Back to Journals » Risk Management and Healthcare Policy » Volume 17
Types of Septic Cardiomyopathy: Prognosis and Influencing Factors - A Clinical Study
Authors Lu NF, Niu HX, Liu AQ, Chen YL, Liu HN, Zhao PH, Shao J, Xi XM
Received 1 December 2023
Accepted for publication 11 April 2024
Published 23 April 2024 Volume 2024:17 Pages 1015—1025
DOI https://doi.org/10.2147/RMHP.S452803
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Nian-Fang Lu,1 Hong-Xia Niu,2 An-Qi Liu,1 Ya-Lei Chen,1 Hu-Nan Liu,1 Pei-Hong Zhao,1 Jun Shao,3 Xiu-Ming Xi4
1Department of Critical Care Medicine, Capital Medical University Electric Teaching Hospital/Beijing Electric Power Hospital, Beijing, People’s Republic of China; 2Department of Emergency, Capital Medical University Electric Teaching Hospital/Beijing Electric Power Hospital, Beijing, People’s Republic of China; 3Department of Critical Care Medicine, Subei People’s Hospital of Jiangsu Province, Yangzhou, People’s Republic of China; 4Department of Critical Care Medicine, Capital Medical University Fuxing Hospital, Beijing, People’s Republic of China
Correspondence: Xiu-Ming Xi, Department of Critical Care Medicine, Capital Medical University Fuxing Hospital, No. 20 Fuxingmenwai Street, Xicheng District, Beijing, 100038, People’s Republic of China, Tel +86 13801244610, Email [email protected] Jun Shao, Department of Critical Care Medicine, Subei People’s Hospital of Jiangsu Province, No. 98 Nantong West Road, Guangling District, Jiangsu, Yangzhou, People’s Republic of China, Tel +86 18051061365, Email [email protected]
Objective: To explore the prognostic outcomes associated with different types of septic cardiomyopathy and analyze the factors that exert an influence on these outcomes.
Methods: The data collected within 24 hours of ICU admission included cardiac troponin I (cTnI), N-terminal pro-Brain Natriuretic Peptide (NT-proBNP); SOFA (sequential organ failure assessment) scores, and the proportion of vasopressor use. Based on echocardiographic outcomes, septic cardiomyopathy was categorized into left ventricular (LV) systolic dysfunction, LV diastolic dysfunction, and right ventricular (RV) systolic dysfunction. Differences between the mortality and survival groups, as well as between each cardiomyopathy subgroup and the non-cardiomyopathy group were compared, to explore the influencing factors of cardiomyopathy.
Results: A cohort of 184 patients were included in this study, with LV diastolic dysfunction having the highest incidence rate (43.5%). The mortality group had significantly higher SOFA scores, vasopressor use, and cTnI levels compared to the survival group; the survival group had better LV diastolic function than the mortality group (p < 0.05 for all). In contrast to the non-cardiomyopathy group, each subgroup within the cardiomyopathy category exhibited elevated levels of cTnI. The subgroup with left ventricular diastolic dysfunction demonstrated a higher prevalence of advanced age, hypertension, diabetes mellitus, coronary artery disease, and an increased mortality rate; the RV systolic dysfunction subgroup had higher SOFA scores and NT-proBNP levels, and a higher mortality rate (P < 0.05 for all); the LV systolic dysfunction subgroup had a similar mortality rate (P > 0.05).
Conclusion: Patients with advanced age, hypertension, diabetes mellitus, or coronary artery disease are more prone to develop LV diastolic dysfunction type of cardiomyopathy; cardiomyopathy subgroups had higher levels of cTnI. The RV systolic dysfunction cardiomyopathy subgroup had higher SOFA scores and NT-proBNP levels. The occurrence of RV systolic dysfunction in patients with sepsis significantly increased the mortality rate.
Keywords: cardiac function, echocardiography, influencing factors, prognosis, sepsis, septic cardiomyopathy
Introduction
Sepsis is a life-threatening organ dysfunction caused by a dysregulated systemic inflammatory response to infection. Every year, more than 18 million cases of severe sepsis are reported globally, and this number is growing.1 Cardiac dysfunction resulting from sepsis is one of the most common complications in clinical settings, with studies showing that the mortality rate for sepsis-induced cardiac dysfunction can reach up to 70%, three to four times higher than in patients without subsequent cardiac dysfunction.2
Previously, it was believed that cardiac dysfunction due to sepsis specifically referred to left ventricular (LV) systolic dysfunction. However, in recent years, with the advancement of echocardiographic technology, researchers have discovered that septic myocardial injury can manifest as various types of cardiac dysfunctions, such as LV diastolic dysfunction, LV systolic dysfunction, and right ventricular (RV) dysfunction, and all can coexist. The timely detection and assessment of different cardiac dysfunctions and their severity, along with proactive treatment, are crucial for improving the prognosis of patients with septic cardiomyopathy.
