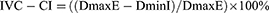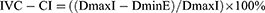Back to Journals » Medical Devices: Evidence and Research » Volume 17
Usefulness of Non-Invasive Parameters (Inferior Vena Cava Diameter, Inferior Vena Cava Collapsibility, Inferior Vena Cava-Aortic Ratio) for Hemodynamic Monitoring in Critically Ill Children: A Systematic Review
Authors Hakim DDL, Meilyana F, Peryoga SU, Arniawati I, Wijaya EA , Martiano MR
Received 20 December 2023
Accepted for publication 19 February 2024
Published 18 March 2024 Volume 2024:17 Pages 123—133
DOI https://doi.org/10.2147/MDER.S454849
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Dzulfikar Djalil Lukman Hakim,* Fina Meilyana,* Stanza Uga Peryoga,* Irma Arniawati,* Elrika Anastasia Wijaya,* Muhamad Rinaldhi Martiano*
Department of Child Health, Faculty of Medicine, Universitas Padjadjaran/Dr. Hasan Sadikin General Hospital, Bandung, West Java, Indonesia
*These authors contributed equally to this work
Correspondence: Elrika Anastasia Wijaya, Tel +6281313369468, Email [email protected]
Purpose: Volume measurement in critically ill children can be conducted using invasive procedure such as Central Venous Pressure (CVP), or non-invasive procedure such as measurement of Inferior Vena Cava (IVC) indices using ultrasonography. However, their accuracy and efficacy are still under scrutiny. We aim to compare CVP and IVC indices as non-invasive parameters in assessing volume status in critically ill children.
Methods: We conducted a systematic review based on literature searching from four electronic databases which were PubMed, Cochrane, ScienceDirect, SpringerLink with keywords: “CENTRAL VENOUS PRESSURE”, “INFERIOR VENA CAVA DIAMETER”, “INFERIOR VENA CAVA COLLAPSIBILITY”, “INFERIOR VENA CAVA AORTIC-RATIO”, “VOLUME STATUS”, “FLUID STATUS”, “CRITICAL ILL”, “CHILDREN”, and “PEDIATRICS”. We included relevant studies in English published from 2000 to 2023 on critically ill children aged 0– 18 years. Comparison between CVP and IVC indices was resumed.
Results: Eight articles were included in this study. Majority of the studies showed a consistent correlation between CVP and IVC indices. IVC-CI was the most common parameter evaluated in the included studies. There was moderate to strong correlations using IVC-CI and IVC-DI, and moderate correlation using IVC-Ao ratio.
Conclusion: We found that non-invasive tools might have a potential role to measure volume in critically ill children equals to CVP. Further high-quality and longitudinal studies are needed to validate these findings and to establish a clear guideline for the non-invasive tool to be used in daily clinical practice.
Keywords: pediatric intensive care, critically ill children, hemodynamic monitoring, non-invasive parameter, ultrasonography
Introduction
Hypovolemic condition is a clinical situation which is commonly found in Pediatric Intensive Care Unit (PICU) and required volume expansion as the mainstay treatment. The prevalence of shock is ranging from 1.5% in hospitalized children, to 44.3% in critically ill children.1 When left untreated or detected late, hypovolemic condition could be fatal and might cause death. Early detection and correction of hypovolemia can restrict hypoxic damage, relieve tissue hypoxia, enhance the clinical outcome of critically ill patients, and thus, might save the patient’s life. However, inappropriate fluid therapy can lead to heart failure.2,3 Thus, in critically ill children, this fluid balance condition needs to be monitored closely, since too much or too little fluid can have a major consequence in the child’s overall prognosis.4
Accurate assessment of volume status forms the backbone of pediatric critical care, playing a crucial role in managing fluid balance and overall hemodynamics of critically ill children. Two prominent strategies are commonly used to ascertain volume status in predicting fluid therapy responsiveness: invasive measurement using Central Venous Pressure (CVP) and non-invasive method using Inferior Vena Cava (IVC) indices, including Inferior Vena Cava Diameter (IVC-DI), IVC Collapsibility (IVC-CI), and the Inferior Vena Cava Aortic Ratio (IVC-Ao).5
Central Venous Pressure, often described as the standard method for hemodynamic monitoring, measured via cathether insertion into a central vein, has long been considered a fundamental gauge of intravascular volume status. It has long been used as a benchmark tool in critical care settings, providing real-time, accurate data to guide fluid management and therapeutic strategies.6,7 However, it requires an invasive procedure with the risk of complications, such as infection, thrombosis, or pneumothorax.8 Moreover, there have been concerns about the reliability of CVP measurements in predicting fluid responsiveness, stirring a quest for an alternative method.
