate (MTX) is a folic acid antagonist, commonly administered for the treatment of a variety of cancers. However, methotrexate toxicity including bone marrow suppression and hepatic and renal toxicity limits its use. Angiotensin AT1 receptor blockers including Valsartan (Val) possess the ability to ameliorate MTX-induced toxicity through various mechanisms. In this study, we explored the potential reno-protective effects of Val against MTX-induced acute kidney injury in rats.
Methods: Twenty-four Wistar rats were randomly segregated into 3 groups. Group 1 served as the control group and received an oral dose of 1mL/kg of normal saline. Group 2 received a single dose of 20 mg/kg of MTX intraperitoneally (IP) for 5 days. Group 3 received a single IP dose of 20 mg/kg of MTX followed by an oral dose of 10 mg/kg of Valsartan for 5 days. At the end of the experiment, the levels of serum kidney biomarkers, inflammatory and oxidative stress markers were accessed. Furthermore, the effect of MTX on kidney tissue histology was examined.
Results and discussion: Our results showed that MTX treatment increased the level of serum kidney and inflammatory biomarkers and decreased the level of antioxidants SOD and GSH while increasing the lipid peroxidation contents. Furthermore, MTX treatment caused structural changes to kidney histology. However, the administration of Val significantly prevented these changes.
Conclusion: Valsartan possesses nephroprotective potential and might serve as a potential therapeutic strategy against MTX-induced kidney injury.
Keywords: acute kidney injury, inflammation, methotrexate, oxidative stress, renal toxicity, renoprotection
Introduction
The complex interactions between oxidants and antioxidant defense pathways lead to oxidative stress, which damages proteins, lipids, nucleic acids, and thiols. There is currently no agreement on a single optimal marker of oxidative stress, despite the fact that the existence of oxidative stress in individuals with chronic renal disease is widely established. Antihypertensive medication effects on oxidative stress have also not been prospectively investigated in patients with chronic renal failure.1,2
Acute kidney injury (AKI) is a serious and potentially life-threatening condition resulting from a sudden decline in kidney function. This can happen for a variety of reasons, including dehydration, infection, and the use of certain medications such as over-the-counter medicines, and prescription drugs, renal/extra-renal surgeries.3 AKI has continuously remained one of the causes of mortality in the world, especially among inpatients receiving treatments for chronic diseases, and this has become a growing concern in public health worldwide with an estimated incidence of 2–7% in hospitalized patients and a mortality rate of up to 50% in those with severe AKI.4
Methotrexate (MTX) is a folate antagonist used to treat several malignant and autoimmune diseases.5 High-dose methotrexate (HDMTX) 500–1000mg/m2 is one of the major causes of kidney toxicity. Patients suffering from obesity, diabetes, heart failure, or older than 60 years are at high risk of AKI induced by MTX or HDMTX.6 At high dosages in acidic conditions, MTX may produce crystals (7-hydroxy-MTX) which are insoluble in urine and precipitate within distal renal tubules. Additionally, substantial deposits of uric acid and calcium may cause acute renal failure caused by tumor rupture while taking chemotherapy.7 The administration of MTX has also been shown to be linked to substantial side effects including depression, gastrointestinal symptoms such as nausea and vomiting, and cardiovascular problems like arrhythmia. MTX may also cause immunosuppression.8
Currently, the available options for the treatment of HDMTX-induced toxicity include the use of glucarpidase and leucovorin.9 Within 15 minutes of treatment, glucarpidase (carboxypeptidase G2) may hydrolyze circulating MTX into its inactive form, deoxyaminopteroic acid, and lower plasma MTX levels by >95%.10 However, there are certain restrictions on the use of glucarpidase for HDMTX treatment. It has a relatively short half-life, limited availability, and is expensive.11 There is therefore a need to search for new drugs devoid of these restrictions.
Valsartan (Val) is an angiotensin II receptor blocker that reduces the activity of the renin-angiotensin-aldosterone system (RAAS) and thus increases renal blood flow and glomerular filtration rate by dilating the efferent arterioles and reducing the intraglomerular pressure.12 Valsartan is well absorbed after oral administration, with a bioavailability of about 25–30%. The time to peak concentration (Tmax) of valsartan is reported to be about 2–4 hours. and its oral absorption is affected by food.13 Valsartan is highly bound to plasma proteins (about 99%) and has a large volume of distribution (about 17 L/kg). It crosses the blood-brain barrier and the placenta and enters the breast milk. Val is metabolized primarily by the liver, with the involvement of cytochrome P450 enzymes (CYP2C9 and CYP3A4).14,15
Previous studies have shown that the introduction of Val to conventional therapy significantly slowed the rate of renal function decline and delayed the need for renal replacement therapy in hypertensive patients with advanced chronic kidney disease (CKD).16 Hence, in this study, we examined the potential reno-protective activity of valsartan against methotrexate-induced acute kidney injury in rats.
Materials and Methods
Drugs and Chemical
Methotrexate and Valsartan were purchased from TargetMol, Massachusetts, United States (MolPort-003-665-521 and MolPort-003-666-608 respectively) and dissolved in DMSO (dimethyl sulfoxide; Millipore, Molsheim, France).