At present, echocardiography stands as the preeminent non-invasive method for diagnosing septic cardiomyopathy.3 Furthermore, in clinical settings, it is the most frequently employed non-invasive technique to evaluate cardiac function, particularly given the recent advancements in tissue Doppler imaging (TDI). TDI is capable of providing a more precise, objective, and quantitative assessment of cardiac function and coordination due to its reduced susceptibility to cardiac preload and afterload.4,5
Septic cardiomyopathy is a reversible myocardial injury caused by sepsis. At present, the treatment for septic myocardial dysfunction mainly focuses on symptomatic supportive treatments such as anti infection, fluid resuscitation, and vasopressor drugs. However, the treatment effect is not satisfactory, so the mortality rate of SCM patients remains high.6
Internationally, research on risk factors for sepsis cardiomyopathy7,8 has not distinguished different types of sepsis cardiomyopathy, and research on the prognosis and risk factors of different types of sepsis cardiomyopathy is extremely rare. The aim of this study is to explore the influencing factors of different types of septic cardiomyopathy, in order to provide early intervention for these risk factors, thereby preventing septic cardiomyopathy and ultimately reducing the mortality rate of septic cardiomyopathy.
The purpose of this research is to examine the prognosis of various types of septic cardiomyopathy and the epidemiological data of septic cardiomyopathy as determined by echocardiography. Additionally, through this study we aim to ascertain the potential influencing factors that may impact distinct subtypes of septic cardiomyopathy. This could provide a theoretical basis for the diagnosis and early prevention of septic cardiomyopathy, consequently leading to a decrease in fatality rates.
Study Participants and Methods
Study Participants
A retrospective study method was employed, involving patients with sepsis admitted to the intensive care units (ICUs) of the State Grid Corporation of China Beijing Electric Power Hospital and the Northern Jiangsu People’s Hospital from June 2017 to June 2023.
The criteria for inclusion in the study were as follows: 1) Patients diagnosed with sepsis according to the sepsis 3.0 diagnostic criteria. Sepsis is diagnosed when a sequential organ failure assessment (SOFA) score of at least 2 points or more is combined with an infection. The diagnostic criteria for septic shock are as follows: necessitating vasopressors to maintain a mean arterial pressure (MAP) of at least 65 mmHg or more following sufficient fluid resuscitation, and having a blood lactate level exceeding 2 mmol/L.9
The criteria for exclusion from the study were as follows: 1) Patients with a history of chronic heart failure; 2) Patients with segmental wall motion abnormalities (possible acute or past myocardial infarction); 3) Patients with a history of dilated cardiomyopathy or hypertrophic obstructive cardiomyopathy; 4) Patients with a history of cardiac valve disease; 5) Patients below 18 years or more than 80 years; 6) Patients with atrial fibrillation; 7) Patients with a hospital stay of less than 24 hours; 8) Patients who did not undergo echocardiography within 24 hours; 9) Patients with unclear echocardiographic images. (see Figure 1)
 |
Figure 1 A flowchart about the criteria for exclusion. |
Study Methods
Data Collection
The echocardiographic data was collected within 24 hours of ICU admission and included the following measurements: LV end-diastolic volume (LVDD), LV ejection fraction (LVEF), e’, E/e’ ratio, LV-Sm, and RV-Sm. Here, e’ refers to the peak early diastolic velocity at the lateral mitral annulus; E/e’ refers to the ratio of early mitral inflow velocity to early diastolic mitral annular velocity; LV-Sm refers to the systolic motion velocity of the mitral annulus in the left ventricle; and RV-Sm refers to the systolic motion velocity of the tricuspid annulus in the right ventricle.
The criteria for septic cardiomyopathy were sepsis + any one of the following echocardiographic results without prior cardiac baseline diseases. The echocardiographic criteria included:
- LV-Sm < 8 cm/s for LV systolic dysfunction cardiomyopathy
- RV-Sm < 12 cm/s for RV systolic dysfunction cardiomyopathy
- E/e’ ratio > 15 or e’< 8 cm/s for LV diastolic dysfunction cardiomyopathy
The previous cardiac baseline diseases included chronic heart failure, segmental wall motion abnormalities, dilated cardiomyopathy, hypertrophic obstructive cardiomyopathy, and heart valve disease.
