Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Trichoscopic Features of Scalp Discoid Lupus Erythematosus versus Lichen Planopilaris: A Systematic Review
Authors Gowda SK , Errichetti E, Thakur V, Panda M, Dash S, Agarwal A, Sethy M, Ayyanar P, Behera B
Received 2 February 2024
Accepted for publication 31 March 2024
Published 9 April 2024 Volume 2024:17 Pages 805—827
DOI https://doi.org/10.2147/CCID.S460742
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Shreya K Gowda,1 Enzo Errichetti,2 Vishal Thakur,1 Maitreyee Panda,3 Siddhartha Dash,4 Akash Agarwal,3 Madhusmita Sethy,5 Pavithra Ayyanar,5 Biswanath Behera1
1Department of Dermatology and Venereology, All India Institute of Medical Sciences, Bhubaneswar, OD, India; 2Institute of Dermatology, Department of Medicine, University of Udine, Udine, Italy; 3Department of Dermatology, IMS and SUM Hospital, Bhubaneswar, OD, India; 4Department of Dermatology, Srirama Chandra Bhanja Medical College and Hospital, Cuttack, OD, India; 5Department of Pathology, All India Institute of Medical Sciences, Bhubaneswar, OD, India
Correspondence: Biswanath Behera, Department of Dermatology and Venereology, All India Institute of Medical Sciences, Bhubaneswar, 751019, India, Email [email protected]
Introduction: Lichen planopilaris (LPP) and discoid lupus erythematosus (DLE) are primary scarring alopecias that pose diagnostic challenges clinically, where trichoscopy features may provide benefit in delineating these two cicatricial alopecia, and also helps in assessing the evolution and therapeutic response. To date, there are few reviews on dermoscopic findings in differentiating these two alopecias.
Methods: A systematic literature review was conducted using the PubMed and Google Scholar databases. The search terms included for scalp DLE were ‘lupus’ OR ‘discoid lupus’ OR “scalp lupus” and for scalp LPP were “lichen planopilaris” OR “scalp follicular lichen planus” OR “lichen planus follicularis” and were combined with “dermoscopy” OR “dermatoscopy” OR “videodermoscopy” OR “video dermatoscopy” OR “trichoscopy”. The differences in the prevalence of dermoscopic features in scalp DLE and LPP were calculated using the Chi-square test.
Results: Of 52 articles, 36 (17 LPP, 19 DLE) were eligible for quantitative analysis. We found predominant peripilar tubular casts and perifollicular erythema with the presence of arborizing vessels in the vicinity of these changes, indicating early LPP. In contrast, follicular red dots, speckled brown pigmentation, and hair diameter variability indicated active DLE. Shiny white areas were common in both the groups in late stages. The target pattern of distribution of blue-grey dots, milky red areas, and irregular white fibrotic dots were seen in LPP, and pink-white background, follicular plugs, perifollicular and interfollicular scale, rosettes, chrysalides, and red spider on yellow dots were detected in DLE. Features such as yellow dots and blue-grey structureless areas were nonspecific and did not have a major role in differentiating DLE from LPP.
Conclusion: This article provides a comprehensive review of the literature and delineates the trichoscopic differences and peculiarities of scalp DLE and LPP, including the correlation of dermoscopic features with histopathological findings.