Animals
Twenty-four male Wistar rats weighing (170 −190 g) were purchased from the animal house of the Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia. All animals were allowed to acclimatize for 1 week at a temperature of 24–26°C, relative humidity 35–75%, and on a 12-hour light and dark cycle.
Experimental Design
Rats were then randomly placed into three groups with eight animals each. Group I was the control group and received 1mL/kg of normal saline. Group II was administered intraperitoneally (IP) with MTX at a dose of 20 mg/kg.8,17 Group III received an IP of 20 mg/kg of MTX and then an oral dose of 10 mg/kg of Val.18 All animal treatments were conducted for five days. Finally, animals were anesthetized by isoflurane and euthanized. Following this, blood was withdrawn through the retro-orbital plexus and kidneys were subsequently collected, rinsed with PBS, and weighed. The left kidneys were frozen at −80°C and used for tissue oxidative stress parameters while the right kidneys were kept in 10% buffered formalin for histopathology. All animal experiments were conducted per the updated Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and approved by the ethics committee of the Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia with a reference number (PH-1442-75).
Determination of Biochemical Parameters
The serum samples were obtained from whole blood after centrifugation at 3000 rpm for 10 minutes and at 4°C. Serum urea, creatinine, albumin, Intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), kidney injury molecule-1(KIM-1), Interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) were quantified with ELISA kit (MyBioSource, California, USA), according to the manufacturer’s instructions.
Measurement of Kidney Tissue Levels of Glutathione, Lipid Peroxidation, and SOD in Tissue Homogenate
Ten percent of kidney homogenate was prepared by weighing 100 mg of frozen kidney tissue in an Eppendorf tube and homogenized with 1 mL of 100 mM phosphate buffer (pH 7.4) containing 1mM EDTA and centrifuged at 14,000 rpm for 15 min at 4°C. The levels of glutathione (GSH) and malondialdehyde (MDA) and superoxide dismutase (SOD) were measured in the supernatant using a commercial kit (MyBioSource, California, USA).
Histopathology
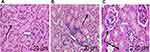
Kidney tissues were fixed in a 10% neutral-buffered formalin solution, followed by dehydration in graded alcohol, clearance in xylene, and embedded in paraffin for 12 hours. Paraffin blocks were then sectioned at 5-µm thickness using a microtome followed by heating in a 60°C oven for 1 hour for fixation on the slide. Slides were deparaffinized in xylene and rehydrated in graded alcohol, and finally rinsed in distilled water for 2 minutes. Subsequently, slides were stained with hematoxylin (stains cell components blue) and eosin (stains cell components pink) solution. Images were taken using a light microscope and tissue sections were examined by a pathologist.
Statistical Analysis
Graphs and statistical analysis with one-way ANOVA followed by Sidak’s multiple comparisons were done using GraphPad Prism V6.0 software (GraphPad Software, San Diego, CA). P<0.05 is considered-significant.
Results
Valsartan Restored the Levels of Serum Biochemical and Kidney Injury Markers
In this study, we investigated the nephroprotective potentials of Valsartan against MTX-induced kidney injury in rats. In achieving the aim of our study, we used an I.P. injection of MTX at a dose of 20 mg/kg body weight. Applying the conversion factor of 6.2,19 this 20 mg/kg in rats has an equivalent dose of 3.23 mg/kg body weight in humans. In a 60kg human, with a conversion factor of 37,20 3.23 mg/kg body is equivalent to 119.51 mg/m2 which is within the physiologically relevant high dose of MTX (500 mg/m2) used in cancer chemotherapy.21
Similarly, the oral dose of Valsartan 10 mg/kg body weight in rats is equivalent to the human dose of 1.61mg/kg. In a 60kg human, this is equivalent to 96.6 mg. This dose is within the physiological dose of 320 mg22 and 400 mg23 of Valsartan used in the treatment of hypertension.
As shown in Figure 1, the MTX group showed significant elevations in serum creatinine, serum urea, albumin, and KIM-1 levels relative to the control (p ˂ 0.05). The administration of rats with Val after MTX administration on the other hand ameliorated these changes (Figure 1).
Valsartan Restored the Levels of Adhesion Molecules of Kidney Injury
Next, we quantified the effect of MTX and Val on adhesion molecules for kidney tissue injury in MTX-induced nephrotoxicity in rats. As shown in Figure 2, rats administered with MTX presented a significant increase in serum ICAM-1 and VCAM-1 compared to the untreated animals in the control group. However, these elevations in the adhesion molecules were significantly prevented with Val administration (Figure 2).
Valsartan Exhibited Anti-Inflammatory Activities
Next, we examined the effects of MTX and Val on serum inflammatory markers in MTX-induced nephrotoxicity in rats. Compared to the control, the MTX-administered group showed significant elevations in serum inflammatory markers (IL-6 and TNF-α) (Figure 3). However, animals given Val following the administration of MTX treatment resulted in a significant decrease in the serum levels of IL-6 and TNF-α compared to MTX-only treated rats (Figure 3).