Sample Collection and Processing Methods
The following information was collected regarding the general health of the patients: age, gender, body mass index (BMI), previous medical conditions (such as hypertension, diabetes mellitus, and coronary artery disease), site of infection, and disease diagnosis. Within twenty-four hours of admission, the patient’s levels of cTnI, NT-proBNP, procalcitonin (PCT), lactate (Lac), SOFA score, vasopressor usage were recorded along with the duration of stay in the ICU (in days), and the prognosis in the ICU.
Statistical Methods
All the data collected in this study were analyzed using SPSS 22.0 software. Normally distributed measurement data were expressed as mean±standard deviation (SD), while non-normally distributed measurement data were expressed as median (interquartil range), and the comparisons were examined by Student-t test and Mann–Whitney test (non parametric distribution). The categorical data were expressed as n(%), and the differences between the two groups were examined by chi-square analysis or Fisher’s exact test. P<0.05 was considered statistically significant.
Results
Patient Demographics
Between June 2016 and June 2023, a total of 310 patients diagnosed with sepsis or septic shock were screened. As per the exclusion criteria, 126 patients were excluded, leaving 184 eligible patients, of whom 57 died and 127 survived. Of these 184 patients, 140 had septic shock, and 44 had sepsis without shock (see Table 1).
 |
Table 1 Comparison of Various Indicators Between the Survival Group and Mortality Group |
In the mortality group, the SOFA scores, the proportion of vasopressor use, and troponin levels were significantly higher compared to the survival group (P<0.05). Regarding echocardiographic parameters, the e’ and RV-Sm were significantly lower in the mortality group than in the survival group (P<0.05). The LVEF and LV-Sm were slightly lower in the survival group compared to the mortality group, but this difference was not statistically significant.
Statistical Data of Different Types of Septic Cardiomyopathy
Out of the 184 patients with sepsis, 95 cases (51.6%) had septic cardiomyopathy; 80 patients had LV diastolic dysfunction; 47 patients had LV systolic dysfunction cardiomyopathy; 22 had RV systolic dysfunction; and 9 patients had a combination of LV systolic, diastolic, and RV dysfunction cardiomyopathy. There were 38 cases of isolated LV diastolic dysfunction; 6 cases of isolated LV systolic dysfunction; and 6 cases of isolated RV dysfunction (see Figure 2).
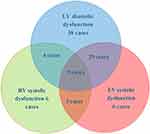 |
Figure 2 Statistical Chart of Different Types of Septic Cardiomyopathy. |
Univariate Analysis of Influencing Factors for Sepsis-Induced LV Diastolic Dysfunction
A few statistically significant differences were observed between the non-cardiomyopathy and LV diastolic dysfunction cardiomyopathy groups with respect to age, prevalence of hypertension and diabetes, incidence of coronary artery disease, troponin levels, and mortality rates (P<0.05). Nevertheless, no statistically significant differences were observed in terms of blood PCT levels, SOFA scores, NT-proBNP levels, or blood lactate levels between the two groups (P > 0.05). LV diastolic dysfunction cardiomyopathy was associated with a higher mortality rate than non-cardiomyopathy (see Table 2).
 |
Table 2 Univariate Analysis of Influencing Factors for Sepsis Left Ventricular Diastolic Dysfunction |
Univariate Analysis of Influencing Factors for Sepsis-Induced LV Systolic Dysfunction
When compared with the non-cardiomyopathy group, the LV systolic dysfunction cardiomyopathy group had higher troponin levels (P<0.05). There were no statistically significant differences for age, BMI, incidence of hypertension, diabetes mellitus, coronary artery disease, SOFA score, NT-proBNP levels, PCT levels, lactate levels, and the number of deaths (P > 0.05, see Table 3).
 |
Table 3 Univariate Analysis of Influencing Factors for Sepsis Left Ventricular Systolic Dysfunction |
Univariate Analysis of Influencing Factors for Sepsis-Induced RV Systolic Dysfunction
Compared to the non-cardiomyopathy group, the RV dysfunction cardiomyopathy group had higher SOFA scores, troponin, and NT-proBNP levels, as well as a higher mortality rate (P<0.05). There were no statistically significant differences in age, gender, BMI, incidence of hypertension, diabetes mellitus, coronary artery disease, PCT, and lactate levels (P > 0.05, see Table 4).
