Back to Journals » Clinical Ophthalmology » Volume 15
Treatment Success Across Different Levels of Preoperative Disease Burden: Stratified Two-Year Outcomes from the Pivotal Trial of iStent inject® Trabecular Micro-Bypass in Primary Open-Angle Glaucoma and Cataract
Authors Singh IP, Sarkisian Jnr S, Hornbeak D, Katz LJ, Samuelson T
Received 21 April 2021
Accepted for publication 23 June 2021
Published 3 August 2021 Volume 2021:15 Pages 3231—3240
DOI https://doi.org/10.2147/OPTH.S316270
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Inder Paul Singh,1 Steven Sarkisian Jnr,2 Dana Hornbeak,3 L Jay Katz,3,4 Thomas Samuelson5 for the iStent
1The Eyes Centers of Racine & Kenosha, Racine, WI, USA; 2Oklahoma Eye Surgeons, Oklahoma City, OK, USA; 3Glaukos Corporation, San Clemente, CA, USA; 4Wills Eye Hospital, Philadelphia, PA, USA; 5Minnesota Eye Consultants, Minneapolis, MN, USA
Correspondence: Inder Paul Singh
The Eye Center of Racine, 3805 B Spring Street, Suite 140, Racine, WI, 53405, USA Tel +1 262 637 0500
Fax +1 262 637 7650
Email [email protected]
Purpose: To examine effectiveness outcomes stratified by preoperative disease burden in the pivotal trial of iStent inject® with cataract surgery (INJ) vs cataract surgery alone (CS).
Materials and Methods: Prospective, 3:1 randomized, single-masked, concurrently-controlled, multicenter trial enrolling 505 subjects with cataract and mild-to-moderate primary open-angle glaucoma who underwent iStent inject implantation with phacoemulsification or phacoemulsification alone, and were followed for 2 years including annual medication washouts. Post hoc stratification was completed for baseline mean diurnal intraocular pressure (BL DIOP; Low-DIOP < 25mmHg, Mid-DIOP ≥ 25 to < 30 mmHg, High-DIOP ≥ 30mmHg) and preoperative medication burden (Low-Med 1 medication, Mid-Med 2 medications, High-Med ≥ 3 medications).
Results: The 24-month primary and secondary effectiveness endpoints were met, with significant treatment-over-control differences in percent of eyes achieving ≥ 20% unmedicated DIOP reduction and in unmedicated DIOP reduction, respectively. In subgroup analyses, the proportions of INJ eyes achieving the primary endpoint remained steady across all BL DIOP (75.4%, 77.1%, 74.4% in Low/Mid/High-DIOP strata, respectively) and preoperative medication levels (76.8%, 70.8%, 79.7% in Low/Mid/High-Med strata, respectively); meanwhile, the proportions of CS eyes diminished with higher BL DIOP (64.5%, 63.6%, 33.3%, respectively) and more medications (69.0%, 63.3%, 29.4%, respectively). Regarding secondary effectiveness, postoperative DIOP reduction increased with higher BL DIOP in INJ eyes (6.2mmHg, 7.8mmHg, 9.8mmHg, respectively) but plateaued in CS eyes (5.2mmHg, 5.8mmHg, 5.4mmHg, respectively). INJ eyes also had consistent DIOP reduction regardless of preoperative medication burden (6.8mmHg, 6.7mmHg, 7.8mmHg, respectively), while DIOP reduction diminished with more medications in CS eyes (6.1mmHg, 5.0mmHg, 3.3mmHg, respectively). Safety was favorable, comparable to phacoemulsification alone.
Conclusion: Significant IOP reductions occurred across all levels of BL DIOP and preoperative medication burden in iStent inject eyes. DIOP reductions increased with higher BL DIOP and remained stable across all levels of preoperative medication burden, suggesting the device’s potential utility in more medically challenging cases.
