Back to Journals » International Medical Case Reports Journal » Volume 17
Unusual Configuration of a Giant Trans-Spatial Pancreatic Pseudocyst with Spontaneous Shrinkage: A Rare Case Report
Authors Abera MT , Damtew HD , Yaynishet YA , Adela AY
Received 21 January 2024
Accepted for publication 24 March 2024
Published 4 April 2024 Volume 2024:17 Pages 281—287
DOI https://doi.org/10.2147/IMCRJ.S458492
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Vinay Kumar
Michael Teklehaimanot Abera,1 Henok Dessalegn Damtew,1 Yodit Abraham Yaynishet,1 Amanuel Yegnanew Adela1– 3
1Department of Radiology, Addis Ababa University, Addis Ababa, Ethiopia; 2Department of Radiology, University of Gondar, Gondar, Ethiopia; 3Ethiopian Federal Police Commission Referral Hospital, Addis Ababa, Ethiopia
Correspondence: Michael Teklehaimanot Abera, Addis Ababa University, College of Health Sciences, Department of Radiology, P.O. Box 9080, Addis Ababa, Ethiopia, Email [email protected]; [email protected]
Abstract: Pancreatic pseudocysts are benign lesions that typically originate within the pancreatic parenchyma, or peripancreatic tissue. They commonly occur following recurrent episodes of pancreatitis or trauma. In this article, we present a case of a giant pancreatic pseudocyst with unusual trans-spatial extensions and spontaneous size decrement in a 40-year-old male patient with a history of alcohol abuse. He presented with chronic epigastric pain, and a physical examination showed only mild abdominal tenderness. Initial computed tomography showed a giant (18.4cm in its largest axis) pancreatic pseudocyst with left subdiaphragmatic and gastrohepatic extensions and concurrent splenic cysts. On follow-up ultrasound, the pseudocyst showed a significant spontaneous size decrement to less than half of its initial size. The giant size and trans-spatial characteristics of the pseudocyst, along with a relatively benign symptomatology and subsequent spontaneous shrinkage, constitute unique aspects of this case.
Keywords: pseudocyst, giant pseudocyst, pancreatitis, multiplanar CT, spontaneous drainage
Introduction
A pancreatic pseudocyst, as implied by its name, is a cyst formed from reactive granulation tissue without a true epithelial layer. It commonly develops as a complication of acute or chronic pancreatitis. While it can arise from a single episode of pancreatitis, it typically indicates recurrent bouts of acute pancreatitis or stems from chronic and persistent inflammation. Alcoholism and gallbladder stone diseases are the usual culprits, with additional implications from abdominal trauma, pancreatic surgery, instrumentation, and various medical causes.2–5
Commonly, pancreatic pseudocysts are restricted to the lesser sac and retroperitoneum.6 Uncommonly, they attain a gigantic size and/or manifest with a wider multi-compartmental and parenchymal extensions, including to the spleen.1,4,7–10 Portal vein thrombosis is an important complication of pancreatitis and carries a future risk of upper gastrointestinal bleeding.11
Ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) all play a role in diagnosing pancreatic pseudocysts. Cross-sectional imaging is important in confirming the diagnosis, ruling out more dangerous pancreatic cystic tumors, and accurately locating the lesion for surgical or other treatments.4 MRI plays a crucial role in defining the status of the pancreatic duct.12
A very rare occurrence with an unclear mechanism is spontaneous symptomatic or silent internal drainage of pseudocyst to the adjacent gastrointestinal tract, which occurs in less than 5% of cases.13
Case History
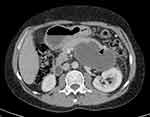
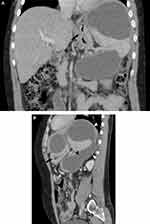
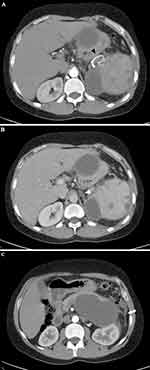