 |
Table 4 Univariate Analysis of Influencing Factors for Sepsis Right Ventricular Systolic Dysfunction |
Discussion
In this study, the ICU mortality rate for 184 patients with sepsis was 30.9%, with 40 deaths among the 95 patients with septic cardiomyopathy, resulting in a mortality rate for septic cardiomyopathy of 42.1%. This rate is notably higher than the mortality rate for patients with sepsis without cardiomyopathy, suggesting that the onset of septic cardiomyopathy significantly increases the risk of death for patients with sepsis. This conclusion is consistent with findings from international studies.2,10
Our results show that among the 184 patients with sepsis, 95 had septic cardiomyopathy, resulting in an incidence rate of 51.6%. Of these, the incidence rate of LV diastolic dysfunction cardiomyopathy was the highest at 42.4% (80 cases). The incidence rate of LV systolic dysfunction cardiomyopathy was 25.5% (47 cases). The study highlights that cardiac dysfunction induced by sepsis is not limited to the left side of the heart, as the involvement of the right side of the heart is also common, with RV systolic dysfunction cardiomyopathy occurring at an incidence rate of 3.3%. There were cases where patients concurrently exhibited LV systolic, diastolic, and RV dysfunction cardiomyopathy, amounting to an incidence rate of 4.9%.
CTnI is an internationally recognized biomarker of myocardial injury. The cTnI of sepsis patients in the death group was significantly higher than that in the survival group. The plasma cTnI concentration of various types of sepsis cardiomyopathy patients was significantly higher than that of the non cardiomyopathy group. The results of this study are consistent with those of domestic and foreign studies.11,12
Due to the increased content of substances such as endotoxins, inflammatory factors, and free radicals in sepsis patients, it is easy to cause varying degrees of damage to myocardial function, which is also one of the mechanisms leading to sepsis cardiomyopathy. These mediators increase the permeability of myocardial cell membranes, and in addition, the activation of intracellular pathways in the myocardium leads to the breakdown of cardiac troponin. Some of the products of cardiac troponin breakdown are released into the bloodstream, causing an increase in systemic cTnI. At this time, myocardial damage becomes more severe, and an increase in blood cTnI concentration is an important factor affecting the prognosis of sepsis cardiomyopathy patients.
LV Diastolic Dysfunction Cardiomyopathy
The results of this study showed that out of 184 patients with sepsis, 95 exhibited signs of septic cardiomyopathy, and of these, 80 patients suffered from LV diastolic dysfunction cardiomyopathy, accounting for 84.2% of the septic cardiomyopathy cases in this study. This indicates that septic cardiomyopathy, particularly LV diastolic dysfunction cardiomyopathy, has a high incidence rate. This finding is consistent with the results obtained from various domestic and international studies.13,14
In this study, the e’ value, which is a specific marker of LV diastolic function, was notably higher in the survival group than in the mortality group, while there was no statistical significance between the two groups in terms of E/e’ and E/A ratios. Since e’ is not influenced by cardiac preload, afterload, or LV systolic function, it reflects the LV diastolic function with greater precision. The higher e’ values in the survival group indicate that patients with LV diastolic dysfunction have a significantly higher mortality rate compared to those without such dysfunction. This aligns with the conclusions of several studies.15,16 Moreover, the study findings showed that the mortality rate in the LV diastolic dysfunction cardiomyopathy group was higher compared to the non-cardiomyopathy group.
E/e’ > 15 is another commonly used index to assess LV diastolic function. However, the E/e’ ratio has limitations in reflecting LV diastolic function accurately due to its susceptibility to various factors such as the transvalvular pressure gradient, age, LV compliance, left atrial size, and cardiac preload and afterload. In this study, no statistical significance was found between the survival and mortality groups regarding E/e’ and E/A.
Further investigations in this study showed significant statistical differences in age, diabetes incidence, hypertension incidence, and coronary artery disease incidence between the non-cardiomyopathy group and the LV diastolic dysfunction cardiomyopathy group. Patients with LV diastolic dysfunction cardiomyopathy tended to be of advanced age and had higher incidences of diabetes mellitus, hypertension, and coronary artery disease, which aligns with the findings of Pulido.17
Advanced Age
The results of this study revealed that there was no statistically significant difference in the age of patients with sepsis between the mortality and the survival groups; however, the age of patients with LV diastolic dysfunction was notably higher. According to studies,18,19 cardiac function gradually deteriorates with increasing age, particularly LV diastolic function, making age a significant factor affecting LV diastolic function. The mechanisms by which advanced age leads to LV diastolic dysfunction may involve a reduced calcium uptake rate in cardiomyocytes and decreased ventricular wall compliance. As early as 2012, Landesberg et al14 discovered a significant correlation between advanced age and LV diastolic dysfunction.
