Back to Journals » OncoTargets and Therapy » Volume 8
Validation of the Memorial Sloan Kettering Cancer Center nomogram for predicting non-sentinel lymph node metastasis in sentinel lymph node-positive breast-cancer patients
Authors Bi X, Wang Y, Li M, Chen P, Zhou Z, Liu Y, Zhao T, Zhang Z, Wang C, Sun X, Qiu P
Received 8 December 2014
Accepted for publication 8 January 2015
Published 23 February 2015 Volume 2015:8 Pages 487—493
DOI https://doi.org/10.2147/OTT.S78903
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Daniele Santini
Xiang Bi,1,* Yongsheng Wang,2 Minmin Li,1,* Peng Chen,2 Zhengbo Zhou,2 Yanbing Liu,2 Tong Zhao,2 Zhaopeng Zhang,2 Chunjian Wang,2 Xiao Sun,2 Pengfei Qiu2
1School of Medicine and Life Sciences, University of Jinan-Shandong Academy of Medical Sciences, Shandong Cancer Hospital, 2Breast Cancer Center, Shandong Cancer Hospital, Jinan, People’s Republic of China
*These authors contributed equally to this study
Background: The main purpose of the study reported here was to validate the clinical value of the Memorial Sloan Kettering Cancer Center (MSKCC) nomogram that predicts non-sentinel lymph node (SLN) metastasis in SLN-positive patients with breast cancer.
Methods: Data on 1,576 patients who received sentinel lymph node biopsy (SLNB) at the Shandong Cancer Hospital from December 2001 to March 2014 were collected in this study, and data on 509 patients with positive SLN were analyzed to evaluate the risk factors for non-SLN metastasis. The MSKCC nomogram was used to estimate the probability of non-SLN metastasis and was compared with actual probability after grouping into deciles. A receiver-operating characteristic (ROC) curve was drawn and predictive accuracy was assessed by calculating the area under the ROC curve.
Results: Tumor size, histological grade, lymphovascular invasion, multifocality, number of positive SLNs, and number of negative SLNs were correlated with non-SLN metastasis (P<0.05) by univariate analysis. However, multivariate analysis showed that tumor size (P=0.039), histological grade (P=0.043), lymphovascular invasion (P=0.001), number of positive SLNs (P=0.001), and number of negative SLNs (P=0.000) were identified as independent predictors for non-SLN metastasis. The trend of actual probability in various decile groups was comparable to the predicted probability. The area under the ROC curve was 0.722. Patients with predictive values lower than 10% (97/492, 19.7%) had a frequency of non-SLN metastasis of 17.5% (17/97).
Conclusion: The MSKCC nomogram can provide an accurate prediction of the probability of non-SLN metastasis, and offers a reference basis about axillary lymph node dissection. Axillary lymph node dissection could be avoided in patients with predictive values lower than 10%.
Keywords: MSKCC nomogram, probability, SLN, axillary lymph node dissection, risk factors
Introduction
Sentinel lymph node biopsy (SLNB) can allow sentinel lymph node (SLN)-negative breast cancer patients to avoid axillary lymph node dissection (ALND). A prospective clinical trial has preliminarily verified its feasibility.1 However, ALND is still the standard treatment mode for patients with positive SLN. Numerous studies have shown that SLN was the unique lymph node with tumor metastasis in 40%–70% patients with breast cancer.2,3 Therefore identifying SLN-positive breast cancer patients with non-SLN metastasis at low risk has become a research hotspot in recent years, as ALND can then be avoided in these patients.
The Memorial Sloan Kettering Cancer Center (MSKCC) nomogram proposed by Van Zee et al is the first model in the world to predict non-SLN metastasis following a positive SLN biopsy.4 This model was validated by a total of 373 cases and the area under the receiver-operating characteristic curve (AUC) was 0.77, which proved to have a good diagnostic value.4
The study reported here retrospectively analyzed the clinical and pathological data of 509 patients with positive SLN to explore the risk factors for non-SLN metastasis and verify the clinical value of the MSKCC model in Chinese breast cancer patients.