Keywords: scalp discoid lupus erythematosus, lichen planopilaris, trichoscopy, dermoscopy, primary cicatricial alopecia
Introduction
Scalp alopecias are broadly classified as scarring and nonscarring types. Primary cicatricial alopecia (PCA) results from the destruction of the hair follicle unit (stem cell niche).1 PCA is further subcategorized based on the inflammatory infiltrate as lymphocytic (discoid lupus erythematosus [DLE], lichen planopilaris [LPP], central centrifugal cicatricial alopecia, pseudopelade of Brocq, and alopecia mucinosa), neutrophilic (acne necrotica, erosive pustular dermatosis, and acne keloidalis nuchae), and mixed infiltrate (dissecting cellulitis of scalp and folliculitis decalvans).2 Early diagnosis and treatment halt the further progression of disease and permanent scarring, hence improving the patient’s quality of life. Scalp DLE and LPP pose diagnostic dilemmas in routine practice, as both present as grey-blue discolored scarring alopecia patches, and many times, a scalp biopsy is necessary to detect the primary cause. Even though histopathology (sensitivity of 40%, 70% for LPP, DLE respectively and specificity of 92%, 63% for LPP, DLE respectively) and direct immunofluorescence (sensitivity of 34%, 83% for LPP, DLE respectively, and a specificity of 95%, 93% for LPP, DLE respectively) are considered the important diagnostic, trichoscopy can be utilized to diagnose scalp pathology and as a prognostic tool during follow-up.3–6
The most common PCA encountered is LPP.7 It usually clinically presents as violaceous papules, which are replaced with follicular plugs and scarring. Eventually, it progresses to white smooth atrophic plaques. The earlier changes of violaceous papules are not seen in all cases. The sites of predilection of LPP, apart from the scalp, are axillae, limb flexures, and inguinal folds.5 Scalp DLE presents as an itchy, erythematous, scaly plaque with follicular plugs in the early active stage. Pigmentary disturbances are seen in the skin of color.8 Dermoscopy is a non-invasive diagnostic tool with an inbuilt illumination and magnifying system that enables the visualization of deeper structure, pigmentary, and vascular patterns of skin, mucosa, nails, and hair (trichoscopy).7
There are currently no documented reports in the literature delineating the distinguishing trichoscopic features between LPP and DLE. This article aims to compare the trichoscopy findings of reported scalp DLE and LPP in terms of frequencies of dermoscopic features of follicular openings, perifollicular pattern, interfollicular areas, vessel pattern, and hair shafts.
Materials and Methodology
This review included original reports, case series, and case reports on videodermoscopic and hand-held dermoscopic findings describing scalp DLE and LPP, described in standardized dermatoscopic terms of any skin type. Frontal fibrosing alopecia and lichen planopilaris involving other areas were excluded from the analysis. The systematic analysis was performed as per PRISMA (“Preferred Reporting Items for Systematic Reviews and Meta Analyses”) guidelines. A detailed search in “PubMed” and “Google Scholar” was performed and all studies published until August 2023 on scalp DLE and LPP fulfilling the inclusion criteria were analyzed. The following search terms were used: “lupus” OR “discoid lupus” OR “scalp lupus” and for scalp LPP, “lichen planopilaris” OR “scalp follicular lichen planus” OR “lichen planus follicularis”. They were combined with “dermoscopy” OR “dermatoscopy” OR “videodermoscopy” OR “videodermatoscopy” OR “trichoscopy”. Trichoscopic features on scalp DLE and lichen planopilaris were divided into five categories: follicular openings, perifollicular surface, interfollicular pattern, vessel pattern, and hair shafts. The differences in the prevalence of dermoscopic features in scalp DLE and LPP were calculated using the Chi-square test. P values less than 0.05 were considered statistically significant.
Results
A total of 36 (17 LPP, 19 DLE) articles with full text and English literature were included for further analysis [Figures 1 and 2]. 1–59 Table 1 depicts the differentiating features of LPP and scalp DLE.
 |
Table 1 Trichoscopic Features of Discoid Lupus Erythematosus and Lichen Planopilaris |
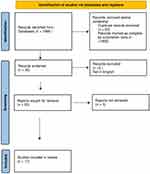 |
Figure 1 PRISMA 2020 flowchart for lichen planopilaris. |
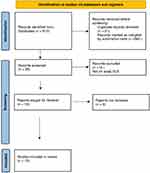 |
Figure 2 PRISMA 2020 flowchart for scalp discoid lupus erythematosus. |
Hair Follicular Features
Absent follicular opening was seen in approximately 45 to 50% of the cases included in the analysis. Follicular red dots (Figure 3) were seen exclusively in DLE (p<0.01).12 White dots can be pinpoint and fibrotic (Figure 4), corresponding to the opening of the eccrine sweat gland and the fibrotic follicular column (Figure 5), respectively.13 These fibrotic white dots were significantly higher in the LPP group (p<0.01). Yellow dots are follicular orifices filled with keratin and/or sebaceous secretions and were found in both groups. Follicular keratotic plugs (Figure 6) are comprised of keratotic mass filling the follicular orifice, corresponding to infundibular hyperkeratosis and plugging (Figure 7).14 The plugs were small, white in lichen planopilaris, and large, yellow color in scalp DLE (p<0.01). Loss of follicular openings in the late stage was seen in both groups.