On the other hand, a rising trend towards less invasive or non-invasive methods has emerged, driven by the desire to mitigate the potential complications of invasive procedures, like infection, thrombosis, or pneumothorax associated with CVP. Recent studies have focused on evaluating hemodynamic monitoring and predicting fluid therapy response using dynamic parameters as an alternative or adjunct. The IVC diameter, its degree of collapsibility, and the IVC-Aortic ratio are dynamic parameters that can be derived from ultrasound imaging and are increasingly being investigated for their correlation with volume status.9 These techniques eliminate the risks associated with invasive procedures. However, their accuracy and efficacy are still under scrutiny.
Despite increasing research, a direct comparison of these methods in pediatric critical care is still lacking. This systematic review aims to comprehensively compare CVP and non-invasive IVC parameters in assessing volume status in critically ill children. By combining and analyzing the findings from multiple studies, we seek to provide a clearer understanding of the benefits and limitations in both techniques and we also hope to be able to improve clinical decision-making in pediatric critical care regarding the measurement of fluid balance using non-invasive method. In the future, perhaps non-invasive method could be considered to be used daily for the fluid balance measurement in critically ill children if it is proven to have a similar performance with the invasive methods.
Materials and Methods
Search Strategy
Literature search was conducted using several online databases: PubMed, Cochrane Library, ScienceDirect, and SpringerLink. The search will include various combinations of keywords and MeSH terms such as: “CENTRAL VENOUS PRESSURE”, “INFERIOR VENA CAVA DIAMETER”, “INFERIOR VENA CAVA COLLAPSIBILITY”, “INFERIOR VENA CAVA AORTIC-RATIO”, “VOLUME STATUS”, “FLUID STATUS”, “CRITICAL ILL”, “CHILDREN”, and “PEDIATRICS”. This systematic review was performed based on the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines. Comparison between the use of CVP and non-invasive procedure (Inferior Vena Cava Diameter, Inferior Vena Cava Collapsibility, or Inferior Vena Cava-Aortic Ratio) in critically ill children were assessed.
Study Selection and Eligibility Criteria
Search results were screened based on titles and abstracts. Duplicated articles were removed. The studies included were limited to articles written in English and published from 2000 to 2023 on critically ill children aged 0–18 years that compare CVP with at least one of the non-invasive parameters (IVC-DI, IVC-CI, IVC-Ao) in critically ill children, with a focus on assessing volume status. Randomized controlled trials, cohort studies, case–control studies, and cross-sectional studies were included. The exclusion criteria were studies that did not directly compare the CVP and the non-invasive parameters, studies that used qualitative methods, abstracts from national/international conferences, guidelines, case reports, case series, commentaries, and review articles.
Data Extraction
For each included study, for the continuous variables, the data of the patient’s characteristics, the sample size, and detail of the study findings were extracted. The data is written in both narratives and numbers.
Quality Assessment and Data Analysis
The quality of the included studies was assessed using appropriate tools based on study design, such as the Cochrane Risk of Bias Tool for randomized controlled trials, the Critical Appraisal Skills Program (CASP) tool for the cohort studies, and the Center for Evidence-Based Management (CEBMa) for the cross-sectional studies. These tools consisted of several checklists based on the research methods used to assess the quality, validity, and relevance of the published articles.