Valsartan Ameliorated Kidney Oxidative Stress
Next, we examined the effects of MTX and Val on oxidative stress markers in MTX-induced nephrotoxicity in rats. Our results showed that rats treated with MTX administered group showed significant elevations in tissue TBARS (p ˂ 0.05) and a significant reduction in the renal tissue levels of GSD and SOD in comparison to untreated animals in group 1 (p ˂ 0.05, Figure 4). However, animals administered with Val following the treatment of MTX revealed a significant increase in the tissue levels of GSH and SOD (Figure 4).
Valsartan Protected Against Structural Changes to Kidney Histology
Next, we examined the effects of MTX and Val on Kidney Histology. As shown in Figure 5, sections from the H&E-stained kidney (cortex and medulla) of the untreated animals in the control group revealed normal histology, comprising the renal corpuscle, glomerular capillaries, and renal tubules. However, nephrotoxicity induced by the administration of MTX in rats showed invasive viable cell aggregation. This is coupled with the decrease in the size of the renal corpuscle, the glomerulus, the dilation of the tubular lumen, and the collapse of the renal parenchyma. In contrast, animals treated with Val after the administration of an MTX revealed a near-normal kidney histology (Figure 5 and Table 1).
 |
Table 1 Semiquantitative Histopathological Scoring Analysis of Changes in the Kidneys of Rats |
Discussion
Accumulated evidence suggests Val possesses the potential to protect against drug-induced acute kidney injury in animal24 and human models.25 In this study, we explored the potential reno-protective activity of valsartan against methotrexate-induced acute kidney injury in rats. Our results showed that the treatment of rats with Val following the administration of MTX led to a decrease in the serum levels of urea, creatinine, albumin, and KIM-1 which were previously elevated due to MTX administration. In addition, our results showed that Val mitigated inflammation and attenuated oxidative stress induced by MTX in rats and also prevented structural changes in kidney histology. These results thus suggest a potential renoprotective role for valsartan.
The serum urea level is a vital index for the evaluation of the functionality of the kidney in clinical settings and provides important information regarding the metabolism of protein. In addition, serum urea is important in the early detection of kidney diseases linked to acute myocardial infarction.26 An increase in the serum level of urea is a sign of kidney malfunction (Kamal, 2014). In this study, a significant increase in the serum urea level was recorded in the MTX-only administered group. This result was similar to the results obtained by Hany et al when they tested the nephroprotective potentials of naringin on renal toxicity caused by MTX.27,28 A sudden increase in serum urea and a significant increase in plasma MTX concentration were laboratory indicators of the development of renal impairment.29 David et al also found that rats treated with 0.125 mg/kg MTX for 14 days resulted in an increase in the serum urea level thus indicating potential kidney damage.30 This MTX-induced increase in the serum urea level was prevented with the administration of Val and hence, signifying a potential renoprotective property.31
Creatinine is an end-product generated from the metabolism of creatine and creatine phosphate metabolism in the muscle.32 In an individual, there is a relatively stable production of creatinine and dependent on the mass of the muscle.33 Creatinine serum (Scr) level is assessed to diagnose an impairment in the function of the kidney.34 A marked elevation in Scr values signifies an AKI.35 In this study, the administration of MTX to rats led to a significant increase in serum creatinine levels. The result is in line with the serum Scr result obtained by Hany et al when they tested the nephroprotective potentials of naringin on renal toxicity caused by MTX.28 The increase in the serum creatine level is due to MTX-induced delay in the renal elimination of creatine. It has been reported that a higher blood creatinine level during an MTX infusion is frequently linked to delayed MTX elimination.36 The elevation in the serum creatinine levels caused by MTX recorded in our study was decreased by the treatment with Val. This indicates that Val mediates the decrease in serum creatinine levels through the attenuation of MTX-induced renal impairment.
Albumin is a major serum protein with numerous important physiological functions which include the maintenance of colloidal osmotic pressure, binding of a large variety of compounds, and providing the majority of plasma antioxidant activity. A decrease in the concentration of serum albumin level below normal has been linked to kidney function decline.37 MTX, being a weak acid has about 50% binding ability with serum albumin.38 While a decrease in serum albumin below the normal level was linked to a decline in kidney function, however, in our study, an increase in serum albumin level above that of the normal control group level was recorded in the MTX-treated group in contrary to other studies who reported a decrease in serum albumin below the normal levels following MTX treatment.39 However, in our study, this increase was reversed to normal or close to normal with the administration of Val. The increase in serum albumin levels observed in the MTX groups may be due to dehydration since previous studies have shown a correlation between high serum albumin levels and dehydration.40 This dehydration might also be due to MTX-induced-kidney damage since relevant studies have reported a link between MTX toxicity and dehydration.41
KIM-1 is a transmembrane glycoprotein. Recent studies on both mice and humans have shown that an increase in the serum KIM-1 level can serve as a biomarker of kidney injury.42 In this study, the serum KIM-1 level increased in the MTX-treated group which is similar to what was observed by Younis et al39 where the authors examined the effect of Geraniol on MTX-induced AKI in rats. The Kim-1’s elevated concentration induced by MTX may function as a compensatory strategy, acting as an adhesion molecule to lessen epithelial loss.43 This increase in the KIM-1 level was significantly prevented with the administration of Val.