Patients and methods
Patients
A total of 509 breast cancer patients with positive SLN who received SLNB in the Shandong Cancer Hospital from December 2001 to March 2014 were enrolled in this study. All the cases were confirmed by histopathology or cytology for invasive breast cancer, and patients had to be clinically axillary lymph node negative, have had no previous systemic treatment, and their SLN biopsy had to have been successful. The method of SLN detection was touch-imprint cytology (TIC) and frozen section. The study was approved by the Medical Ethics Committee of the Shandong Cancer Hospital and all patients enrolled in the study signed informed consent.
Evaluation method of sentinel lymph nodes
We subcutaneously injected 99mTc-labeled sulfur colloid into the primary tumor or around the biopsy cavity 3–18 hours before the operation and lymphoscintigraphy was performed 30 minutes before surgery.5,6 After successful anesthesia, 2–4 mL of 1% methylthioninium was similarly injected.
An intraoperative gamma detector (Neoprobe® neo2000® Gamma Detection System, Mannotome, Cincinnati, OH, USA) and blue dye were used to identify SLNs. The fresh tissue containing the SLNs was sent for intraoperative pathological examination by TIC and frozen section. TIC was performed from alternative cut surfaces on a glass slide and the whole material was then submitted to frozen section. Patients with negative SLNs only received SLNB. However, if SLNB failed or SLNs were positive, ALND was considered inevitable.
Memorial Sloan Kettering Cancer Center nomogram for predicting non-sentinel lymph node metastases
The MSKCC nomogram is a computerized model that is used to estimate the probability of non-SLN metastasis. We logged in to the MSKCC website at http://nomograms.mskcc.org/Breast/BreastAdditionalNonSLNMetastasesPage.aspx, and entered the appropriate information of each patient, then we could obtain the predicted probability. The MSKCC model contains nine independent variables: frozen section performed?, pathological size, tumor type and grade, number of positive SLNs, SLN method of detection, number of negative SLNs, lymphovascular invasion, multifocality, and estrogen-receptor status. The predictive accuracy was assessed by drawing a receiver-operating characteristic (ROC) curve and calculating the AUC. The value of AUC is between 0.5 and 1.0. In the case of AUC >0.5, the closer AUC is to 1.0, the better the effect of diagnosis. The AUC has a lower accuracy at 0.5–0.7 and a superior accuracy in the range of 0.7–0.9; AUC has high accuracy at more than 0.9. AUC =0.5 illustrates the diagnostic methods do not work and there is no diagnostic value. AUC <0.5 does not conform to reality; it is rarely seen in practice.
Statistical analysis
SPSS software (v 17.0; IBM Corporation, Armonk, NY, USA) was used to analyze the data of this study. The χ2 test or Fisher’s exact test was performed to compare the rate between categorical variables, and the independent t-test was used to compare the difference in means between groups. A multivariable logistic regression analysis was used to determine the independent predictors of non-SLN metastasis. Two-sided P-values <0.05 were considered to indicate significant difference.
Results
Relative factors for non-sentinel lymph node metastasis
Data on 1,576 patients who received SLNB in the Shandong Cancer Hospital from December 2001 to March 2014 were collected in this study, and data on 509 SLN-positive breast cancer patients were analyzed to evaluate the risk factors for non-SLN metastasis. A total of 198 patients were determined to have non-SLN metastasis (non-SLN positive, 38.9%, 198/509) and 311 patients were determined to be without non-SLN metastasis (non-SLN negative, 61.1%, 311/509).
Univariate analysis showed that tumor size, histological grade, lymphovascular invasion, multifocality, number of positive SLNs, and number of negative SLNs were related to non-SLN metastasis (P<0.05) (Table 1). However, multivariate analysis showed that tumor size (P=0.039), histological grade (P=0.043), lymphovascular invasion (P=0.001), number of positive SLNs (P=0.001), and number of negative SLNs (P=0.000) were independent predictors for non-SLN metastasis (Table 2).