All studies which met the criteria were identified using the selected keywords. Then, title screening was done, followed by abstract screening. Complete article screening was done for studies that met the inclusion criteria.
Results
The article selection flowchart is depicted in Figure 1. Our initial database search yielded a total of 260 studies. After removing duplicates, 254 studies remained. Upon title and abstract screening, 15 studies were selected. Two studies were published older than the time period of this review, three did not contain the required data, and the remaining two did not have children (0–18 years old) as the study sample. The other seven studies were excluded based on the exclusion criteria and eligibility check. In the end, eight studies met our inclusion criteria and were included in this systematic review.
 |
Figure 1 Article Search and Selection Flowchart. |
Eight studies included in our review were published between 2013 and 2023. They encompassed 433 critically ill children from different countries, including the United States, Indonesia, Iran, India, and Turkey. The sample sizes of the studies ranged from 15 to 107 participants. The age of the participants ranged from neonates to 18 years old. All studies compared CVP with at least one of the non-invasive parameters: IVC-CI, IVC-DI, or IVC-Ao ratio for assessing volume status. The study characteristics of all eight journal articles included in this systematic review are summarized in Table 1.
 |
Table 1 Study Characteristics |
The majority of the included studies demonstrated a statistically significant correlation between CVP and each of the non-invasive parameters. One out of eight studies reported a positive correlation between CVP and IVC-DI, six out of eight reported a negative correlation between CVP and IVC-CI, and two out of eight studies reported a negative correlation between CVP and IVC-DI. As for the IVC-Aortic ratio, one out of eight studies noted a positive correlation with CVP. However, a study by Lorraine et al revealed neither IVC-CI nor IVC/Aorta ratio correlated with CVP in assessing intravascular volume.11
Inferior Vena Cava Collapsibility Index (IVC-CI) was the most commonly studied non-invasive parameter. In all studies, IVC measurements were taken from the supine position, and the USG probe was placed on the subxiphoid area. However, there were some differences among the studies regarding the patient population, the use of 2D and M-mode USG, the use of mechanical ventilation, and the IVC-CI or IVC-DI calculation. Half of the included studies used maximum IVC diameter on expiration and minimum IVC diameter on inspiration as variables of the IVC-CI formula, and two studies used maximum IVC diameter on inspiration and minimum IVC diameter on expiration as variables of the IVC-DI formula. It suggested that the phase of the respiratory cycle was considered. A study by Mugloo et al calculated IVC-CI as a ratio without regard to respiratory cycle phases due to the impossibility of time inspiration and expiration in neonates as the study sample.17
Correlation between CVP and IVC-CI was assessed by Babaie et al, Garcia et al, Ali et al, Vaish et al, Aslan et al, and Mugloo et al. Almost all studies used ultrasonography to measure the IVC diameter before calculating IVC-CI, except for the study by Mugloo et al that used echocardiography. All studies reported a negative correlation between CVP and IVC-CI with variable correlation coefficient values (Table 1). Most studies showed the moderate-strong level of correlation between CVP and IVC-CI. Those suggested that a higher percentage of IVC-CI could indicate lower CVP in dehydrated or low-volume status in critically ill children. All studies provided p-value <0.05 for the statistically significant correlation reported.10,12,14–17
In addition to IVC-CI, IVC-DI was assessed by Dalimunthe et al and Aslan et al. Both studies also reported a negative correlation between CVP and IVC-DI with strong level (correlation coefficient; r=−0.623, p=0.003) and moderate level (r=−0.412, p=0.004) of correlation, respectively. Those suggested that a higher percentage of IVC-DI could indicate lower CVP in dehydrated or low-volume status in critically ill children.13,16 A study by Vaish et al showed that IVC diameter during inspiration had a weak positive correlation following 6 hours and 12 hours of resuscitation (r =0.312, p=0.05; r=0.292, p=0.04, respectively).15 Lastly, a study by Babaie et al presented a moderate positive correlation (r=0.423, p<0.001) between IVC-Ao ratio (1.09 ± 0.4) and CVP (10.64 ± 3.85 mm/Hg) in critically ill children with dehydration.10 All the above studies provided p-values less than 0.05 for the statistically significant correlation reported.