Intercellular adhesion molecule-1 (ICAM-1) is a cell surface glycoprotein present in endothelial cells and leukocytes. An increase in ICAM-1 has been linked to diabetes mellitus and diabetic nephropathy (DN) and thus serves as a biomarker for DN.44 The overexpression of ICAM-1 is mostly induced by inflammatory cytokines.45 The MTX-induced increase in ICAM-1 level observed in this study could be a result of an increase in the expression of inflammatory cytokines like interleukin-1β, and the TNF-α that are produced by monocytes and macrophages.46 Our observations agreed with those of Gu et al since an increase in ICAM-1 was also observed in the MTX-only group in their study.44 This increase was ameliorated with Val treatment.
Furthermore, Vascular Cell Adhesion Molecule-1 (VCAM-1) is a cell adhesion molecule that assists in the regulation of inflammation-associated vascular adhesion and the migration of leukocytes in endothelial cells like macrophages and T cells.47 The expression of VCAM-1 is usually activated by pro-inflammatory cytokines such as TNFα and ROS.47,48 Therefore, an increase in the serum VCAM-1 level could be a result of the increase in the serum levels of the inflammatory biomarkers which was demonstrated in this study following the treatment with MTX. This result is in tandem with what was obtained by Wang et al, who showed an increase in the expression of VCAM-1 following 150mg/kg/day of MTX in rats.49 However, in this study, this increase was prevented with the administration of Val thus confirming its nephroprotective effects against MTX.
In this study, animals administered with a single dose of MTX experienced invasive cell aggregation and a reduction in the size of the renal corpuscle, glomerulus, the dilation of the tubular lumen, and a collapse of the renal parenchyma. All these observations agree with17,28,50 whose studies also showed similar effects of MTX on the kidney tissues. Furthermore, treatment of the animals with MTX and with Val showed a significant improvement in the histological structure of the kidney.
IL-6 is a representative cytokine with a pleiotropic effect on inflammation, hematopoiesis, and immune response but a continuous increase in the production of IL-6 can result in the development of several immune-mediated diseases.51 MTX increased IL-6 production might be due to the reduced serum IL-6 clearance.52 An increase in the IL-6 serum level was observed in the MTX group similar to the study by Abdel-Daim et al.53 The treatment of the MTX group with Val attenuated the MTX-induced increase in IL-6 concentrations. In addition, TNFα is a pro-inflammatory cytokine that activates the expression of inflammatory molecules and other cytokines and cell adhesion molecules. TNFα activates the expression of ICAM-1 and VCAM-1.47 An earlier study has shown that there is a relationship between elevated TNFα levels and advanced malignant growth and inflammation.54 The pleiotropic TNF-α is elevated in conditions of chronic inflammation like diabetes and hypertension. The activity and expression of transporters are both impacted by TNF-α, which also changes renal hemodynamics and nephron transport. By promoting immune cell infiltration and cell death, it also contributes to organ damage.55 The increase in the levels of TNFα in the MTX-administered rats might be due to infiltration of the kidney cells by macrophages.56 Several previous studies reported an increase in TNFα concentration in the MTX-only treated group which supports the result of this study.39,53,57 The MTX-induced increase in TNF-α was reversed by Val. Hence, the decrease in the tissue levels of TNF-α by Val might be one mechanism through which it mediates its reno-protective potentials.
Oxidative stress ensures when there is an imbalance between the generation of reactive oxygen species (ROS) and the antioxidant defense system58 resulting in molecular damage to macromolecules such as DNA, lipids, proteins, and/or a disturbance of redox signaling and regulation.59 Since a number of these proteins function as transcriptional and regulatory factors for cellular activities. Thus, any structural changes can trigger signaling cascades that change how the cell functions, resulting in mutagenicity and genotoxicity.60
TBARS is formed as a byproduct of lipid peroxidation. It is now used as a standard marker for lipid peroxidation induced by oxidative stress.61 MTX generates ROS, which results in lipid peroxidation and impairs mitochondrial function.62 It was hypothesized that the destruction and damage to cell membranes caused by oxygen-free radicals, or lipid peroxidation, was a significant source of the tissue damage brought on by MTX.63 In this study, the MTX-administered group showed an increase in TBARS levels which was similar to the result obtained by Dalaklioglu et al who showed an increase in TBARS levels in the livers of rats following the administration of MTX.64 Our result showed that Val significantly prevented the increase in renal TBARS levels and had protective impacts against lipid peroxidation. We theorize that their ability to scavenge free radicals appears to be helping to prevent lipid peroxidation and confers nephroprotection.