Keywords: microinvasive glaucoma surgery/MIGS, glaucoma, iStent inject, trabecular micro-bypass, IOP, stratification, severity
Introduction
Already the leading cause of irreversible blindness globally, glaucoma is expected to increase in prevalence from approximately 65 million people in 2013 to nearly 120 million in 2040.1 The cornerstone of glaucoma treatment is IOP reduction, the only proven way to limit the progressive optic nerve damage associated with the disease. IOP reduction is unequivocally beneficial, with 1 mmHg of IOP reduction equating to 11–19% reduced risk of disease progression2,3 and 10% reduced risk of glaucoma development in pre-glaucomatous eyes.4 Currently-available glaucoma therapies range from medications and laser trabeculoplasty (more conservative) to filtering procedures like trabeculectomy and tube placement (more invasive). Between these extremes, a class of procedures known as micro- or minimally-invasive glaucoma surgery (MIGS) may offer a lower-risk alternative for reducing IOP and medication burden. Recent years have witnessed a marked rise in the use of MIGS procedures as an earlier intervention prior to (or ideally instead of) filtering procedures.5 MIGS procedures seek to avoid the disadvantages of ocular hypotensive medication (eg, ocular surface disease, poor compliance, side effects, cost) and the risks of filtering surgeries (eg, dysesthesia, bleb leaks, hypotony, blebitis, and lifetime endophthalmitis risk).6–10
To-date, the most extensive body of MIGS evidence pertains to the first US Food and Drug Administration (FDA)-approved MIGS device, the Glaukos iStent® Trabecular Micro-Bypass. With up to 7 years of postoperative follow-up, iStent studies have often focused on patients with mild to moderate POAG undergoing cataract surgery, but an increasing number of studies have assessed iStent for different glaucoma types (eg, pseudoexfoliative, angle-closure, pigmentary), more advanced severity, standalone cases, and in combination with other procedures or stents.11–23 The more recent iStent inject® Trabecular Micro-Bypass (CE Mark 2010, FDA approval 2018) contains two stents that create two patent bypasses through the diseased trabecular meshwork. This device has been studied both with and without concomitant cataract extraction, and in studies with up to 5 years of follow-up.24–39
Both iStent and iStent inject are implanted ab internally and enhance the physiologic trabecular outflow pathway of aqueous humor, thereby decreasing IOP. Targeting the natural outflow pathway helps avoid the risks of suprachoroidal, bleb-forming, subconjunctival, and/or ab externo procedures. Among trabecular MIGS, the micro-scale iStent inject is the only modality that avoids tissue destruction or removal, and that has the smallest known footprint designed to preserve angle structures. Importantly, stent implantation does not preclude additional medical or surgical therapies should they be needed later in this lifelong disease.
Several preoperative characteristics may shape the goal of glaucoma surgery; some of these also may predict postoperative outcomes. A common factor evaluated in the literature is baseline IOP. Studies in glaucomatous and healthy eyes have shown a consistent association between higher baseline IOP and greater postoperative IOP reduction.40–43 In addition, several publications have shown this IOP association following iStent or iStent inject implantation.15–18,26,27 Fewer studies have evaluated the relationship between preoperative medication burden and postoperative IOP reduction.44,45 However, to our knowledge, no MIGS pivotal trials to-date have specifically explored outcomes with stratification by preoperative IOP and medications.
The present pivotal trial was a large randomized study evaluating iStent inject implantation with cataract surgery versus cataract surgery alone in patients with mild to moderate POAG and cataract. At two years postoperative, both the primary and secondary effectiveness endpoints were met (≥20% unmedicated DIOP reduction and mean unmedicated DIOP reduction, respectively).24 The current manuscript examines these outcomes with stratification by baseline DIOP and preoperative number of medications, thereby assessing device viability across the spectrum of preoperative treatment burden and surgical goals.
Materials and Methods
Study Design, Endpoints, and Participants
This prospective, 3:1 randomized, single-masked, controlled, multicenter US pivotal trial evaluated the two-year safety and effectiveness of iStent inject in patients with mild to moderate POAG and cataract. The study design was in alignment with the FDA Guidance on Premarket Studies of Implantable Minimally Invasive Glaucoma Devices (December 2015) and the ANSI Z80.27–2014 Standard for Implantable Glaucoma Devices. The trial was approved by the Western Institutional Review Board (IRB) and followed the tenets of the Declaration of Helsinki, including written informed consent of all subjects. The study was registered with the National Library of Medicine (clinicaltrials.gov, NCT00323284).