Diagnostic performance characteristics of IVC parameters for predicting CVP among spontaneously breathing patients were described in 3 studies by Babaie et al, Lorraine et al, and Garcia et al. Babaie et al which revealed that higher IVC-CI (≥50% or ≥0,5) was 45.5% sensitive and 91.7% specific with a positive predictive value of 71.4 and a negative predictive value of 78.6 to predict CVP <8 mmHg, as well as lower IVC-Ao (≤80% or ≤0,8) is 50.8% sensitive and 87.5% specific with a positive predictive value of 64.7 and a negative predictive value of 79.2 to predict CVP <8 mmHg. Lorraine et al showed that IVC-CI 50% or greater correlated with CVP of 8 mmHg or less with a sensitivity of 14.3%, specificity of 82.8%, positive predictive value of 37.5%, and a negative predictive value of 57.1%, as well as IVC-Ao index 80% or lesser correlated with CVP of 8 mmHg or less with a sensitivity of 17.6%, specificity of 80.8%, positive predictive value of 37.5%, and a negative predictive value of 60%, although it was not statistically significant. Garcia et al found that 2D IVCCI ≤ 0.24 had 94% sensitivity, 79% specificity, and 88.9% negative predictive value (95% CI; 65.5%–96.9%) to detect CVP≥10 mmHg during first measurement, as well as 2D IVC-CI≤0.26 had 91% sensitivity, 54% specificity, and 87.5% negative predictive value (95% CI; 69.8%–95.5%) to detect CVP≥10 mmHg.10–12
Discussion
This systematic review included 8 cross-sectional studies and aimed to compare the effectiveness of Central Venous Pressure (CVP) and non-invasive parameters (IVC diameter, IVC-CI, IVC-DI, and IVC/Ao ratio) in assessing volume status in critically ill children. Whether using CVP or non-invasive parameters which had been mentioned before, IVC needs to be examined by both parameters. The size and shape of IVC fluctuate with CVP and intravascular volume variations. Several factors may affect the IVC size. Under normal physiologic conditions, IVC diameter decreases, and venous return increases during inspiration due to negative intra-thoracic and positive intra-abdominal pressure. This condition is reversed in patients receiving positive pressure ventilation (PPV). American Society of Echocardiography (ASE) recommends the patient’s supine position to assess IVC diameter as it is directly affected.18 All studies in our review had applied this recommendation. The IVC diameter is best visualized by M-mode sonography.18 Most studies in our review also reported the use of M-mode sonography. A study by Garcia et al compared 2D and M-mode in visualizing IVC diameter. However, 2D sonography assessment gave statistically significant and more consistent results with a strong correlation than M-mode sonography.12
Central venous pressure (CVP) represents the pressure exerted in the thoracic vena cava near the right atrium by the blood returning to the heart from the body’s organs and tissues. Central venous pressure is an essential indicator of a patient’s volume status and cardiac function and is commonly used in critical care practice to guide fluid management.6 Generally, low CVP values had been assumed to indicate hypovolemia or dehydration, while high CVP values indicated hypervolemia or congestive heart failure. Trends of CVP measurements over time or changes in response to a fluid challenge may provide more reliable information regarding intravascular volume status, especially in shock or critically ill patients.7 However, measurement of CVP is not without its limitations. The procedure is invasive and can be influenced by factors such as the patient’s body position, intra-abdominal pressure, and mechanical ventilation. Possible complications, such as infection, thrombosis, and pneumothorax, make the usage of CVP measurement require careful consideration.8