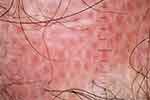 |
Figure 3 Trichoscopy of scalp discoid lupus erythematosus shows follicular red dots, interfollicular pink-white structureless area, and polymorphous vessels. |
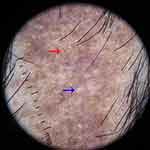 |
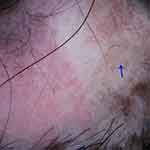
Figure 4 Trichoscopy of lichen planopilaris shows fibrotic white dots (blue arrow) and pinpoint white dots (red arrow). |
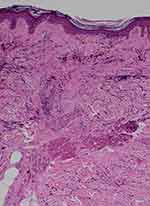 |
Figure 5 Histopathology of lichen planopilaris shows wedge-shaped perifollicular fibrosis with loss of elastic fibers (blue triangle) corresponding to the white fibrotic dots (VVG, X100). |
 |
Figure 7 Histopathology of scalp discoid lupus erythematosus shows follicular dilatation, plugging, and perifollicular and interfollicular dilated vessels (H & E, X50). |
Perifollicular Features
Perifollicular erythema (Figure 8) was seen predominantly in LPP and scalp DLE; however, this finding was not seen in DLE involving other than the scalp.15 Perifollicular scales (Figure 6) were significantly higher in DLE than in LPP. Perifollicular tubular casts are cylindrical scales encircling the hair shaft, which is approximately 3 mm in length, and were seen in LPP predominantly (p<0.01).16 Perifollicular blue-grey dots were seen in both LPP and scalp DLE, but the characteristic “target pattern” (Figure 9) of distribution of the dots was seen in LPP due to characteristic interface changes and melanin incontinence around the hair follicle (Figure 10) (p<0.01). Perifollicular gray to blue-gray structureless areas were seen in both groups.
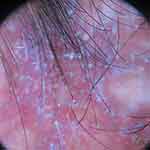 |
Figure 8 Trichoscopy of lichen planopilaris shows perifollicular scales and perifollicular and interfollicular erythema. |
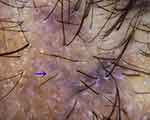 |
Figure 9 Trichoscopy of lichen planopilaris shows target sign (blue arrow). |
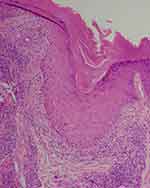 |
Figure 10 Histopathology of scalp discoid lupus erythematosus shows periinfundibular pigment incontinence corresponding to the perifollicular blue-gray dots (H & E, X100). |
Interfollicular Areas
In this study, pigmentation patterns identified were honeycomb and speckled brown pigmentation, which correspond to melanin deposits in epidermal ridges and melanophages, respectively, and these findings were seen in DLE. The optical phenomenon of polarization in the follicular and perifollicular structures, which leads to white rosettes (Figure 11), was seen significantly in scalp DLE (p<0.01).18 The background was pink-white (p<0.01) and milky white (p=0.02) in scalp DLE and LPP, respectively. Chrysalides are shiny white lines that correlate with stromal fibrosis and were seen in longstanding DLE (p<0.01).19 Interfollicular structureless white areas were seen in both groups, which correlates with epidermal acanthosis and dermal fibrosis in scarring alopecias. Interfollicular white scales and yellow scales (p<0.01) and interfollicular erythema were seen significantly in scalp DLE (p<0.01). Interfollicular scattered blue-grey dots, brown globules, red globules, and blue structureless areas were seen significantly in LPP (Figure 7). Scattered blue-grey dots in the interfollicular area were seen in LPP (p<0.01). Erosions and ulcerations were described only in one case of scalp DLE.20
 |
Figure 11 Trichoscopy of scalp discoid lupus erythematosus shows rosette and shiny-white lines. |
Blood Vessel Patterns
Six morphologies of blood vessels were found on trichoscopy of scalp DLE and LPP: arborizing, dotted, comma-shaped, linear, hairpin, and polymorphous vessels (Figure 12). Vasculature was more prominent in scalp DLE than LPP. Thin arborizing vessels around the large yellow dot are termed as a red spider (Figure 8) in the yellow dot, which is a highly specific finding of DLE (p=0.02), and this is not seen in skin of color due to masking of cutaneous vasculature. Dotted and hairpin vessels were seen to be significantly higher in scalp DLE (p<0.01).20
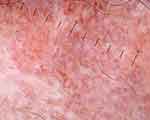 |
Figure 12 Trichoscopy of scalp discoid lupus erythematosus shows polymorphous vessels. |
Hair Shaft Features
Black dots result from the breakage of the hair shaft at emergence from the scalp.19 Black dots were exclusively seen in DLE. In a study, hair diameter variability was seen predominantly in scalp DLE (p<0.01) at the periphery of the alopecia patch.21 Hair tufting (Figure 10) is defined as tufts of more than six hair shafts originating from the single follicular orifice.22 The emergence of two to three hair shafts from follicular opening was seen in LPP (p<0.01). “Lonely hair sign”, an isolated single hair in the frontal area, was seen in LPP (Figure 13). Pili torti was seen in scalp DLE (p<0.01).16 Regrowing hairs (Figure 14) and tapered hairs were seen significantly in LPP (p<0.01).