Hypertension
Research indicates that hypertension has the potential to stimulate the neuroendocrine system, which may result in various physiological changes including inflammatory stress, myocardial ischemia and hypoxia, disturbances in cellular calcium homeostasis, hypertrophy of cardiomyocytes, proliferation of the extracellular matrix, cardiac remodeling, and LV hypertrophy. These modifications have the potential to induce myocardial diastolic dysfunction and reduced compliance.20
Diabetes Mellitus
Early characteristics of diabetic cardiomyopathy include abnormal cardiac diastolic function. Insulin resistance, hyperinsulinemia, and hyperglycemia are independent risk factors for the development of diabetic cardiomyopathy. The pathophysiological mechanisms behind diabetic cardiomyopathy include systemic metabolic disorder, excessive activation of the renin-angiotensin-aldosterone system, oxidative stress, inflammation, and immune dysregulation.21
Coronary Artery Disease
Clinical research indicates that LV diastolic function is significantly impaired in the early stages of cardiomyocyte ischemia and hypoxia, whereas LV systolic function is generally unaffected. In patients with hypertension complicated by coronary artery disease, hypertension exacerbates the reduction in LV diastolic function caused by the coronary condition through various mechanisms. As coronary artery disease progresses over time, with increasing scope of lesions and ongoing cardiomyocyte ischemia and hypoxia, coupled with advancing age, these factors contribute to increased LV stiffness, dyssynchronous movement of the left ventricle, and disorders of ventricular filling, among other manifestations. Ultimately, this cascade of events results in diminished LV diastolic function.22 Therefore, advanced age, hypertension, diabetes mellitus, and coronary artery disease can interact with one another, ultimately causing LV diastolic dysfunction.
In patients with cardiovascular diseases, abnormalities in ventricular diastolic function occur earlier in comparison to ventricular systolic dysfunction. Ventricular diastolic dysfunction is an early manifestation of certain cardiac disorders. Regional ventricular diastolic dysfunction is frequently present in patients with advanced age, hypertension, diabetes mellitus, and coronary artery disease, despite the absence of early myocardial morphological changes. Myocardial diastolic function primarily depends on the active relaxation of the ventricles and passive filling, with the capacity for active relaxation mainly caused by alterations in cardiomyocyte calcium cycling. Severe sepsis can induce cardiomyocyte diastolic dysfunction through multiple pathways such as circulating myocardial depressant substances, intracellular Ca2+ homeostasis imbalance, abnormalities in myocardial energy metabolism, mitochondrial dysfunction, and cardiomyocyte apoptosis, among others. Among these, the imbalance of Ca2+ homeostasis is the most direct cause of impaired cardiomyocyte diastolic function.23,24 Severe sepsis can exacerbate the underlying myocardial diastolic dysfunction in patients with advanced age, hypertension, diabetes mellitus, or coronary artery disease, leading to overt cardiac diastolic dysfunction.
LV Systolic Dysfunction Cardiomyopathy
LVEF is the most frequently utilized clinical index for evaluating LV systolic function. However, its accuracy is highly dependent on the quality of the echocardiographic images. When measuring LV systolic function with TDI, a technique distinct from LVEF measurement, the Doppler shift signal of myocardial tissue is assessed. Studies have confirmed that the longitudinal motion of the mitral annulus is less affected by the preload and afterload of the heart, and the volume status of the body. Due to the fact that the acquisition of the mitral annulus is not constrained by image quality, TDI offers a straightforward and rapid advantage. TDI thus presents a novel approach for clinically accurate, speedy, and non-invasive quantitative evaluation of LV function. The results of this study showed that among 47 cases of LV systolic dysfunction cardiomyopathy, the incidence rate of isolated LV systolic dysfunction cardiomyopathy was only 3.3% (6 cases). Most patients with LV systolic dysfunction also had concomitant LV diastolic dysfunction, totaling 38 cases, accounting for 80.8% of all patients with LV systolic dysfunction. This suggests that ventricular contraction and relaxation are two interdependent processes in the cardiac cycle, and LV systolic dysfunction is commonly accompanied by LV diastolic dysfunction.