Key aspects from the complete inclusion/exclusion criteria, sample size calculations, randomization, effectiveness endpoints, medications, and postoperative follow-up24 are summarized here. Subjects were randomized in a 3:1 ratio to the treatment group (cataract surgery + iStent inject, n=387, INJ) or control group (cataract surgery only, n=118, CS) after completion of uncomplicated cataract surgery; the randomization scheme was based on a computer-generated list. Throughout postoperative follow-up, subjects and the technicians performing postoperative measurements were masked to treatment assignment. The primary effectiveness endpoint was a ≥ 20% reduction from baseline in medication-free mean diurnal DIOP at Month 24. The secondary effectiveness endpoint was the unmedicated 24-month DIOP reduction from baseline. IOP measurements were taken by Goldmann applanation using a standard 2-person method common in glaucoma studies;4 mean DIOP was calculated as the mean of three individual IOP measurements on the same day (at approximately 8:00 am, 12:00 pm, and 4:00 pm), and was performed at baseline and at Months 6, 12, and 24. Safety parameters included best spectacle-corrected visual acuity (BSCVA), slit-lamp and fundus examinations, gonioscopy, pachymetry, specular microscopy, visual field testing, adverse events (AEs), and complications. Patients underwent surgery from January 2012 to August 2015. Postoperatively, study visits occurred at 6 hours, Day 1, Week 1, and at Months 1, 3, 6, 11, 12, 18, 23, and 24. Prior to the preoperative baseline visit, and at the Month 11 and Month 23 postoperative visits, subjects using ocular hypotensive medication(s) were instructed to undergo medication washout in order to permit unmedicated DIOP assessment at baseline and at Months 12 and 24, respectively.
Key inclusion criteria included a diagnosis of mild to moderate POAG (with mean deviation not worse than −12dB), cataract requiring surgery, screening IOP ≤24 mmHg on 1–3 ocular hypotensive medications, and unmedicated baseline mean DIOP (BL DIOP) 21–36 mmHg. Patients were excluded if they had traumatic, uveitic, neovascular, angle-closure, or vascular-disorder-associated glaucoma; history of prior incisional glaucoma surgery, argon laser trabeculoplasty (ALT), iridectomy, or iridotomy, or completion of selective laser trabeculoplasty (SLT) within 90 days prior to screening; or ocular disease or visual field status that would preclude safe medication washout.
Study Device and Surgical Implantation Technique
As detailed previously,24 the iStent inject device and implantation technique may be summarized as follows. Each single-use iStent inject injector is pre-loaded with two biocompatible titanium stents. Each stent has a central lumen and four side lumens to facilitate multidirectional outflow, and is designed to carry the entirety of aqueous humor production by the human body (average 2.5 µL/min).46,47 The placement of two stents enables access to up to six clock-hours of collector channels, and has shown the ability to reactivate formerly dormant collector channels and enhance flow through active collector channels.48
Following phacoemulsification cataract extraction and intraocular lens insertion, the iStent inject injector is advanced under direct gonioscopy through the existing corneal incision to the nasal trabecular meshwork, where the first stent is implanted through the meshwork into Schlemm’s canal. While remaining in the eye, the injector tip is repositioned to implant the second stent approximately 2 to 3 clock hours away from the first stent, and proper placement and seating are confirmed for both stents. Viscoelastic is then removed and sealing of the corneal incision is ensured. Following surgery, patients were prescribed topical antibiotics (for one week) and prednisolone acetate 1% (tapered over four weeks).
Statistical Analyses
For the baseline DIOP subgroup post hoc analyses, eyes were divided into three groups (Low-DIOP <25 mmHg, Mid-DIOP ≥25 to <30 mmHg, and High-DIOP ≥30mmHg). For the preoperative medication subgroup analyses, eyes also were divided into three groups (Low-Med, 1 medication; Mid-Med, 2 medications; and High-Med: ≥3 medications). Within each IOP or medication level, the mean reductions in IOP and medications were compared between the INJ and CS groups using a two-sample t-test. The proportion of eyes achieving a ≥20% DIOP reduction versus baseline was compared between INJ and CS eyes within each DIOP or medication stratum using a two-sided chi-square test. If 25% of the cells had expected counts less than 5, then Fisher’s exact test was used.