 |
Figure 13 Trichoscopy of lichen planopilaris shows lonely hair sign. |
 |
Figure 14 Trichoscopy of lichen planopilaris shows regrowing vellus hair. |
Table 2 and Table 3 summarize the findings of LPP and scalp DLE described in various studies.
 |
Table 2 The Summary of Studies on Trichoscopy of Lichen Planopilaris |
 |
Table 3 Trichoscopic Studies of Scalp Discoid Lupus Erythematosus Included in the Analysis |
Discussion
Trichoscopy is a non-invasive diagnostic tool for unveiling the diagnosis of different scarring alopecias, although histopathology with DIF is considered the standard tool in diagnosing PCA. Among the PCA, scalp DLE, and LPP create a diagnostic dilemma in daily practice. Our review focuses on the key features that help distinguish these two scalp pathologies and may avoid invasive tests.
Follicular and Perifollicular Features
Although the absence of follicular opening is considered characteristic of cicatricial alopecia, this finding is not appreciated in the early lesions of DLE or LPP. As per the analysis, it was seen in around 45% to 50% of the cases in both groups. In the early stages of the disease, the follicular opening may be intact, even in the scarring type. Follicular keratotic plugs correlate with the hyperkeratosis and plugging of the follicular ostia with keratotic material on histology. They were initially attributed as a sign of early and active DLE. However, these findings were recorded in even later stages.24 Fibrotic white dots are irregularly blurred round structures that tend to merge, which later progress to form white structureless areas. Fibrotic white dots correspond to fibrotic tracts arranged vertically in histopathology.25 Fibrotic white dots must be differentiated from pinpoint white dots, which are regularly spaced sharply demarcated small dots with a peripheral hyperpigmented halo. These pinpoint white dots correspond to eccrine duct openings or follicular openings.26 Yellow dots are better visualized in polarized mode reflection and correlate with the follicular ostia filled with sebaceous secretions.27 Follicular red dots are erythematous polycyclic structures seen around the hair follicle, which correlate with dilated follicular openings with perifollicular vasculature and extravasated erythrocytes on histopathology and were only found in DLE. Yellow dots in scalp DLE were reported to be large, and thin arborizing vessels were described at the periphery of yellow dots, which was specific for inactive longstanding DLE and termed as “red spider on a yellow dots”. This finding was not visible in skin of color.20
Perifollicular white and yellow scales were significantly noted in DLE. Scales of LPP are usually adherent and encircle the hair shafts and later tend to climb along the proximal portion of hair shafts, referred to as a “perifollicular tubular cast”. These are better appreciated in polarized mode and were thought to be specific to LPP but later were described in pemphigus foliaceous, folliculitis decalvans, and scalp DLE. Peripilar cast corresponds to perifollicular hyperkeratosis on histopathology.17 In LPP, interface dermatitis in a shaped manner limited to follicular area sparing interfollicular areas on histopathology leads to a target pattern of blue-gray dots on trichoscopy and helps discriminate it from scalp DLE.