In general, most studies supported the use of sonographic IVC-CI as an alternative for CVP in volume status assessment. Our study review found a consistent but inverse moderate to strong correlation, in line with physiological expectations, as increased IVC collapsibility often suggests a low CVP or volume status. The collapsibility of IVC can be measured as an index to describe the variation of IVC diameter through the respiratory cycle. Measurement of maximum IVC diameter during expiration (DmaxE) and minimum IVC diameter during expiration (DminI) using M-mode was the important thing in calculating IVC-CI based on the formula below:
The above formula was used in most included studies to define IVC-CI in the study method, except in a study by Mugloo et all, which cannot use this criterion due to its dependence on the respiratory cycle, which is impossible to apply in neonates as the study sample. However, this study still uses sonography assessment of maximum and minimum IVC diameter without referring to inspiration and expiration.17 Higher percentage of IVC-CI (≥50%) may indicate an association to lower CVP in hypovolemic patients. A collapsibility index of more than 40% may indicate the responsiveness to fluid therapy in critically ill patients. On the contrary, IVC-CI of less than 15% shows inadequate responsiveness to fluid therapy.19 Three out of eight included studies represent IVC-DI as an index with a moderate to strong correlation to CVP. IVC distensibility index (IVC-DI) is calculated based on the following formula:
The higher percentage of IVC-DI could indicate lower CVP in dehydrated or low-volume status in critically ill children.20 The formula of IVC-DI and IVC-CI are generally similar, except for the measurement timing, which depends on the respiratory cycle. Therefore, the IVC-DI is usually measured in mechanically ventilated patients, while IVC-CI is generally measured in spontaneously breathing patients.16
Only a study by Vaish et al assessed IVC diameter during inspiration which has a weak positive correlation following 6 hours and 12 hours of resuscitation.15 This means that there was a considerable increase in the IVC diameter over 12 hours of resuscitation and IVC diameter during inspiration best correlated with CVP towards the end of resuscitation in critically ill children with shock. The clinical utility of these parameters warrants further exploration, particularly in settings where invasive monitoring is not readily available or feasible.
In our review, only a study by Babaie et al showed a moderate positive correlation between the IVC-Aortic ratio and CVP.10 IVC-Aortic (IVC-Ao) ratio as the element of the ultrasound examination is relatively new in evaluating volume status. Ultrasound examination is performed by placing the transducer just below the xiphoid process. The IVC diameter is assessed in the intrahepatic segment, about 3 cm below the diaphragm, during the expiration. The Ao diameter is visualized at the same level by moving the probe with a swinging motion to the left of the patient’s body. A normal value of the IVC-Ao index ranges from approximately 0.8 to 1.2. The IVC-Ao index of less than 0.8 indicates that the patient requires fluid therapy, while a value of more than 1.2 indicates that the patient is most likely overhydrated.19 IVC diameter and aorta are different for every child due to several factors, such as age, gender, body weight, and body surface area. As explained before, IVC diameter highly depends on respiratory changes (intra-thoracic and intra-abdominal pressure changes) and fluid deficiency state. Meanwhile, aortic diameter is relatively stable despite dehydration because of lower compliance than IVC. This explains the use of aortic diameter as an internal control for every child.5 However, the use of the IVC-Aortic ratio is relatively less explored in pediatric patients, and more studies are still needed to establish its reliability and validity.