In this study, the sepsis mortality group had slightly higher LVEF and LV-Sm compared to the survival group, however, the difference was not statistically significant. The group with LV systolic dysfunction cardiomyopathy had a slightly higher mortality rate compared to the non-cardiomyopathy group, yet there was no statistical significance between the two groups. Li et al,25 studied 61 patients with septic shock and found that the mortality group had a significantly higher LV-Sm within 24 hours (11.0 cm/s [9.1 to 12.5]) compared to the survival group (7.8 cm/s [5.5 to 9.0]). When LV-Sm was included in multivariate regression analysis, it was identified as an independent risk factor for 28-day mortality in patients with septic shock. Our study results showed that the LV, LVEF, and LV-Sm in the survival group were slightly lower than those of the mortality group, but there was no statistical significance between the two groups. The conclusion drawn in this study is incongruent with that of Li et al, primarily due to differences in patient selection criteria. Li et al specifically focused on patients with septic shock, whereas our experiment included patients with sepsis, encompassing a broader range beyond those with septic shock. As early as 2010, Sturgess et al26 also studied the role of LV-Sm in patients with sepsis and found no statistical significance between the mortality group and the survival group regarding LV-Sm, a finding that aligns with the conclusions of our study.
RV Systolic Dysfunction Cardiomyopathy
Cardiac suppression caused by sepsis occurs not only in the left side of the heart but is also common in the right side of the heart, yet sepsis-related right cardiac suppression is often overlooked.
The development of sepsis-associated right cardiac suppression is multifactorial, such as disturbances in microcirculation, myocardial ischemia, release of inflammatory mediators, and endotoxins, among others. RV dysfunction depends on dual factors: the contractility of the right ventricle itself and the RV afterload. Thus, the RV systolic function is closely related to the extent of the patient’s lung injury. Endotoxins may lead to hypoxemia, hypercapnia, and acidosis, all of which can induce pulmonary vasoconstriction and pulmonary hypertension, thereby causing RV dysfunction.27,28
The findings of this study showed that the incidence rate of RV systolic dysfunction cardiomyopathy was 11.9% (22 cases), which is significantly lower than the 31% incidence rate of RV systolic dysfunction reported by Pulido et al.17 The possible reasons for this statistical discrepancy include: 1) The data from ultrasonography is greatly affected by human factors, and the small sample size of our experiment may lead to certain biases; 2) The study by Harmankaya et al revealed that the variability of RV-Sm values between different operators ranges between 2.9%–5.1%.29 Additionally, the criteria for RV dysfunction in the study by Pulido et al17 were Sm = < 15 cm/s, whereas in our experiment, we used RV-Sm = < 12 cm/s as the standard for RV dysfunction (consistent with the criteria used in the study by Furian30) leading to inconsistencies in the criteria. All the aforementioned factors could contribute to the variability in the reported incidence rates.
Compared to the non-cardiomyopathy group, patients with RV dysfunction cardiomyopathy showed higher SOFA scores, troponin levels, and NT-proBNP, as well as a higher mortality rate.
Patients with right heart dysfunction and sepsis cardiomyopathy have a significant increase in blood NT proBNP, which may be due to the following reasons: 55% of the infection sources in this study were pulmonary infections. Meanwhile, compared to the left ventricle, the right ventricle is a thin-walled structure with high compliance/low resistance pulmonary circulation. During sepsis, abnormal increases in pulmonary vascular resistance may occur due to factors such as hypoxic vasoconstriction, pulmonary infection, acute respiratory distress syndrome, hypercapnia, and mechanical ventilation. Liquid overload further increases the preload of the right ventricle, leading to a significant increase in NT proBNP levels in patients with sepsis and right heart dysfunction compared to those without cardiomyopathy.
Our previous research indicates that serum prealbumin is an independent risk factor for death in patients with sepsis. The SOFA score is one of the two most common scores in critical care medicine that reflects the severity of critically ill patients’ conditions, with higher scores indicating more severe disease. Therefore, RV dysfunction cardiomyopathy is associated with the severity of disease in patients in the intensive care unit (ICU) with sepsis and correlates with mortality rates in the ICU. The conclusions of this study are consistent with previous reports.28,31
Nevertheless, there are some limitations to this study. It is a retrospective study involving two centers. Furthermore the sample size was limited to 184 patients; moving forward, it is anticipated that additional centers will collaborate in order to incorporate a greater number of patients, thereby enhancing the persuasiveness of the data. It is necessary to assess the reproducibility of the echocardiographic data collected in this study. Echocardiographic measurements are prone to operator subjectivity, which inevitably introduces data collection bias. In this study, we only investigated the correlations and differences of echocardiographic parameters within 24 hours of ICU admission without long-term observation and analysis.