Interfollicualr Features
The pink-white background was seen significantly in scalp DLE, which corresponds to partial fibrosis and inflammatory infiltration. The blue-white veil is characterized by central white areas and peripheral irregular patches of blue-brown pigment with an overlying “ground-glass” hue corresponding to hyperkeratosis overlying lichenoid infiltrate with melanophages in the upper dermis.11,31 Speckled brown pigmentation in the interfollicular area (Figure 4) correlates with the involvement of interfollicular interface dermatitis with melanin incontinence, which was seen significantly in active DLE.
Vessels
Thick arborizing vessels have a diameter more than the diameter of the hair shafts and were reported in scalp DLE with a mean vessel thickness of 114±28 mm. These correspond to subpapillary plexus oh histology. This was seen in both groups, but the distribution around the follicle points towards LPP, and the presence of vasculature in the interfollicular area indicates scalp DLE.24 As per Tosti et al, nonfollicular red dots visualized on hand-held dermoscopy correspond to coiled vessels on videodermoscopy, a specific indication of active DLE.13 Hairpin vessels are seen in the normal scalp, but in DLE, the density of the vasculature was significantly increased in our review.32
Hair Shaft Features
Black dots represent cadavarized or broken hair before the emergence of hair from the scalp surface. Vellus hairs are thin hair shafts (less than 0.03mm in diameter) with less pigmentation, while the regrowing hairs and circular pigtail hairs are darkly pigmented upright shafts with tapered ends. Both vellus hairs and regrowing hairs were seen predominantly in LPP. Pili torti on trichoscopy shows flattening at irregular intervals, with twisting at 180°, and was described in scalp DLE.2
Limitations of this review were inconsistent and variability in the terminologies of dermoscopic features, the inclusion of all tools [dermatoscope and videodermatoscope], and data on variability in the findings on polarized and nonpolarized light were not assessed. While the study included participants of various skin types, the specific nuances and differences within skin of color may not have been explicitly examined or emphasized. Nevertheless, the broader literature recognizes the importance of considering skin type diversity and its potential impact on trichoscopic findings. Detailed analysis of variation in the trichoscopic findings in skin of color could be extrapolated, and further stratification of the scalp and non-scalp DLE and follicular LP could be evaluated.
Conclusion
In conclusion, this systematic review indicates that trichoscopy can be an effective tool in differentiating the two common forms of cicatricial alopecias: the presence of follicular red dots, perifollicular scales, pink-white background, speckled brown pigmentation, chrysalides, white rosettes, and vessels indicate scalp DLE and follicular white fibrotic dots, perifollicular tubular casts, blue-gray dots/globules in a target pattern, and milky white areas point LPP. In the future, a large comparison study involving both fair and dark-skinned individuals and using standardized trichoscopic terms will be able to further explore the distinguishing trichoscopic features of scalp DLE from LPP.
Acknowledgment
We thank the patient for granting permission for clinical photography.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Olga Warszawik M. Trichoscopy of cicatricial alopecia. J Drugs Dermatol. 2012;11:753–758.
2. Stefanato CM. Histopathology of alopecia: a clinicopathological approach to diagnosis. Histopathology. 2010;56(1):24–38. doi:10.1111/j.1365-2559.2009.03439.x
3. Rudnicka L, Olszewska M, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651–656.
4. Trachsler S, Trüeb RM. Value of direct immunofluorescence for differential diagnosis of cicatricial alopecia. Dermatology. 2005;211(2):98–102. doi:10.1159/000086436
5. Bharti S, Dogra S, Saikia B, Walker RM, Chhabra S, Saikia UN. Immunofluorescence profile of discoid lupus erythematosus. Indian J Pathol Microbiol. 2015;58(4):479–482. doi:10.4103/0377-4929.168850
6. Bary AA, Eldeeb M, Hassan E. Cicatricial alopecia: do clinical, trichoscopic, and histopathological diagnosis agree? Acta Dermatovenerol Alp Pannonica Adriat. 2021;30:129–137.