Results
Effectiveness
The preoperative characteristics of the overall cohort from this randomized controlled pivotal trial were provided in the prior publication.24 The distribution and demographic characteristics of the current stratified IOP and medication subgroups are provided in Tables 1 and 2, respectively.
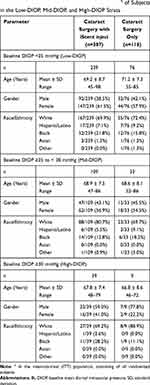 |
Table 1 Preoperative Demographic Characteristicsa of Subjects in the Low-DIOP, Mid-DIOP, and High-DIOP Strata |
 |
Table 2 Preoperative Demographic Characteristicsa of Subjects in the Low-Med, Mid-Med, and High-Med Strata |
The study met both the primary and secondary effectiveness endpoints: a significantly higher proportion of INJ eyes (75.8%) than CS eyes (61.9%) achieved a ≥ 20% reduction in medication-free DIOP from baseline at 24 months (p=0.005), and the mean reduction in medication-free DIOP from baseline to 24 months was significantly greater in treatment versus control eyes (p<0.001), respectively. Notably, the final IOP of INJ eyes without the use of any medications was 17.1 mmHg, comparable to or lower than other trabecular MIGS randomized controlled trials (RCTs).24,49 Furthermore, iStent inject eyes reduced their mean medication burden by 75% (versus 47% in CS eyes), with 84% of stent eyes becoming medication-free at two years (vs 67% of control eyes), and a 50% lower final mean medication burden in stent eyes than control eyes (0.4 versus 0.8 medications, respectively; p<0.001).
Within the baseline DIOP strata, significant treatment-vs.-control differences in the proportion of eyes achieving a ≥ 20% DIOP reduction from baseline were observed regardless of BL DIOP (p<0.05 for all 3 IOP strata). In the three BL DIOP strata of the CS group (Low-DIOP n=76, Mid-DIOP n=33, High-DIOP n=9), the proportion of eyes reaching the endpoint was 64.5% in the Low-DIOP stratum, 63.6% in the Mid-DIOP stratum, and 33.3% in the High-DIOP stratum. In contrast, within the BL DIOP strata of the INJ group (Low-DIOP n=239, Mid-DIOP n=109, High-DIOP n=39), the proportions of INJ eyes achieving the endpoint remained steady across all levels of BL DIOP (75.4% in Low-DIOP stratum, 77.1% in Mid-DIOP stratum, and 74.4% in High-DIOP stratum) (Figure 1). In other words, iStent inject eyes with higher DIOP preoperatively appeared to be just as likely to achieve treatment success as iStent inject eyes with lower preoperative DIOP, a consistency that was not observed in the cataract-only CS group.
 |
Figure 1 Proportion of Subjects with 24-Month Medication-Free Mean Diurnal Intraocular Pressure (DIOP) Reduction ≥20% from Baseline, Stratified By Baseline DIOP. |
With regard to the secondary effectiveness outcome, the amount of postoperative DIOP reduction plateaued in CS eyes (5.2 mmHg in Low-DIOP stratum, 5.8 mmHg in Mid-DIOP stratum, and 5.4 mmHg in High-DIOP stratum) while it increased with higher baseline DIOP in INJ eyes (6.2 mmHg, 7.8 mmHg, and 9.8 mmHg in the three strata, respectively) (Figure 2).