Another study by Lorraine et al revealed a contrary result with no correlation between IVC-CI and IVC-Ao ratio with CVP in assessing intravascular volume.11 However, the study explains that it can be caused by several confounding factors leading to selection bias, consisting of Positive Pressure Ventilation (PPV) use, sedative and vasoactive agent. Positive pressure ventilation leads to increased intrathoracic pressure during inspiration, decreased systemic venous return, and increased volume of venous blood in the IVC. The IVC diameter widens during inspiration and contracts during expiration in an intubated patient. The dimension and distensibility of the IVC are consequently affected.21 In a meta-analysis study by Si et al, the accuracy of IVC diameter was investigated as a predictor of fluid responsiveness in mechanically ventilated patients when the tidal volume was 8 mL/kg and PEEP 5 cmH2O.22 Therefore, IVC measurements to estimate CVP in mechanically ventilated patients should consider the use and setting of mechanical ventilation.21
This review has several limitations. Current studies comparing CVP and IVC indices in critically ill children are still limited. Since most of the study designs in the studies included in this review are cross-sectional study, we were unable to infer the causality of the variables. Further studies are required to establish standard measurement techniques and understand the factors influencing this correlation between CVP and non-invasive parameters in hemodynamic monitoring in critically ill children.
Aside from the consideration of using these non-invasive methods routinely for volume status measurement in children, the development of scoring for volume status prediction in children might be considered, as had been done in adults. Studies done by Hayıroğluet al had already developed a scoring system to predict long-term atrial fibrillation diagnosis through surface electrocardiograph and a formula to predict echocardiographic diastolic dysfunction through electrocardiographic index.23,24 Similar scoring method for volume status assessment in children could be considered to be developed in the future.
Conclusion
Our findings support the potential role of these non-invasive parameters as adjuncts to CVP for assessing volume status in critically ill children. Non-invasive parameters have lower risks and complications compared to the invasive procedure, thus, it might even be superior to be used for hemodynamic monitoring in critically ill children. However, due to the heterogeneity and limitations of the included studies, further high-quality, longitudinal studies are needed to validate these findings and to establish clear guidelines for their use in clinical practice. Furthermore, more studies regarding non-invasive parameters as a tool to monitor hemodynamic condition are still needed.
We also suggest that a scoring system to predict volume status in children could be considered to be done in the future.
Funding
The authors thank Universitas Padjadjaran for the financial help received in publishing this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Assies R, Snik I, Kumwenda M, et al. Etiology, pathophysiology and mortality of shock in children in low (middle) income countries: a systematic review. J Trop Pediatr. 2022;68(4):fmac053. doi:10.1093/tropej/fmac053
2. Gui J, Guo J, Nong F, et al. Impact of individual characteristics on sonographic IVC diameter and the IVC diameter/aorta diameter index. Am J Emerg Med. 2015;33(11):1602–1605. doi:10.1016/j.ajem.2015.06.047
3. Elbaih AH, Safi MR. Approach to critical ill child. Medicine. 2021;10(1):1.
4. Airapetian N, Maizel J, Alyamani O, et al. Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients? Crit Care. 2015;19(1):1–8. doi:10.1186/s13054-015-1100-9
5. Kusumastuti NP, Latief A, Pudjiadi AH. Inferior vena cava/abdominal aorta ratio as a guide for fluid resuscitation. J Emerg Trauma Shock. 2021;14(4):211. doi:10.4103/JETS.JETS_154_20
6. Magder S. Central venous pressure: a useful but not so simple measurement. Crit Care Med. 2006;34(8):2224–2227. doi:10.1097/01.CCM.0000227646.98423.98