It has been demonstrated that MTX inhibits the cytosolic NADP-dependent dehydrogenases and NADP malic enzymes, suggesting that the MTX may lessen the availability of NADPH in cells by obstructing pentose cycle enzymes. Due to interaction with the pentose phosphate shunt, MTX may potentially inhibit nucleic acid metabolism. Owing to the considerable decrease in GSH levels caused by MTX, the antioxidant enzyme defense system is less effective, which makes cells more sensitive to ROS. Therefore, MTX’s harmful effects are partially brought on by its direct hazardous action through boosting ROS generation.65 Previous studies showed that MTX caused a decrease in the GSH level which confirms our findings in the group treated with HDMTX.39,53,57 This decrease was prevented with Val treatment.
Superoxide dismutase (SOD) is an antioxidant enzyme that helps in the physiological defense strategies in animals against ROS and free radicals.66 The deficiency of SOD can lead to overwhelming oxidative stress which leads to carcinogenesis.67 Superoxide radicals are converted into hydrogen peroxide by superoxide dismutase, while water and oxygen gas are produced as a result of catalase. Increased oxidative stress in the cell results in a decrease in the activity of the SOD and glutathione peroxidase (GSH-Px) enzymes. Previous research using MTX indicated that a decline in SOD and GSH-Px activity led to an even greater rise in oxidative stress in the tissues.68 Our results showed that MTX caused a decrease in the tissue SOD level which agrees with Erdogan et al observation who examined Rutin’s preventive potentials in a rat hepatotoxicity model caused by a single dose of 20 mg/kg MTX.69 The administration of Val increased the tissue SOD level. Valsartan might mediate this increase in kidney SOD levels through the removal of excess free radicals (like O2·– and the peroxyl radical) as well as an improvement in the antioxidant status70 and could partly account for the potential renoprotective effects of Val.
The possible mechanism for the renoprotective effect of valsartan against MTX-induced kidney injury could be partly due to its action as an angiotensin II receptor blocker (ARB). Valsartan, as an ARB, blocks the angiotensin II type 1 (AT1) receptor, which is involved in vasoconstriction, sodium retention, and inflammation.71 By inhibiting the AT1 receptor, valsartan may likely prevent the renal vasoconstriction and inflammation caused by MTX, thereby reducing kidney damage. In addition, valsartan’s antioxidant properties may contribute to its renoprotective effects by reducing oxidative stress and lipid peroxidation, as indicated by the observed increase in antioxidant levels and decrease in lipid peroxidation contents in this study.
Conclusion
This study sheds light on the renoprotective potential of Valsartan on MTX-induced kidney injury in rats. We showed that the administration of MTX to rats leads to its uptake by the cells. Prolonged MTX treatment results in the accumulation of this compound in the kidney cells thereby causing an increase in the level of reactive oxygen species which under a compromised antioxidant system resulted in oxidative stress. An accumulation of MTX also leads to inflammation. However, these effects were prevented with the administration of Val which could partly be due to its antioxidant potential coupled with its anti-inflammatory ability.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Annuk M, Zilmer M, Lind L, Linde T, Fellström B. Oxidative stress and endothelial function in chronic renal failure. J Am Soc Nephrol. 2001;12(12):2747–2752. doi:10.1681/ASN.V12122747
2. Himmelfarb J, McMonagle E, McMenamin E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int. 2000;58(6):2571–2578. doi:10.1046/j.1523-1755.2000.00443.x
3. Pazhayattil GS, Shirali AC. Drug-induced impairment of renal function. Internat J Nephrol Renov Dis. 2014;457–468. doi:10.2147/IJNRD.S39747
4. Rolland A-L, Garnier A-S, Meunier K, Drablier G, Briet M. Drug-induced acute kidney injury: a study from the French medical administrative and the French national pharmacovigilance databases using capture-recapture method. J Clin Med. 2021;10(2):168. doi:10.3390/jcm10020168
5. Alsdorf WH, Karagiannis P, Langebrake C, Bokemeyer C, Frenzel C. Standardized supportive care documentation improves safety of high‐dose methotrexate treatment. Oncologist. 2021;26(2):e327–e332. doi:10.1002/onco.13603
6. Amitai I, Rozovski U, El‐Saleh R, et al. Risk factors for high‐dose methotrexate associated acute kidney injury in patients with hematological malignancies. Hematolog Oncol. 2020;38(4):584–588. doi:10.1002/hon.2759
7. Cosmai L, Porta C, Ronco C, Gallieni M. Acute kidney injury in oncology and tumor Lysis syndrome. Crit Care Nephrol. 2019;2019:234–250. e1.