 |
Figure 2 Average 24-Month Medication-Free Mean Diurnal Intraocular Pressure (DIOP) Change from Baseline, Stratified By Baseline DIOP. |
Outcomes also were analyzed in three subgroups based on the number of preoperative medications, which served as a proxy for disease treatment burden. In the medication strata of the CS group (Low-Med n=71, Mid-Med n=30, High-Med n=17), the proportion of eyes achieving the primary endpoint was 69.0% in the Low-Med stratum, 63.3% in the Mid-Med stratum, and 29.4% in the High-Med stratum. Meanwhile, within the medication strata of the INJ group (Low-Med n=224, Mid-Med n=98, High-Med n=65), the proportion of treatment eyes achieving the endpoint remained steady regardless of preoperative medication burden (76.8%, 70.8%, and 79.7% in the three strata, respectively) (Figure 3). In other words, iStent inject eyes with higher preoperative medication burden appeared to be just as likely to achieve treatment success as iStent inject eyes with fewer preoperative medications, a consistency that was not observed in the control group.
With regard to the secondary effectiveness outcome, the amount of postoperative DIOP reduction diminished with higher preoperative medication burden in CS eyes (6.1 mmHg in Low-Med stratum, 5.0 mmHg in Mid-Med stratum, and 3.3 mmHg in High-Med Stratum), whereas it remained stable regardless of preoperative medication burden in INJ eyes (6.8 mmHg, 6.7 mmHg, and 7.8 mmHg in the three strata, respectively) (Figure 4).
 |
Figure 4 Average 24-Month Medication-Free Mean Diurnal Intraocular Pressure (DIOP) Change from Baseline, Stratified By Number of Ocular Hypotensive Medications at Screening. |
Safety
As reported in the pivotal publication,24 safety was excellent in the iStent inject treatment group, comparable to phacoemulsification alone. This included results for BSCVA, visual field MD, C:D ratio, and endothelial cell stability. There were no unanticipated adverse events and no cases of significant inflammatory responses, myopic shift, choroidal hemorrhage or effusion, hypotony, stent dislocation or migration, significant hyphema, corneal decompensation, shallow anterior chamber, cyclodialysis, or endophthalmitis. The rate of peripheral anterior synechiae was low (1.8%). Over two years of follow-up, the rate of incisional glaucoma surgery was 1% in both INJ and CS eyes.
Discussion
Given the range of available therapies, choosing an appropriate intervention must account for patients’ preoperative characteristics and individual surgical goals. Such factors may be ocular (eg, baseline IOP, medications, glaucoma severity) as well as non-ocular (eg, medication compliance, ocular surface health, and quality of life39). The ocular factors (specifically IOP and medications) undergoing stratified analysis in this study show consistency with the majority of the literature.40–43 For example, two prominent publications of phacoemulsification cataract surgery in healthy41 and glaucomatous42 eyes showed that the amount of postoperative IOP reduction was proportional to preoperative IOP, with approximately three-fold higher percent reduction in the highest-IOP subgroups than in the 15–17 mmHg subgroup. This trend also is widely recognized in glaucomatous eyes undergoing combined phacoemulsification and glaucoma procedures,50,51 and specifically in eyes undergoing iStent or iStent inject implantation either with or without phacoemulsification.15–18,26,27
In contrast to the predominant consensus in the literature, four recent non-controlled, non-randomized studies have suggested that success rates are higher in eyes with lower, rather than higher, disease burden: for example, in eyes with lower baseline IOP44,52 or lower IOP during the first postoperative month;45 lower number of preoperative medications;44,45,52 or lower glaucoma severity according to VF MD.45,53 However, attention must be paid to how these studies defined postoperative success; specifically, none of the studies’ success criteria were adjusted for patients’ preoperative IOP/medication burden or individual goals for surgery. As such, the criteria were not necessarily consistent with expectations of doctors and patients in real-world clinical practice. For example, Guedes et al and Rothschild et al required eyes to be on zero medications while achieving a fixed IOP value of <18 mmHg or ≤18 mmHg, respectively; Chansangpetch et al required eyes to be at a set postoperative IOP value of 18/15/12 mmHg for mild/moderate/severe cases, respectively; and Konopinska required eyes to be on zero medications postoperatively. Given these criteria, it is no surprise that eyes with lower preoperative IOP or medications are more likely to achieve the success threshold; this can be expected, as not all eyes had the same starting point.