7. Berlin DA, Bakker J. Starling curves and central venous pressure. Crit Care. 2015;19(1):1–8. doi:10.1186/s13054-015-0776-1
8. Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5):e1331–44. doi:10.1542/peds.2015-1507
9. Steiner MJ, DeWalt DA, Byerley JS. Is this child dehydrated? JAMA. 2004;291(22):2746–2754. doi:10.1001/jama.291.22.2746
10. Babaie S, Behzad A, Mohammadpour M, Reisi M. A comparison between the bedside sonographic measurements of the inferior vena cava indices and the central venous pressure while assessing the decreased intravascular volume in children. Adv Biomed Res. 2018;7. doi:10.4103/abr.abr_213_17
11. Ng L, Khine H, Taragin BH, Avner JR, Ushay M, Nunez D. Does bedside sonographic measurement of the inferior vena cava diameter correlate with central venous pressure in the assessment of intravascular volume in children? Pediatr Emerg Care. 2013;29(3):337–341. doi:10.1097/PEC.0b013e31828512a5
12. Garcia RU, Meert KL, Safa R, Aggarwal S. Inferior vena cava collapsibility index to assess central venous pressure in perioperative period following cardiac surgery in children. Pediatr Cardiol. 2021;42:560–568. doi:10.1007/s00246-020-02514-9
13. Dalimunthe LR, Adriansyah R, Trisnawati Y. Correlation between central venous pressure and inferior vena cava distensibility index for assessment of volume status in critically ill children. Int J Res. 2022;97(1):9. doi:10.47119/IJRP100971320222956
14. Ali MK, Das A, Naim E. To evaluate the relation between central venous pressure and inferior vena cava collapsibility in cases of pediatric shock. Indian J Child Health. 2018;562–565. doi:10.32677/IJCH.2018.v05.i09.004
15. Vaish H, Kumar V, Anand R, Chhapola V, Kanwal SK. The correlation between inferior vena cava diameter measured by ultrasonography and central venous pressure. Indian J Pediatr. 2017;84:757–762. doi:10.12669/pjms.302.4375
16. Aslan N, Yildizdas D, Horoz OO, Coban Y, Arslan D, Sertdemir Y. Central venous pressure, global end-diastolic index, and the inferior vena cava collapsibility/distensibility indices to estimate intravascular volume status in critically ill children: a pilot study. Aust Crit Care. 2021;34(3):241–245. doi:10.1016/j.aucc.2020.08.005
17. Mugloo MM, Malik S, Akhtar R. Echocardiographic inferior vena cava measurement as an alternative to central venous pressure measurement in neonates. Indian J Pediatr. 2017;84:751–756.
18. Safadi S, Murthi S, Kashani KB. Use of ultrasound to assess hemodynamics in acutely ill patients. Kidney360. 2021;2(8):1349. doi:10.34067/KID.0002322021
19. Piotrkowski J, Buda N, Januszko-Giergielewicz B, Kosiak W. Use of bedside ultrasound to assess fluid status: a literature review. Pol Arch Intern Med. 2019;129(10):692–699. doi:10.20452/pamw.14962
20. Lujan Varas J, Martinez Díaz C, Blancas R, et al. Inferior vena cava distensibility index predicting fluid responsiveness in ventilated patients. Intensive Care Med Exp. 2015;3(1):1. doi:10.1186/2197-425X-3-S1-A600
21. Ciozda W, Kedan I, Kehl DW, Zimmer R, Khandwalla R, Kimchi A. The efficacy of sonographic measurement of inferior vena cava diameter as an estimate of central venous pressure. Cardiovasc Ultrasou. 2015;14(1):1–8. doi:10.1186/s12947-016-0076-1
22. Si X, Xu H, Liu Z, et al. Does respiratory variation in inferior vena cava diameter predict fluid responsiveness in mechanically ventilated patients? A systematic review and meta-analysis. Anesth Analg. 2018;127(5):1157–1164. doi:10.1097/SHK.0000000000000801
23. Hayıroğlu Mİ, Çınar T, Selcuk M, et al. The significance of the morphology-voltage-P-wave duration (MVP) ECG score for prediction of in-hospital and long-term atrial fibrillation in ischemic stroke. J Electrocardiol. 2021;69:44–50. doi:10.1016/j.jelectrocard.2021.09.006
24. Hayıroğlu Mİ, Tufan Ç, Vedat Ç, et al. A simple formula to predict echocardiographic diastolic dysfunction—electrocardiographic diastolic index. Herz. 2021;46:159–165. doi:10.1007/s00059-020-04972-6
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.