Predictive ability of the Memorial Sloan Kettering Cancer Center nomogram
As mentioned earlier, we calculated the predicted probability of non-SLN metastasis for each patient by logging on to the MSKCC website and entering the appropriate information online. In order to evaluate the accuracy of the MSKCC model, we drew a trend line, with the X-axis and the Y-axis representing predicted probability and probability of non-SLN metastasis, respectively. Then we found that the trend of actual probability in various decile groups corresponded to the predicted probability, and there was no significant deviation (Figure 1).
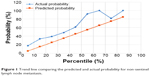  | Figure 1 Trend line comparing the predicted and actual probability for non-sentinel lymph node metastasis. |
In addition, we drew an ROC curve and the AUC was 0.722 (Figure 2). This shows that fairly precise results of non-SLN metastasis can be predicted by the MSKCC model. Patients with predictive values lower than 10% (97/492, 19.7%) had a frequency of non-SLN metastasis of 17.5% (17/97).
Discussion
The development of SLNB was a breakthrough in the field of breast surgery in the 1990s. It is an effective method of evaluating the status of axillary lymph nodes in patients with early-stage breast cancer, and mainly used in clinically axillary lymph node-negative patients for lymph-node staging.7,8 ALND may not be necessary for SLN-negative breast cancer patients, but for SLN-positive patients, ALND is still the traditional mode of treatment of breast cancer. In recent years there have been calls to reassess this traditional mode through the study of axillary lymph node metastasis. On the one hand, studies have shown that 40%–70% patients only exist SLN metastasis and the ALN of this part of patients are negative, so this subgroup of patients cannot benefit from ALND. Further, ALND not only increases the operation complication rate and medical costs, but also prolongs hospitalization in such situations.9 Also important is that ALND has considerably more side effects for the patient than SLN alone. On the other hand, SLNB exists false negative rate of 0–29%, which will affect lymph-node staging and the treatment of breast cancer. Therefore, it is of great clinical importance to make the prediction model used with SLN-positive patients clear so as to avoid ALND.
As the earliest model proposed to predict non-SLN metastasis, the MSKCC nomogram has been validated by multiple agencies around the world and the value of the AUC ranged from 0.58 to 0.86. However, some of the differences between the validation results are caused by certain inconsistencies among the case models of different research centers and the composition of the MSKCC classic case model (Table 3). Degnim et al thought MSKCC nomogram could predict the risk of non-SLN metastasis and the AUC was 0.86,10 while the AUC obtained by Klar et al was only 0.58.11 Lambert et al considered the discrepancy in SLN searching method, pathologic evaluation method, and biological characteristics of tumors were the important factors that led to change in predicting accuracy.12 Yet, between different medical institutions, there exists great difference in the detection method of intraoperative SLN. Lambert et al obtained an accurate result using the MSKCC nomogram by using TIC to evaluate SLN, and suggested that TIC can be used appropriately instead of frozen section in nomogram.12 However, when TIC was applied in the same way by Kocsis et al they failed to verify the nomogram.13 In our study, all patients who received SLNB were submitted for TIC and frozen section, which had good accuracy. We thought that the difference in the detection method of intraoperative SLN might affect the predictive accuracy of MSKCC nomogram.