Regardless of how success is defined in any given study, from a clinical perspective, it is unlikely that patients with high preoperative IOP and/or medication burden would realistically expect to achieve normotensive IOP with zero medications after MIGS surgery. In these patients (who comprise a substantial portion of the glaucoma population), an operative “success” may be defined differently based upon patients’ individual needs: for example, reducing IOP (with stable medications), reducing medications (with stable IOP), reducing exposure to topical drops and harmful preservatives, conserving conjunctival and trabecular tissue, and/or avoiding filtration surgery. Thus, to apply a single uniform postoperative goal to all eyes regardless of preoperative glaucoma status is inappropriate for many patients, as it would not take into account preoperative characteristics (in particular IOP and medications) nor surgical goals. A more appropriate threshold of success might be percent or amount of IOP reduction (rather than a fixed absolute IOP value), as well as reduction in medications, as these would accommodate heterogeneous preoperative characteristics. Not surprisingly, it is such baseline-sensitive endpoints that the FDA requires, given their clinical relevance and appropriateness.
Some authors have postulated that medication burden and/or IOP level can be considered a proxy for the degree of glaucomatous pathology in the trabecular meshwork, suggesting that eyes with more preoperative medications or with higher baseline IOP are more likely to have dysfunctional or dormant trabecular outflow networks.45 However, this hypothesis is countered by the stratified results in the present large, randomized, controlled trial, in which greater IOP reductions were observed in eyes with higher baseline IOP and medications. It is also countered by aqueous angiography findings in eyes following iStent inject implantation,48 which show clear reactivation of formerly dormant outflow areas, thereby suggesting that both trabecular and non-trabecular outflow networks may be restored via a trabecular (physiologic) intervention, without employing higher-risk suprachoroidal, subconjunctival, or more extensive tissue-disrupting MIGS procedures.
At every level of disease severity, a core principle of surgical glaucoma treatment is to implement the safest, least invasive technology that will achieve the treatment objectives. Consistent with these objectives, the safety profile of iStent inject was excellent, as summarized in the prior pivotal publication. Secondary filtration surgery occurred in very few eyes. Importantly, there were no complications as seen with filtering surgeries, such as endophthalmitis, hypotony, bleb infections, bleb leaks, and subconjunctival fibrosis.6–10
The main study limitations are that surgeons could not be masked to treatment assignment (given the surgical nature of the treatment intervention), and that data included the surgeons’ learning curve with the technology. The former issue was counterbalanced by the use of a masked 2-person IOP measurement method, and the masking of subjects and technicians performing postoperative measurements; the latter aspect suggests that real-world outcomes of present-day surgeons actually may prove to be better than those observed in the study. Another limitation is the modest sample sizes within certain IOP or medication subgroups, as the study was not specifically designed to analyze these subgroups.
Conclusions
This pivotal study of the second-generation iStent inject trabecular micro-bypass device demonstrated significant, sustained, and safe clinical benefit of device implantation with cataract surgery in subjects with mild to moderate POAG. The IOP findings are meaningful given the well-established importance of IOP reduction; the medication reduction is also highly relevant, given the association of medications with ocular surface disease, increased future surgical failure, poor compliance, costs, diminished quality of life, and side effects.39,54–57
The additional stratification completed in the current analysis provides a greater understanding of iStent inject utility in patients with a range of IOP and medication levels along the disease spectrum. By grouping patients according to preoperative glaucoma treatment burden (specifically IOP and medications), a variety of possible surgical goals could be represented. Such goals could range from simply reducing medications to avoiding filtering surgery. In the present stratified analyses, it became apparent that the treatment-over-control advantage was greater, rather than smaller (as is sometimes assumed), in eyes with higher preoperative DIOP and more preoperative medications.
In addition, as previously described,24 the safety outcomes observed in the pivotal trial were excellent, comparable to cataract surgery alone. This high safety, combined with the stratified effectiveness outcomes revealed in the present report, reinforce the viability of iStent inject with cataract surgery as an initial intervention prior to riskier, more invasive treatments. The stratified results suggest that treatment viability may extend to patients across the spectrum of preoperative treatment burden. By analyzing different stratified subgroups, from well-controlled cases aiming for medication reduction to uncontrolled cases aiming to reduce IOP and avoid filtrating surgery, the current analysis informs surgeons of the postoperative IOP results that could reasonably be expected for patients at each level of disease burden.