8. Yang M, Kim J, Kim J-S, et al. Hippocampal dysfunctions in tumor-bearing mice. Brain Behav Immun. 2014;36:147–155. doi:10.1016/j.bbi.2013.10.022
9. Song Y, Liu L, Liu B, et al. Interaction of nobiletin with methotrexate ameliorates 7-OH methotrexate-induced nephrotoxicity through endoplasmic reticulum stress-dependent PERK/CHOP signaling pathway. Pharmacol Res. 2021;165:105371. doi:10.1016/j.phrs.2020.105371
10. Ramsey LB, Balis FM, O’Brien MM, et al. Consensus guideline for use of glucarpidase in patients with high‐dose methotrexate induced acute kidney injury and delayed methotrexate clearance. oncologist. 2018;23(1):52–61. doi:10.1634/theoncologist.2017-0243
11. Domingo-González A, Osorio S, Landete E, Monsalvo S, Díez-Martín JL. A second administration of glucarpidase in a different cycle of high-dose methotrexate: is it safe and effective in adults? J Oncol Pharm Pract. 2021;27(3):734–738. doi:10.1177/1078155220946464
12. Acanfora D, Ciccone MM, Scicchitano P, Acanfora C, Casucci G. Neprilysin inhibitor–angiotensin II receptor blocker combination (sacubitril/valsartan): rationale for adoption in SARS-CoV-2 patients. Europ Heart J Cardiovas Pharmacoth. 2020;6:135–136. doi:10.1093/ehjcvp/pvaa028
13. Chella N, Shastri N, Tadikonda RR. Use of the liquisolid compact technique for improvement of the dissolution rate of valsartan. Acta Pharmaceutica Sinica B. 2012;2(5):502–508. doi:10.1016/j.apsb.2012.07.005
14. Hedaya MA, Helmy SA. Pharmacokinetic interactions of valsartan and hydrochlorothiazide: an open-label, randomized, 4-period crossover study in healthy Egyptian male volunteers. Clin Ther. 2013;35(6):846–861. doi:10.1016/j.clinthera.2013.04.014
15. Wu Q, Wang X, Chen Q, et al. Pharmacokinetics and bioequivalence of two formulations of valsartan 80 mg capsules: a randomized, single dose, 4-period crossover study in healthy Chinese volunteers under fasting and fed conditions. Drug Des Devel Ther. 2020;14:4221–4230. doi:10.2147/DDDT.S253078
16. Yasuda T, Endoh M, Suzuki D, et al. Effects of valsartan on progression of kidney disease in Japanese hypertensive patients with advanced, predialysis, chronic kidney disease: Kanagawa Valsartan Trial (KVT). Hypertens Res. 2013;36(3):240–246. doi:10.1038/hr.2012.183
17. Yuksel Y, Yuksel R, Yagmurca M, et al. Effects of quercetin on methotrexate-induced nephrotoxicity in rats. Hum Exp Toxicol. 2016;36(1):51–61. doi:10.1177/0960327116637414
18. Kusaka H, Sueta D, Koibuchi N, et al. LCZ696, angiotensin II receptor-neprilysin inhibitor, ameliorates high-salt-induced hypertension and cardiovascular injury more than valsartan alone. Am J Hypertens. 2015;28(12):1409–1417. doi:10.1093/ajh/hpv015
19. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharma. 2016;7(2):27. doi:10.4103/0976-0105.177703
20. Jacob S, Nair AB, Morsy MA. Dose conversion between animals and humans: a practical solution. Indian J Pharm Educ Res. 2022;56:600–607. doi:10.5530/ijper.56.3.108
21. Howard SC, McCormick J, Pui C-H, Buddington RK, Harvey RD. Preventing and managing toxicities of high-dose methotrexate. oncologist. 2016;21(12):1471–1482. doi:10.1634/theoncologist.2015-0164
22. Zappe DH, Crikelair N, Kandra A, Palatini P. Time of administration important? Morning versus evening dosing of valsartan. J Hypert. 2015;33(2):385. doi:10.1097/HJH.0000000000000397
23. Engeli S, Stinkens R, Heise T, et al. Effect of sacubitril/valsartan on exercise-induced lipid metabolism in patients with obesity and hypertension. Hypertension. 2018;71(1):70–77. doi:10.1161/HYPERTENSIONAHA.117.10224
24. Sun X, Luan Q, Qiu S. Valsartan prevents glycerol-induced acute kidney injury in male albino rats by downregulating TLR4 and NF-κB expression. Int J Biol Macromol. 2018;119:565–571. doi:10.1016/j.ijbiomac.2018.07.149
25. Komers R, Anderson S. Treatment of hypertension in diabetic patients with nephropathy. Curr Diab Rep. 2001;1(3):251–260. doi:10.1007/s11892-001-0043-5
26. Wei D, Ge M. The spatial distribution of BUN reference values of Chinese healthy adults: a cross-section study. Internat J Biometeorol. 2018;62:2099–2107. doi:10.1007/s00484-018-1585-4
27. Alum EU, Famurewa AC, Orji OU, et al. Nephroprotective effects of Datura stramonium leaves against methotrexate nephrotoxicity via attenuation of oxidative stress-mediated inflammation and apoptosis in rats. Avicenna J Phytomed. 2023;13(4):377. doi:10.22038/AJP.2023.21903
28. Hany E, Abdullah MA, Manal A, Ashraf MA, Mahmoud K. Nephroprotective effect of naringin in methotrexate induced renal toxicity in male rats. Biomed Pharmacother. 2021;143:1–15.