7. Mehta P, Malakar S. Trichoscope as a monitoring tool for therapeutic efficacy in lichen planopilaris. Int J Dermoscopy. 2017;1:38–39. doi:10.5005/jp-journals-10061-0009
8. Panjwani S. Early diagnosis and treatment of discoid lupus erythematosus. J Am Board Fam Med. 2009;22(2):206–213. doi:10.3122/jabfm.2009.02.080075
9. Vendramini DL, Silveira BR, Duque-Estrada B, et al. Isolated body hair loss: an unusual presentation of lichen planopilaris. Skin Appendage Disord. 2017;2:97–99. doi:10.1159/000449229
10. Lacarrubba F, Musumeci ML, Ferraro S, et al. A three-cohort comparison with videodermatoscopic evidence of the distinct homogeneous bushy capillary microvascular pattern in psoriasis vs atopic dermatitis and contact dermatitis. J Eur Acad Dermatol Venereol. 2016;30:701–703. doi:10.1111/jdv.12998
11. Cervantes J, Hafeez F, Miteva M. Blue-white veil as novel dermatoscopic feature in discoid lupus erythematosus in 2 African-American patients. Skin Appendage Disord. 2017;3:211–214. doi:10.1159/000477354
12. Tosti A, Torres F, Misciali C, et al. Follicular red dots: a novel dermoscopic pattern observed in scalp discoid lupus erythematosus. Arch Dermatol. 2009;145(12):1406–1409. doi:10.1001/archdermatol.2009.277
13. Abraham LS, Pineiro-Maceira J, Duque-Estrada B, et al. Pinpoint white dots in the scalp: dermoscopic and histopathologic correlation. J Am Acad Dermatol. 2010;63(4):721–722. doi:10.1016/j.jaad.2009.12.011
14. Jha AK, Sonthalia S, Sarkar R. Dermoscopy of discoid lupus erythematosus. Indian Dermatol Online J. 2016;7(5):458. doi:10.4103/2229-5178.190493
15. Souissi A, Ben Tanfous A, Azzouz H, et al. When trichoscopy enlightens clinics. Int J Dermatol. 2016;55(11):1278–1280. doi:10.1111/ijd.13139
16. Choundhary SV, Tarafdar PP, Jawade S, Singh A. A point to note in pili torti. Int J Trichol. 2018;10:95–97. doi:10.4103/ijt.ijt_111_16
17. Mathur M, Acharya P, Karki A, Shah J, Kc N. Tubular Hair Casts in Trichoscopy of Hair and Scalp Disorders. Int J Trichol. 2019;11:14–19. doi:10.4103/ijt.ijt_77_18
18. Ankad BS, Shah SD, Adya KA. White rosettes in discoid lupus erythematosus: a new dermoscopic observation. Dermatol Pract Concept. 2017;7:9–11. doi:10.5826/dpc.0704a03
19. Tosti A. Lonely hair sign: not specific for frontal fibrosing alopecia-reply. Arch Dermatol. 2012;148:1208–1209. doi:10.1001/archdermatol.2012.1873
20. Żychowska M. Dermoscopy of discoid lupus erythematosus–a systematic review of the literature. Int J Dermatol. 2021;60:818–828. doi:10.1111/ijd.15365
21. Gomez-Quispe H, de Las Heras-Alonso ME, Lobato-Berezo A, et al. Trichoscopic findings of discoid lupus erythematosus alopecia: a cross-sectional study. J Am Acad Dermatol. 2020;S0190-9622:31021–31025.
22. Qi S, Zhao Y, Zhang X, Li S, Cao H, Zhang X. Clinical features of primary cicatricial alopecia in Chinese patients. Indian J Dermatol Venereol Leprol. 2014;80:306–312. doi:10.4103/0378-6323.136833
23. Sillani C, Bin Z, Ying Z, Zeming C, Jian Y, Xingqi Z. Effective treatment of folliculitis decalvans using selected antimicrobial agents. Int J Trichol. 2010;2:20–23. doi:10.4103/0974-7753.66908
24. Miteva M, Tosti A. Dermoscopy guided scalp biopsy in cicatricial alopecia. J Eur Acad Dermatol Venereol. 2013;27(10):1299–1303. doi:10.1111/j.1468-3083.2012.04530.x
25. Tawfik SS, Sorour OA, Alariny AF, et al. White and yellow dots as new trichoscopic signs of severe female androgenetic alopecia in dark skin phototypes. Int J Dermatol. 2018;57:1221–1228. doi:10.1111/ijd.14140
26. Inui S, Itami S, Murakami M, Nishimoto N. Dermoscopy of discoid lupus erythematosus: report of two cases. J Dermatol. 2014;41(8):756–757. doi:10.1111/1346-8138.12547