Data Sharing Statement
Additional details from this clinical trial are available on the US FDA website and the clinicaltrials.org clinical trials registry, including the complete Summary of Safety and Effectiveness Data (SSED) document. Should further inquiries regarding de-identified individual participant data arise, a response and supporting documentation may be made available by the authors (Dr. Dana Hornbeak, [email protected]) on reasonable request.
Disclosures
Dr Singh:
Consultant with Aerie, Alcon, Allergan, Ellex, Glaukos, New World Medical.
Dr Sarkisian:
Consultant/advisor to Alcon Laboratories, Allergan, Beaver Visitec International, Inc., Glaukos Corporation, Katena Products, Inc., New World Medical Inc., Omeros, Santen Inc., Sight Sciences, Inc.; grants from Alcon Laboratories, Glaukos Corporation, Sight Sciences, Inc.; and Alconies Fees.
Dr Hornbeak:
Employee of Glaukos Corporation.
Dr Katz:
Personal fees from Glaukos Corporation, Allergan, Aerie Pharmaceuticals, Mati Therapeutics, Aerpio Therapeutics; grants and personal fees from Diopsys, Alcon, Bausch & Lomb, Grants from Heidelberg Engineering, outside the submitted work.
Dr Samuelson:
Support from Glaukos (during the conduct of the study); Consultant with Ivantis, Alcon Surgical, MicroOptix, Santen, Allergan (outside the submitted work).
Funding
Glaukos Corporation (San Clemente, CA, USA) participated in the design and conduct of the study; the collection, management, and analysis of data; and the preparation of the manuscript.
References
1. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi:10.1016/j.ophtha.2014.05.013
2. Chauhan BC, Mikelberg FS, Balaszi AG, et al. Canadian Glaucoma Study: 2. risk factors for the progression of open-angle glaucoma. Arch Ophthalmol. 2008;126(8):1030–1036. doi:10.1001/archopht.126.8.1030
3. Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma Trial. Arch Ophthalmol. 2003;121(1):48–56. doi:10.1001/archopht.121.1.48
4. Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–720. doi:10.1001/archopht.120.6.714
5. Rathi S, Andrews CA, Greenfield DS, Stein JD. Trends in glaucoma surgeries performed by glaucoma subspecialists versus nonsubspecialists on medicare beneficiaries from 2008 through 2016. Ophthalmology. 2021;128(1):30–38. doi:10.1016/j.ophtha.2020.06.051
6. Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC, Tube Versus Trabeculectomy Study Group. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804–814. doi:10.1016/j.ajo.2011.10.024
7. Jampel HD, Musch DC, Gillespie BW, Lichter PR, Wright MM, Guire KE, Collaborative Initial Glaucoma Treatment Study Group. Perioperative complications of trabeculectomy in the collaborative initial glaucoma treatment study (CIGTS). Am J Ophthalmol. 2005;140(1):16–22. doi:10.1016/j.ajo.2005.02.013
8. Wilson MR, Mendis U, Paliwal A, et al. Long-term follow-up of primary glaucoma surgery with Ahmed glaucoma valve implant versus trabeculectomy. Am J Ophthalmol. 2003;136(3):464e470. doi:10.1016/S0002-9394(03)00239-3
9. Kim EA, Law SK, Coleman AL, et al. Long-term bleb-related infections after trabeculectomy: incidence, risk factors, and influence of bleb revision. Am J Ophthalmol. 2015;159(6):1082e1091. doi:10.1016/j.ajo.2015.03.001
10. Rulli E, Biagioli E, Riva I, et al. Efficacy and safety of trabeculectomy vs nonpenetrating surgical procedures: a systematic review and meta-analysis. JAMA Ophthalmol. 2013;131(12):1573–1582. doi:10.1001/jamaophthalmol.2013.5059
11. Samuelson TW, Katz LJ, Wells JM, Duh Y-J, Giamporcaro JE. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–467. doi:10.1016/j.ophtha.2010.07.007
12. Ziaei H, Au L. Manchester iStent study: long-term 7-year outcomes. Eye (Lond). 2020. PMID: 33139875. doi:10.1038/s41433-020-01255-6.