A 40-year-old male patient was referred to our radiology department for a post-contrast abdominopelvic CT, with the initial impression of a large left upper quadrant localized hydatid cyst (based on a prior abdominal ultrasound). He reported a one-year history of vague left upper quadrant abdominal pain and the recent onset of dyspepsia. This was his first-ever hospital visit. He works as a daily laborer in construction and has been consuming approximately 50–75 mL of spirited alcohols (locally available brands with 30–40% ABV) on most days for over 10 years. Additionally, he experienced occasional recurrent epigastric pain, which were not severe enough to hinder his daily activities. At the time of presentation, he exhibited a normal build, was afebrile, normotensive, and had a regular pulse rate of 70/min and a breathing rate of 16/min. An Abdominal examination revealed moderate tenderness over his epigastric and left upper quadrant regions, with no signs of chronic liver disease. Laboratory examinations, including CBC, hepatitis viral markers, liver and renal function tests, and bilirubin levels, were all within the normal range. Serum Amylase and Lipase levels were also within the correct range with 72 IU/L and 90 IU/L respectively. Subsequently, pre- and post-contrast multiplanar CT scans of the abdomen and pelvis in arterial, portal, and delayed phases (Figures 1–3) were performed. It showed a very large, homogeneous cystic lesion arising from the pancreas and extending to the left sub-diaphragmatic and gastro-hepatic spaces. It shows no internal-enhancing solid component or daughter cysts. It exerts a considerable mass effect on the spleen, which also contains multiple parenchymal cysts, and the stomach. Adjacent small intraperitoneal cysts and left perinephric inflammation were evident. The pancreas had a small punctate calcification but no ductal dilation.
The CT examination confidently diagnosed a giant pancreatic pseudocyst with trans-spatial extension, concurrent splenic pseudocysts, and perilesional inflammatory changes. The patient’s past habits also fit the radiological diagnosis. Further assessment with MRI for pancreatic ductal communication was suggested, but the patient could not afford. Subsequently, surgical drainage was offered as the size the pseudocyst was very large and the patient had symptoms. Surgery was planned after three months (usual waiting time for procedures in our center due to large backlog of cases).
After three months, the patient had clinical improvements in terms of his pain and appetite. A follow-up ultrasound (Figure 4) clearly indicated a significant reduction in the size and extension of the previously observed large pseudocyst. A residual cyst was visible in the left subdiaphragmatic region only, exhibiting a linear extension that terminates in the splenic hilum/pancreatic tail region. The other abdominal compartments and the splenic parenchyma appeared normal, showing no cystic lesions. A search for ascetic fluid in the peritoneal cavity yielded no findings. Severe fatty liver, aligning with the patient’s history of excessive alcohol use, was also detected. Since the patient did not undergo any procedures to drain the cystic lesion, we assume that the subsequent improvement occurred spontaneously. Based on the clinical and radiologic improvements, we recommended a conservative approach with follow-up imaging. We strongly advised discontinuing alcohol.
Discussion
Pseudocysts typically result from acute and chronic pancreatitis, with an incidence ranging from 5 to 80 per 100,000.2 A study from Ethiopia5 looked at 64 people who had acute pancreatitis. The most common causes were alcohol (44.4%) and gallstones (24.1%), with unknown causes coming in at 22.2%. The study also revealed that the age group of 20–40 years old accounted for 66.6% of the investigated cases. Pseudocysts usually take at least four weeks to develop following acute pancreatitis and are often small, confined to the lesser sac, or the anterior and posterior retroperitoneum.6
Pancreatic pseudocysts are classified as giant when they reach 10cm. As they progress in size and extension, complications such as gastric compression (leading to dyspepsia and weight loss) and blockage of extrahepatic biliary ducts (causing jaundice) may arise.7,8 The dyspepsia reported by our patient is likely linked to gastric compression.