Conclusion
Septic cardiomyopathy occurs at a high rate in patients with sepsis, particularly with a high incidence rate of LV diastolic dysfunction cardiomyopathy. Elevated troponin is a risk factor for various types of septic cardiomyopathy, while advanced age, hypertension, diabetes mellitus, and coronary artery disease are risk factors for LV diastolic dysfunction cardiomyopathy. High SOFA scores and high NT-proBNP levels are risk factors for RV systolic dysfunction. When patients with sepsis develop RV systolic dysfunction, their mortality rate significantly increases.
The impact of sepsis on the heart is gaining increasing attention. As an adverse cardiac complication, septic cardiomyopathy significantly influences the prognosis of sepsis. However, most of the research on the risk factors of septic cardiomyopathy focuses on LV dysfunction cardiomyopathy, with studies on RV dysfunction being extremely rare. Even more scarce is research that separately investigates the prognosis and influencing factors of LV diastolic dysfunction, LV systolic dysfunction, and RV systolic dysfunction within the same study. Early identification of the influencing factors of different septic cardiomyopathies and the implementation of proactive intervention measures are of immense importance for improving cardiac function and the prognosis of patients with sepsis.
Abbreviations
LVDD, Left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; E, early diastolic flow peak velocity of the mitral valve; A, diastolic flow peak velocity of the mitral valve; e’, early diastolic peak velocity of mitral valve annulus; LV-Sm, left ventricular systolic mitral annular velocity; RV-Sm, and right ventricular systolic tricuspid annular velocity; cTnI, Cardiac troponin I; NT-proBNP, N-terminal probrain natriuretic peptide; SOFA, Sepsis-related organ failure assessment; PCT, Procalcitonin; ICU, Intensive care unit; ELISA, Enzyme linked immunosorbent assay; RV, Right ventricular; LV, Left ventricular.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author Xiu-Ming Xi upon reasonable request.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of Beijing Electric Power Hospital. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff who implemented the intervention and evaluation components of the study.
Funding
This work was supported by Scientific Research Project of Jiangsu Commission of Health of the People’s Republic of China (grant number: Z2020055), and the Ministry of Science and Technology of the People’s Republic of China (grant number: 2012BAI11B05).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Evans L, Rhodes A, Alhazzani W, et al. Executive summary: surviving sepsis campaign: international guidelines for the management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):1974–1982. doi:10.1097/CCM.0000000000005357
2. Hollenberg SM, Singer M. Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol. 2021;18(6):424–434. doi:10.1038/s41569-020-00492-2
3. Ravikumar N, Sayed MA, Poonsuph CJ, et al. Septic cardiomyopathy: from basics to management choices. Curr Probl Cardiol. 2021;46(4):100767. doi:10.1016/j.cpcardiol.2020.100767
4. L’Heureux M, Sternberg M, Brath L, et al. Sepsis-induced cardiomyopathy: a comprehensive review. Curr Cardiol Rep. 2020;22(5):35. doi:10.1007/s11886-020-01277-2
5. Walley KR. Sepsis-induced myocardial dysfunction. Curr Opin Crit Care. 2018;24(4):292–299. doi:10.1097/MCC.0000000000000507
6. Carbone F, Liberale L, Preda A, Schindler TH, Montecucco F. Septic cardiomyopathy: from pathophysiology to the clinical setting. Cells. 2022;11(18):2833. doi:10.3390/cells11182833
7. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
8. Xin Y, Ge Y, Chang L, Ni Y, Liu H, Zhu J. Risk factors of postoperative septic cardiomyopathy in perioperative sepsis patients. BMC Anesthesiol. 2022;22(1):193. doi:10.1186/s12871-022-01714-5
9. Lu NF, Jiang L, Zhu B, et al. Elevated plasma histone H4 levels are an important risk factor in the development of septic cardiomyopathy. Balkan Med J. 2020;37(2):72–78. doi:10.4274/balkanmedj.galenos.2019.2019.8.40
10. Meluzin J, Spinarova L, Bakala J, et al. Pulsed Doppler tissue imaging of the velocity of tricuspid annular systolic motion; a new, rapid, and non-invasive method of evaluating right ventricular systolic function. Eur Heart J. 2001;22(4):340–348. doi:10.1053/euhj.2000.2296