29. Al-Rashidy AH, Salem RR, Alhosary AA, et al. Role of erythropoietin in methotrexate-induced nephrotoxicity in adult male albino rats. J Nephropharmacol. 2018;7(2):156–163. doi:10.15171/npj.2018.31
30. David AVA, Satyanarayana N, Parasuraman S, Bharathi S, Arulmoli R. Ameliorative effect of quercetin on methotrexate induced toxicity in Sprague-Dawley rats: a histopathological study. Indian J Pharm Educ. 2016;50:200–2008. doi:10.5530/ijper.50.3.30
31. Rizwan F, Yesmine S, Banu SG, Chowdhury IA, Hasan R, Chatterjee TK. Renoprotective effects of stevia (Stevia rebaudiana Bertoni), amlodipine, valsartan, and losartan in gentamycin-induced nephrotoxicity in the rat model: biochemical, hematological and histological approaches. Toxicol Rep. 2019;6:683–691. doi:10.1016/j.toxrep.2019.07.003
32. Kashani K, Rosner MH, Ostermann M. Creatinine: from physiology to clinical application. Eur J Internal Med. 2020;72:9–14. doi:10.1016/j.ejim.2019.10.025
33. Bargnoux A-S, Kuster N, Cavalier E, et al. Serum creatinine: advantages and pitfalls. J Lab Precis Med. 2018;3(8):71. doi:10.21037/jlpm.2018.08.01
34. Kamal A. Estimation of blood urea (BUN) and serum creatinine level in patients of renal disorder. Indian J Fundam Appl Life Sci. 2014;4(4):199–202.
35. Corbett M, Duarte A, Llewellyn A, et al. Point-of-care creatinine tests to assess kidney function for outpatients requiring contrast-enhanced CT imaging: systematic reviews and economic evaluation. Health Technol Assess. 2020;24(39):1. doi:10.3310/hta24390
36. Yang S-L, Zhao F-Y, Song H, Shen D-Y, X-J X. Methotrexate associated renal impairment is related to delayed elimination of high-dose methotrexate. Sci World J. 2015;2015:1–8. doi:10.1155/2015/751703
37. Lang J, Katz R, Ix JH, et al. Association of serum albumin levels with kidney function decline and incident chronic kidney disease in elders. Nephrol Dial Transplant. 2018;33(6):986–992. doi:10.1093/ndt/gfx229
38. Mohassel L, Griffin S, Binder N, Barnett SD, Cuevo R. Evaluation of methotrexate clearance in adult and pediatric patients with hypoalbuminemia. Blood. 2019;134:2906. doi:10.1182/blood-2019-121892
39. Younis NS, Elsewedy HS, Shehata TM, Mohamed ME. Geraniol averts methotrexate-induced acute kidney injury via keap1/Nrf2/HO-1 and MAPK/NF-κB Pathways. Curr Issues Mol Biol. 2021;43(3):1741–1755. doi:10.3390/cimb43030123
40. Levitt DG, Levitt MD. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gene Med. 2016;Volume 9:229–255. doi:10.2147/IJGM.S102819
41. Herfarth HH, Long MD, Isaacs KL. Methotrexate: underused and ignored? Dig Dis. 2013;30(Suppl. 3):1.
42. Sabbisetti VS, Waikar SS, Antoine DJ, et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014;25(10):2177. doi:10.1681/ASN.2013070758
43. Arun M. Acute Kidney Injury in the Medical Wards of Government Rajaji Hospital, Madurai: A Prospective Study. Madurai: Madurai Medical College; 2013.
44. Gu HF, Ma J, Gu KT, Brismar K. Association of intercellular adhesion molecule 1 (ICAM1) with diabetes and diabetic nephropathy. Front Endocrinol. 2013;3:179. doi:10.3389/fendo.2012.00179
45. Bui TM, Wiesolek HL, Sumagin R. ICAM-1: a master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leuc Biol. 2020;108(3):787–799. doi:10.1002/JLB.2MR0220-549R
46. Kandemir FM, Kucukler S, Caglayan C, Gur C, Batil AA, Gülçin İ. Therapeutic effects of silymarin and naringin on methotrexate‐induced nephrotoxicity in rats: biochemical evaluation of anti‐inflammatory, antiapoptotic, and antiautophagic properties. J Food Biochem. 2017;41(5):e12398. doi:10.1111/jfbc.12398
47. Kong D-H, Kim YK, Kim MR, Jang JH, Lee S. Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. Int J Mol Sci. 2018;19(4):1057. doi:10.3390/ijms19041057
48. Cook-Mills JM, Marchese ME, Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signaling. 2011;15(6):1607–1638. doi:10.1089/ars.2010.3522
49. Wang Y, Zhao -T-T, Zhao H-Y, Wang H. Melatonin protects methotrexate-induced testicular injury in rats. Eur Rev Med Pharmacol Sci. 2018;22(21):1.