Of course, the MSKCC nomogram does not include the size of SLN metastases, but D’Eredita’ et al regarded this as an important predictor of non-SLN metastasis.14 Van la Parra et al also concluded that the method of detection is a substitution for size of metastasis in the MSKCC nomogram.15 However, the nomogram will not work well if the assigned method of detection does not correlate with size. The size category of SLN metastasis can be used in applying the nomogram to patients in whom SLN histologic analysis is performed by a very different procedure than that used to develop the MSKCC nomogram. This results in an improved predictive accuracy.15 Coutant et al analyzed 246 patients with SLN micrometastasis, and the AUC was 0.72, which showed a satisfactory predictive value.16 Pal et al simplified the predictive factors and emphasized the predictive value of SLN metastasis size for non-SLN metastasis, then improved the prediction accuracy in their study population.17 Kohrt et al collected data on 171 patients with breast cancer in 2008, and analyzed 92.5% of these patients with SLN micrometastasis or isolated tumor cells, then established a new Stanford nomogram, which got high accuracy in 77 patients with SLN macrometastasis.18 Nevertheless, Alran et al analyzed patients with SLN micrometastasis, but found no predictive value, with an AUC of 0.53.19 Even so, many research scholars still consider that MSKCC nomograms can be used to assess the risk of non-SLN metastasis and provide reliable prediction analysis.20,21
Multivariate analysis of our study showed that tumor size, histological grade, lymphovascular invasion, number of positive SLNs, and number of negative SLNs were independent predictors of non-SLN metastasis. But multifocality only displayed statistically significant differences in univariate analysis (P<0.001) and no significant differences were found by logistic regression model (P=0.064, odds ratio =0.364, 95% confidence interval 0.125–1.063). These results may be due to the fact that molybdenum target X-ray and ultrasonic examination were routinely performed in our study, which could preliminarily screen out the patients with multifocal breast cancer. However, magnetic resonance imaging (MRI) – which can find more multicentricity – was not a routine examination before the operations in our study. Additionally, the imperfection of pathological examination of the surgical specimen in our study would also affect the final results. And we also believe that racial disparities and the difference in research method between our study and the MSKCC model are factors leading to the inconsistency of the results. This study demonstrates that the risk factors of non-SLN metastasis are essentially in agreement with the variables of the MSKCC model, and this result also indirectly validates the effectiveness of the MSKCC nomogram.
Our study evaluated the accuracy of the MSKCC model for predicting non-SLN metastasis by drawing an ROC curve and calculating the AUC. The AUC was 0.722, which indicates the MSKCC nomogram could provide a reliable prediction method. Many researchers believe that patients with predictive values lower than 10% can avoid ALND.12,16 In our study, patients with predictive values lower than 10% (97/492, 19.7%) had a frequency of non-SLN metastasis of 17.5% (17/97). In addition, these 17 patients all underwent breast conserving surgery, a tumor size ≤2.5 cm, and ≤2 positive SLNs. The research of the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial showed that such patients could avoid ALND.22 Therefore, our research is consistent with the findings of research abroad.
Obviously, the MSKCC model has its shortcomings. First, in terms of the current domestic diagnosis and treatment of breast cancer, the pathological diagnosis of breast primary lesions mainly relies on rapid intraoperative frozen section, and cannot provide all the variables required for the model. Even if we use preoperative core needle biopsy, the judgment of lymphovascular invasion and the status of the hormone receptor, etc., often take longer. Second, the MSKCC nomogram just provides the probability of non-SLN metastasis, rather than a clinical decision.
Conclusion
MSKCC nomograms can be widely used in SLN-positive patients for the prediction of non-SLN metastasis, and exhibit high reference values for clinical decision making. However, they cannot be used as a clinical application of standard. Furthermore, whether the model is suitable for the Chinese population still needs further verification. So we should establish a special statistics model, and use a large multi-center sample for validation of the process of the nomograms applied in clinic, then optimize and select predictive factors with statistical and clinical significance in order to provide a more accurate and practical forecasting model for different populations. Only in this way can the nomogram guide clinical work better.
Disclosure
The authors report no conflicts of interest in this work.