As evident from the provided images, multiplanar CT proves advantageous in clearly depicting the origin, extent, size, orientation, local mass effect, and extra pancreatic inflammatory changes of the lesion. The multi-compartmental nature of our case emphasizes the indispensability of the multi-planar reformatting capability of cross-sectional imaging.6,8 Two additional imaging findings—pancreatic calcification and multiple splenic cysts—strongly hint at the presence of chronic and/or recurrent underlying dispositions.9,10
As seen in our case, the non-visualization of the splenic vein with the replacement of short tubular-enhancing vessels implies splenic vein thrombosis and is a crucial finding with prognostic implications. Although not immediately symptomatic, portal vein thrombosis can lead to portal vein hypertension and upper gastrointestinal bleeding,11 warranting future clinical and radiologic follow-ups. In cases of voluminous pseudocysts, suspicion of an associated rupture of the pancreatic duct arises. Establishing the duct’s status is crucial for management, as duct-communicating pseudocysts are less likely to resolve spontaneously.12
A case of pseudocyst with an unusual trans-compartmental involvement was reported by Sadat et al13 in which a mediastinal extension of a pancreatic pseudocyst was diagnosed with chest CT in a chronic pancreatitis (following alcohol) patient who presented with severe reflux symptoms. Posterior tearing of the main pancreatic duct allows for retroperitoneal fluid collection that can potentially reach the posterior mediastinum through mostly the esophageal or aortic hiatuses. Tracking to the pleural space is common. In another report7 a giant pseudocyst, with the largest locule measuring 30cm and filling almost the entire abdominopelvic cavity, occurred in the background of acute pancreatitis. It extended to the right subdiaphragmatic space and displaced the heart. These 2 cases demonstrate how a pancreatic pseudocysts can reach a huge size and extend across anatomic barriers. In both cases multiplanar CT was crucial.
There are multiple surgical options for giant pseudocysts, including open, external, internal, and endoscopic drainage. One of the procedure is cystogastrostomy. It is performed to insert a stent between the cyst cavity and gastric lumen. Both endoscopic and open methods are effective. In most cases endoscopic treatment leads to a shorter hospital stay.14
Less likely alternative diagnoses from the provided CT might include lymphatic malformations and cystic pancreatic tumors. Even though lymphatic malformation looks like a fluid-attenuating lesion with insinuating extensions, it rarely affects the pancreas, causing direct peritoneal extension and the inflammatory changes that go along with it. Pancreatic tumors, especially mucinous types, can attain significant size but usually present with an enhanced soft tissue component.1
Despite initial observations, the giant pseudocyst in our case decreased in size without intervention, an uncommon occurrence with no definitive known mechanism. Spontaneous improvements and resolutions, though rare, are well described. The rate of spontaneous rupture or drainage of pancreatic pseudocysts is small, less than 5%.15 Severe symptoms often occur when there is communication with the colon16 and peritoneum.17 Silent or asymptomatic drainage is even rarer, usually associated with drainage to upper alimentary tract structures.15–17 In our case, the absence of alarming symptoms, such as sepsis or acute abdominal pain, suggests spontaneous drainage to an adjacent upper GI tract like the stomach or duodenum. In such cases, a conservative approach with close clinical and radiologic follow-up is appropriate unless intervention becomes necessary for developing symptoms or if the pseudocyst does not resolve. The subsequent choice of imaging will depend on clinical symptoms, with ultrasound sufficient if the patient remains stable If alarming symptoms arise, an abdominal CT scan may be required.15
Conclusion
While the majority of pseudocysts are typically small and confined to the lesser sac, instances of unusual size attainment with multicompartmental extension can occur. Assessing such cases is best done by cross-sectional imaging with multiplanar reconstruction, as their extent may not be fully appreciated with sonographic examination alone. Furthermore, an asymptomatic spontaneous reduction in the size of giant pseudocysts is exceedingly rare but may occur as a result of drainage to adjacent hollow viscera.
Abbreviations
CT, Computed Tomography; MRI, Magnetic Resonance Imaging; CBC, Complete blood count; ABV, Alcohol by volume.
Data Sharing Statement
The datasets used during the current study are available from the corresponding author on reasonable request.
Ethical Consideration
To publish this case report and related images, we received written informed consent from the patient. Institutional permit is not required for a case report.
Acknowledgment
We thank the patient for allowing the publication of this case report.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; they took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
There was no specific grant provided for this work by public, private, or nonprofit funding organizations.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chauhan A, Elsayes KM, Sagebiel T, Bhosale PR. The pancreas. cross-sectional imaging of the abdomen and pelvis: a practical algorithmic approach. 2015:189–227.