27. Choundhary SV, Tarafdar PP, Jawade S, Singh A. A point to note in pili torti. Int J Trichol. 2018;10:95–97.
28. Lallas A, Apalla Z, Lefaki I, et al. Dermoscopy of discoid lupus erythematosus. Br J Dermatol. 2013;168(2):284–288. doi:10.1111/bjd.12044
29. Massi D, De Giorgi V, Carli P, Santucci M. Diagnostic significance of the blue hue in dermoscopy of melanocytic lesions: a dermoscopic-pathologic study. Am J Dermatopathol. 2001;23:463–469. doi:10.1097/00000372-200110000-00013
30. Liebman TN, Rabinovitz HS, Dusza SW, Marghoob AA. White shiny structures: dermoscopic features revealed under polarized light. J Eur Acad Dermatol Venereol. 2012;26(12):1493–1497. doi:10.1111/j.1468-3083.2011.04317.x
31. Jain N, Doshi B, Khopkar U. Trichoscopy in alopecias: diagnosis simplified. Int J Trichol. 2013;5:170–178. doi:10.4103/0974-7753.130385
32. Lajevardi V, Mahmoudi H, Moghanlou S, Ansari M, Teimourpour A, Daneshpazhooh M. Assessing the correlation between trichoscopic features in lichen planopilaris and lichen planopilaris activity index. Australas J Dermatol. 2019;60(3):214–218. doi:10.1111/ajd.13022
33. Eftekhari H, Azimi SZ, Rafiei R, et al. Dermoscopic features of lichen planopilaris in Northern Iran: a prospective observational study. Int J Dermatol. 2019;58(12):1406–1414. doi:10.1111/ijd.14589
34. Estrada BD, Tamler C, Sodré CT, Barcaui CB, Pereira FB. Dermoscopy patterns of cicatricial alopecia resulting from discoid lupus erythematosus and lichen planopilaris. Anais brasileiros de dermatologia. 2010;85:179–183. doi:10.1590/S0365-05962010000200008
35. Farag AM, Salem RM, Abdelrahman AM, EL-Adawy DM. Trichoscopic Findings of Frontal Fibrosing Alopecia. Benha J Appl Sci. 2020;5:71–74. doi:10.21608/bjas.2020.136217
36. Panchaprateep R, Ruxrungtham P, Chancheewa B, Asawanonda P. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: a retro‐prospective cohort study. J Dermatol. 2020;47:1301–1311. doi:10.1111/1346-8138.15517
37. Thakur BK, Verma S, Raphael V. Clinical, trichoscopic, and histopathological features of primary cicatricial alopecias: a retrospective observational study at a tertiary care centre of North East India. Int j Trichol. 2015;7(3):107. doi:10.4103/0974-7753.167459
38. Starace M, Brandi N, Alessandrini A, Bruni F, Piraccini BM. Frontal fibrosing alopecia: a case series of 65 patients seen in a single Italian centre. J Eur Acad Dermatol Venereol. 2019;33(2):433–438. doi:10.1111/jdv.15372
39. Arshdeep BM, Kubba A, Kubba R. Lichen planopilaris beyond scalp: a case series with dermoscopy‐histopathology correlation. Int J Dermatol. 2018;57:e127–31. doi:10.1111/ijd.14168
40. Ankad BS, Beergouder SL, Moodalgiri VM. Lichen planopilaris versus discoid lupus erythematosus: a trichoscopic perspective. Int J Trichol. 2013;5(4):204. doi:10.4103/0974-7753.130409
41. Friedman P, Sabban EC, Marcucci C, Peralta R, Cabo H. Dermoscopic findings in different clinical variants of lichen planus. Is dermoscopy useful? Dermatol Practical Conceptual. 2015;31:51–55. doi:10.5826/dpc.0504a13
42. Thompson CT, Velasco MA, Tosti A. Three-dimensional imaging of a peripilar cast and compound follicle in frontal fibrosing alopecia. JAAD Case Rep. 2022;23:46–48. doi:10.1016/j.jdcr.2022.02.036
43. Shim WH, Jwa SW, Song M, et al. Dermoscopic approach to a small round to oval hairless patch on the scalp. Ann Dermatol. 2014;26:214–220. doi:10.5021/ad.2014.26.2.214
44. Góes HF, Dias MF, Salles SD, Lima CD, Vieira MD, Pantaleão L. Lichen planopilaris developed during childhood. Anais Brasileiros de Dermatologia. 2017;92:543–545. doi:10.1590/abd1806-4841.20174890
45. Zeeshan M, Chaudhary RK, Roy PK, Jha AK. Dermoscopy in selected disorders of scarring alopecia. J Pakistan Assoc Dermatol. 2018;28:449–451.