References
Giuliano AE, Haigh PI, Brennan MB, et al. Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol. 2000;18(13):2553–2559. | ||
Eldweny H, Alkhaldy K, Alsaleh N, et al. Predictors of non-sentinel lymph node metastasis in breast cancer patients with positive sentinel lymph node (Pilot study). J Egypt Natl Canc Inst. 2012;24(1):23–30. | ||
Meretoja TJ, Leidenius MH, Heikkilä PS, et al. International multicenter tool to predict the risk of nonsentinel node metastases in breast cancer. J Natl Cancer Inst. 2012;104(24):1888–1896. | ||
Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10(10):1140–1151. | ||
Wang L, Yu JM, Wang YS, et al. Preoperative lymphoscintigraphy predicts the successful identification but is not necessary in sentinel lymph nodes biopsy in breast cancer. Ann Surg Oncol. 2007;14(8):2215–2220. | ||
Sun X, Liu JJ, Wang YS, et al. Roles of preoperative lymphoscintigraphy for sentinel lymph node biopsy in breast cancer patients. Jpn J Clin Oncol. 2010;40(8):722–725. | ||
Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349(9069):1864–1867. | ||
Lyman GH, Giuliano AE, Somerfield MR, et al; American Society of Clinical Oncology. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23(30):7703–7720. | ||
Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer. 2006;106(1):4–16. | ||
Degnim AC, Reynolds C, Pantvaidya G, et al. Nonsentinel node metastasis in breast cancer patients: assessment of an existing and a new predictive nomogram. Am J Surg. 2005;190(4):543–550. | ||
Klar M, Jochmann A, Foeldi M, et al. The MSKCC nomogram for prediction the likelihood of non-sentinel node involvement in a German breast cancer population. Breast Cancer Res Treat. 2008;112(3):523–531. | ||
Lambert LA, Ayers GD, Hwang RF, et al. Validation of a breast cancer nomogram for predicting nonsentinel lymph node metastases after a positive sentinel node biopsy. Ann Surg Oncol. 2006;13(3):310–320. | ||
Kocsis L, Svébis M, Boross G, et al. Use and limitations of a nomogram predicting the likelihood of non-sentinel node involvement after a positive sentinel node biopsy in breast cancer patients. Am Surg. 2004;70(11):1019–1024. | ||
D’Eredita’ G, Troilo VL, Fischetti F, Rubini G, Berardi T. Comparison of two models for predicting non-sentinel lymph node metastases in sentinel lymph node-positive breast cancer patients. Updates Surg. 2011;63(3):163–170. | ||
van la Parra RF, Ernst MF, Bevilacqua JL, et al. Validation of a nomogram to predict the risk of nonsentinel lymph node metastases in breast cancer patients with a positive sentinel node biopsy: validation of the MSKCC breast nomogram. Ann Surg Oncol. 2009;16(5):1128–1135. | ||
Coutant C, Olivier C, Lambaudie E, et al. Comparison of models to predict nonsentinel lymph node status in breast cancer patients with metastatic sentinel lymph nodes: a prospective multicenter study. J Clin Oncol. 2009;27(17):2800–2808. | ||
Pal A, Provenzano E, Duffy SW, Pinder Se, Purushotham AD. A model for predicting non-sentinel lymph node metastatic disease when the sentinel lymph node is positive. Br J Surg. 2008;95(3):302–309. | ||
Kohrt HE, Olshen RA, Bermas HR, et al; Bay Area SLN Study. New models and online calculator for predicting non-sentinel lymph node status in sentinel lymph node positive breast cancer patients. BMC Cancer. 2008;8:66. | ||
Alran S, De Rycke Y, Fourchotte V, et al; Institut Curie Breast Cancer Study Group, Sigal-Zafrani B. Validation and limitations of use of a breast cancer nomogram predicting the likelihood of non-sentinel node involvement after positive sentinel node biopsy. Ann Surg Oncol. 2007;14(8):2195–2201. | ||
Scow JS, Degnim AC, Hoskin TL, Reynolds C, Boughey JC. Assessment of the performance of the Stanford Online Calculator for the prediction of nonsentinel lymph node metastasis in sentinel lymph node-positive breast cancer patients. Cancer. 2009;115(18):4064–4070. | ||
Qiu PF, Liu JJ, Wang YS, et al. Risk factors for non-sentinel lymph node metastasis and validation study of the MSKCC nomogram in patients with breast cancer. Chinese Journal of Practical Surgery. 2012;32:945–949. | ||
Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–575. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