11. Mehta NJ, Khan IA, Gupta V, et al. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol. 2004;95(1):13–17. doi:10.1016/j.ijcard.2003.02.005
12. Ebelt H, Werdan K. Septic shock and septic cardiomyopathy. Med Klin Intensivmed Notfmed. 2012;107(1):24–28. doi:10.1007/s00063-011-0031-8
13. Landesberg G, Jaffe AS, Gilon D, et al. Troponin elevation in severe sepsis and septic shock: the role of left ventricular diastolic dysfunction and right ventricular dilatation. Crit Care Med. 2014;42(4):790–800. doi:10.1097/CCM.0000000000000107
14. Landesberg G, Gilon D, MerozY G, et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur Heart J. 2012;33(7):895–903. doi:10.1093/eurheartj/ehr351
15. Mourad M, Chow-Chine L, Faucher M, et al. Early diastolic dysfunction is associated with intensive care unit mortality in cancer patients presenting with septic shock. Br J Anaesth. 2014;112(1):102–109. doi:10.1093/bja/aet296
16. M ST, Franci D, Schweller M, et al. Left ventricle tissue Doppler imaging predicts disease severity in septic patients newly admitted in an emergency unit. J Emerg Med. 2015;49(6):907–915. doi:10.1016/j.jemermed.2015.06.054
17. Pulido JN, Afessa B, Masaki M, et al. Clinical spectrum, frequency, and significance of myocardial dysfunction in severe sepsis and septic shock. Mayo Clin Proc. 2012;87(7):620–628. doi:10.1016/j.mayocp.2012.01.018
18. Kitzman DW. Diastolic dysfunction in the elderly. Genesis and diagnostic and therapeutic implications. Cardiol Clin. 2000;18(3):597–617. doi:10.1016/S0733-8651(05)70164-8
19. Zhang Y, Safar ME, Iaria P, et al. Prevalence and prognosis of left ventricular diastolic dysfunction in the elderly: the PROTEGER Study. Am Heart J. 2010;160(3):471–478. doi:10.1016/j.ahj.2010.06.027
20. Nadruz W, Shah AM, Solomon SD. Diastolic dysfunction and hypertension. Med Clin North Am. 2017;101(1):7–17.
21. Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61(1):21–28. doi:10.1007/s00125-017-4390-4
22. Ge H. Is diastolic dysfunction a new windsock in the risk stratification of patients with coronary heart disease? Int J Cardiol. 2022;346:103–104. doi:10.1016/j.ijcard.2021.11.037
23. Rudiger A, Singer M. Mechanisms of sepsis induced cardiac dysfunction. Crit Care Med. 2007;35(6):1599–1608. doi:10.1097/01.CCM.0000266683.64081.02
24. Cohen RI, Wilson D, Liu SF. Nitric oxide modifies the sarcoplasmic reticular calcium release channel in endotoxemia by both guanosine 3’, 5’(cyclic)phosphate dependent and independent pathways. Crit Care Med. 2006;34(1):173–181. doi:10.1097/01.CCM.0000194722.12260.F9
25. Weng L, Liu YT, Du B, et al. The prognostic value of left ventricular systolic function measured by tissue Doppler imaging in septic shock. Crit Care. 2012;16(3):R71. doi:10.1186/cc11328
26. Sturgess DJ, Marwick TH, Joyce C, et al. Prediction of hospital outcome in septic shock: a prospective comparison of tissue Doppler and cardiac biomarkers. Crit Care. 2010;14(2):R44. doi:10.1186/cc8931
27. Alhamdi Y, Zi M, Abrams ST, et al. Circulating histone concentrations differentially affect the predominance of left or right ventricular dysfunction in critical illness. Crit Care Med. 2016;44(5):e278–e288. doi:10.1097/CCM.0000000000001413
28. Lu NF, Shao J, Niu HX, et al. Early diastolic peak velocity of mitral valve annulus and right ventricular systolic tricuspid annular velocity as predictors in assessing prognosis of patients with sepsis. Risk Manag Healthc Policy. 2023;17(16):921–930. doi:10.2147/RMHP.S407929
29. Harmankaya A, Akilli H, Gul M, et al. Assessment of right ventricular functions in patients with sepsis, severe sepsis and septic shock and its prognostic importance: a tissue Doppler study. J Crit Care. 2013;28(6):1111–1117. doi:10.1016/j.jcrc.2013.07.059
30. Furian T, Aguiar C, Prado K, et al. Ventricular dysfunction and dilation in severe sepsis and septic shock: relation to endothelial function and mortality. J Crit Care. 2012;27(3):319. doi:10.1016/j.jcrc.2011.06.017
31. Lu NF, Jiang L, Zhu B, et al. Elevated plasma histone H4 level predicts increased risk of mortality in patients with sepsis. Ann Palliat Med. 2020;9(3):1084–1091. doi:10.21037/apm-20-1011
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