46. Batra P, Sukhdeo K, Shapiro J. Hair loss in lichen planopilaris and frontal fibrosing alopecia: not always irreversible. Skin Appendage Disorders. 2020;6(2):125–129. doi:10.1159/000505439
47. Kaliyadan F, Ameer AA. Localized and linear lichen planopilaris over the face and scalp with associated alopecia–clinical and dermoscopy pattern. Dermatol Online J. 2015;21:13030. doi:10.5070/D3219028698
48. Rossi A, Iorio A, Scali E, et al. Frontal fibrosing alopecia and lichen planopilaris: clinical, dermoscopic and histological comparison. Eur J Inflammation. 2013;11(1):311–314. doi:10.1177/1721727X1301100134
49. Andziukeviciute J, Makstiene J, Valiukeviciene S. Trichoscopy as an Additional Diagnostic Tool for Monitoring of Lichen planopilaris. Aktuelle Dermatologie. 2016;280–282.
50. Rakowska A, Slowinska M, Kowalska-Oledzka E, et al. Trichoscopy of cicatricial alopecia. J Drugs Dermatol. 2012;11:753–758.
51. Melo DF, De Carvalho N, Ardigò M, et al. Concordance among in vivo reflectance confocal microscopy, trichoscopy, and histopathology in the evaluation of scalp discoid lupus. Skin Res Technol. 2020;26:675–682. doi:10.1111/srt.12852
52. Nikam VV, Mehta HH. A nonrandomized study of trichoscopy patterns using nonpolarized (contact) and polarized (noncontact) dermatoscopy in hair and shaft disorders. Int J Trichol. 2014;6(2):54. doi:10.4103/0974-7753.138588
53. Chiramel MJ, Sharma VK, Khandpur S, Sreenivas V. Relevance of trichoscopy in the differential diagnosis of alopecia: a cross-sectional study from North India. Indian J Dermatol Venereol Leprol. 2016;82:651. doi:10.4103/0378-6323.183636
54. Karadag O, Güleç AT. Evaluation of a handheld dermatoscope in clinical diagnosis of primary cicatricial alopecias. Dermatol Therapy. 2019;9:525–535. doi:10.1007/s13555-019-0304-3
55. Abedini R, Kamyab Hesari K, Daneshpazhooh M, Ansari MS, Tohidinik HR, Ansari M. Validity of trichoscopy in the diagnosis of primary cicatricial alopecias. Int J Dermatol. 2016;55(10):1106–1114. doi:10.1111/ijd.13304
56. Melo DF, Müller Ramos P, Iorizzo M, et al. Epidemiological, Clinical, Trichoscopic, and Histopathological Features of Lupus Erythematous Mimicking Alopecia Areata: a Multicenter Retrospective Study. Skin Appendage Disorders. 2022;8:236–240. doi:10.1159/000520825
57. Mikiel D, Polańska A, Żaba R, Adamski Z, Dańczak‐Pazdrowska A. Suitability of high‐frequency ultrasonography (20 MHz) in evaluation of various forms of primary cicatricial alopecia in relation to trichoscopy—pilot study. Skin Res Technol. 2021;27:774–784. doi:10.1111/srt.13018
58. Lanuti E, Miteva M, Romanelli P, Tosti A. Trichoscopy and histopathology of follicular keratotic plugs in scalp discoid lupus erythematosus. Int j Trichol. 2012;4(1):36. doi:10.4103/0974-7753.96087
59. Nascimento LLD, Enokihara MMSES, Vasconcellos MRA. Coexistence of chronic cutaneous lupus erythematosus and frontal fibrosing alopecia. An Bras Dermatol. 2018;93:274–276. doi:10.1590/abd1806-4841.20186992
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.