50. Halil A, Ozlem O, Hamit YE, Bunyamin A, Ercan B, Necat Y. The impact of gallic acid on the methotrexate-induced kidney damage in rats. J Food Drug Anal. 2017;25:890–897. doi:10.1016/j.jfda.2017.05.001
51. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspect Biol. 2014;6(10):a016295–a016295. doi:10.1101/cshperspect.a016295
52. Dennen P, Altmann C, Kaufman J, et al. Urine interleukin-6 is an early biomarker of acute kidney injury in children undergoing cardiac surgery. Critical Care. 2010;14(5):1–13. doi:10.1186/cc9289
53. Abdel-Daim MM, Khalifa HA, Abushouk AI, Dkhil MA, Al-Quraishy SA. Diosmin attenuates methotrexate-induced hepatic, renal, and cardiac injury: a biochemical and histopathological study in mice. Oxid Med Cell Longev. 2017;2017:1–10. doi:10.1155/2017/3281670
54. Al Obeed OA, Alkhayal KA, Al Sheikh A, et al. Increased expression of tumor necrosis factor-α is associated with advanced colorectal cancer stages. World J Gastroenterol. 2014;20(48):18390. doi:10.3748/wjg.v20.i48.18390
55. Ramseyer VD, Garvin JL. Tumor necrosis factor-α: regulation of renal function and blood pressure. Am J Physiol Renal Physiol. 2013;304(10):F1231–F1242. doi:10.1152/ajprenal.00557.2012
56. Awad AS, You H, Gao T, et al. Macrophage-derived tumor necrosis factor-α mediates diabetic renal injury. Kidney Int. 2015;88(4):722–733. doi:10.1038/ki.2015.162
57. Owumi SE, Ajijola IJ, Agbeti OM. Hepatorenal protective effects of protocatechuic acid in rats administered with anticancer drug methotrexate. Hum Exp Toxicol. 2019;38(11):1254–1265. doi:10.1177/0960327119871095
58. Masschelin PM, Cox AR, Chernis N, Hartig SM. The impact of oxidative stress on adipose tissue energy balance. Front Physiol. 2020;10:1638. doi:10.3389/fphys.2019.01638
59. Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L. The role of oxidative stress during inflammatory processes. Biol Chem. 2014;395(2):203–230. doi:10.1515/hsz-2013-0241
60. Chatterjee S. Oxidative stress, inflammation, and disease. In: Oxidative Stress and Biomaterials. Elsevier; 2016:35–58.
61. Khoubnasabjafari M, Ansarin K, Jouyban A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. Bioimpacts. 2015;5(3):123. doi:10.15171/bi.2015.20
62. Ulusoy HB, Öztürk İ, Sönmez MF. Protective effect of propolis on methotrexate-induced kidney injury in the rat. Renal Failure. 2016;38(5):744–750. doi:10.3109/0886022X.2016.1158070
63. Kose E, Sapmaz HI, Sarihan E, Vardi N, Turkoz Y, Ekinci N. Beneficial effects of montelukast against methotrexate-induced liver toxicity: a biochemical and histological study. Sci World J. 2012;2012:1–6. doi:10.1100/2012/987508
64. Dalaklioglu S, Genc G, Aksoy N, Akcit F, Gumuslu S. Resveratrol ameliorates methotrexate-induced hepatotoxicity in rats via inhibition of lipid peroxidation. Hum Exp Toxicol. 2013;32(6):662–671. doi:10.1177/0960327112468178
65. Swayeh NH, Abu-Raghif AR, Qasim BJ. The protective effects of felodipine on methotrexate-induced hepatic toxicity in rabbits. Iraqi J Med Sci. 2016;14(2):1.
66. Stephenie S, Chang YP, Gnanasekaran A, Esa NM, Gnanaraj C. An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. Journal of Functional Foods. 2020;68:103917. doi:10.1016/j.jff.2020.103917
67. Saed GM, Diamond MP, Fletcher NM. Updates of the role of oxidative stress in the pathogenesis of ovarian cancer. Gynecologic Oncol. 2017;145(3):595–602. doi:10.1016/j.ygyno.2017.02.033
68. Clavo B, Rodríguez-Esparragón F, Rodríguez-Abreu D, et al. Modulation of oxidative stress by ozone therapy in the prevention and treatment of chemotherapy-induced toxicity: review and prospects. Antioxidants. 2019;8(12):588. doi:10.3390/antiox8120588
69. Erdogan E, Ilgaz Y, Gurgor PN, Oztas Y, Topal T, Oztas E. Rutin ameliorates methotrexate induced hepatic injury in rats. Acta Cir Bras. 2015;30:778–784. doi:10.1590/S0102-865020150110000009
70. Vardi N, Parlakpinar H, Ates B, Cetin A, Otlu A. The protective effects of Prunus armeniaca L (apricot) against methotrexate-induced oxidative damage and apoptosis in rat kidney. J Physiol Biochem. 2013;69:371–381. doi:10.1007/s13105-012-0219-2
71. Silva IVG, de Figueiredo RC, Rios DRA. Effect of different classes of antihypertensive drugs on endothelial function and inflammation. Int J Mol Sci. 2019;20(14):3458. doi:10.3390/ijms20143458
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.