2. Banks PA. Epidemiology, natural history, and predictors of disease outcome in acute and chronic pancreatitis. Gastrointest Endosc. 2002;56(6 Suppl):S226–30. PMID: 12447272. doi:10.1067/mge.2002.129022
3. Lankisch PG, Assmus C, Pflichthofer D, Struckmann K, Lehnick D. Which etiology causes the most severe acute pancreatitis? Int J Pancreatol. 1999;26(2):55–57. PMID: 10597400. doi:10.1007/BF02781731
4. Bradley EL, Clements JL, Gonzalez AC. The natural history of pancreatic pseudocysts: a unified concept of management. Am J Surg. 1979;137(1):135–141. PMID: 758840. doi:10.1016/0002-9610(79)90024-2
5. Dansa A, Kotisso B. Acute pancreatitis at a tertiary hospital in Addis Ababa, Ethiopia: a 4-year retrospective study. East Central Afr J Surg. 2019;24:2.
6. Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the international symposium on acute pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128(5):586–590. PMID: 8489394. doi:10.1001/archsurg.1993.01420170122019
7. Udeshika WAE, Herath HMMTB, Dassanayake SUB, Pahalagamage SP, Kulatunga A. A case report of giant pancreatic pseudocyst following acute pancreatitis: experience with endoscopic internal drainage. BMC Res Notes. 2018;11(1):262. PMID: 29703250; PMCID: PMC5923028. doi:10.1186/s13104-018-3375-9
8. Podgurski L, Hou G, Shaffer K. CT imaging of a pancreatic pseudocyst: clinical and anatomic implications. Radiol Case Rep. 2015;2(4):107. PMID: 27303493; PMCID: PMC4895868. doi:10.2484/rcr.v2i4.107
9. Verma A, Yadav A, Sharma S, et al. A rare splenic pseudocyst. J Surg Case Rep. 2013;2013(9):rjt086. PMID: 24963908; PMCID: PMC3813775. doi:10.1093/jscr/rjt086
10. Fishman EK, Soyer P, Bliss DF, Bluemke DA, Devine N. Splenic involvement in pancreatitis: spectrum of CT findings. AJR Am J Roentgenol. 1995;164(3):631–635. PMID: 7863884. doi:10.2214/ajr.164.3.7863884
11. Koo BC, Chinogureyi A, Shaw AS. Imaging acute pancreatitis. Br J Radiol. 2010;83(986):104–112. PMID: 20139261; PMCID: PMC3473535. doi:10.1259/bjr/13359269
12. Gouyon B, Lévy P, Ruszniewski P, et al. Predictive factors in the outcome of pseudocysts complicating alcoholic chronic pancreatitis. Gut. 1997;41(6):821–825. PMID: 9462217; PMCID: PMC1891603. doi:10.1136/gut.41.6.821
13. Sadat U, Jah A, Huguet E. Mediastinal extension of a complicated pancreatic pseudocyst; a case report and literature review. J Med Case Rep. 2007;1(1):12. PMID: 17459155; PMCID: PMC1863421. doi:10.1186/1752-1947-1-12
14. Billari WR, Roche D, DiGennaro JV, Shallcross MJ. Inpatient management and treatment of a giant pancreatic pseudocyst: a case report. Cureus. 2021;13(11):e19990. PMID: 34987890; PMCID: PMC8716118. doi:10.7759/cureus.19990
15. Clements JL, Bradley EL, Eaton SB. Spontaneous internal drainage of pancreatic pseudocysts. AJR Am J Roentgenol. 1976;126(5):985–991. PMID: 178244. doi:10.2214/ajr.126.5.985
16. Urakami A, Tsunoda T, Hayashi J, Oka Y, Mizuno M. Spontaneous fistulization of a pancreatic pseudocyst into the colon and duodenum. Gastrointest Endosc. 2002;55(7):949–951. PMID: 12024164. doi:10.1067/mge.2002.124555
17. Hanna WA. Rupture of pancreatic cysts. Report of a case and review of the literature. Br J Surg. 1960;47(205):495–498. PMID: 14399534. doi:10.1002/bjs.18004720508
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.