13. Neuhann TH, Hornbeak DM, Neuhann RT, Giamporcaro JE. Long-term effectiveness and safety of trabecular micro-bypass stent implantation with cataract surgery in patients with glaucoma or ocular hypertension: 5-year outcomes. J Cataract Refract Surg. 2019;45(3):312–320. doi:10.1016/j.jcrs.2018.10.029
14. Gallardo MJ, Supnet RA. Three-year outcomes of combined trabecular micro-bypass and phacoemulsification in a predominantly Hispanic population with primary open-angle glaucoma. Clin Ophthalmol. 2019;13:869–879. doi:10.2147/OPTH.S189071
15. Ferguson TJ, Mechels KB, Dockter Z, et al. iStent trabecular microbypass stent implantation with phacoemulsification in patients with open-angle glaucoma: 6-year outcomes. Clin Ophthalmol. 2020;14:1859–1866. doi:10.2147/OPTH.S247910
16. Ferguson T, Swan R, Ibach M, Schweitzer J, Sudhagoni R, Berdahl JP. Evaluation of a trabecular microbypass stent with cataract extraction in severe primary open-angle glaucoma. J Glaucoma. 201827(1):71–76. doi:10.1097/IJG.0000000000000825.
17. Ferguson TJ, Swan RJ, Bleeker A, et al. Trabecular microbypass stent implantation in pseudoexfoliative glaucoma: long-term results [published online ahead of print, 2020 May 7]. J Cataract Refract Surg. 2020. doi:10.1097/j.jcrs.0000000000000243
18. Ferguson TJ, Ibach M, Schweitzer J, et al. Trabecular microbypass stent implantation in pseudophakic eyes with open-angle glaucoma: long-term results. J Cataract Refract Surg. 2019;45(4):414–420. doi:10.1016/j.jcrs.2018.11.005
19. Ferguson TJ, Ibach M, Schweitzer J, Karpuk KL, Stephens JD, Berdahl JP. Trabecular microbypass stent implantation with cataract extraction in pigmentary glaucoma. Clin Exp Ophthalmol. 2020;48(1):37–43. doi:10.1111/ceo.13638
20. Nitta K, Yamada Y, Morokado S, Sugiyama K. iStent trabecular micro-bypass stent implantation with cataract surgery in a Japanese glaucoma population. Clin Ophthalmol. 2020;15(14):3381–3391. doi:10.2147/OPTH.S274281
21. Fechtner RD, Voskanyan L, Vold SD, et al. Five-year, prospective, randomized, multi-surgeon trial of two trabecular bypass stents versus prostaglandin for newly-diagnosed open-angle glaucoma. Ophthalmol Glaucoma. 2019;2(3):156–166. doi:10.1016/j.ogla.2019.03.004
22. Chen DZ, Sng CCA, Sangtam T, et al. Phacoemulsification vs phacoemulsification with micro-bypass stent implantation in primary angle closure and primary angle closure glaucoma: a randomized single-masked clinical study. Clin Exp Ophthalmol. 2020;48(4):450–461. doi:10.1111/ceo.13721.
23. Schweitzer JA, Hauser WH, Ibach M, et al. Prospective interventional cohort study of ocular surface disease changes in eyes after trabecular micro-bypass stent(s) implantation (iStent or iStent inject) with phacoemulsification. Ophthalmol Ther. 2020;9(4):941–953. doi:10.1007/s40123-020-00290-6
24. Samuelson TW, Sarkisian SR
25. Shalaby WS, Jia J, Katz LJ, Lee D. iStent inject®: a comprehensive survey of the literature [published online ahead of print, 2020 Jul 10]. J Cataract Refract Surg. 2020. doi:10.1097/j.jcrs.0000000000000325.
26. Ferguson TJ, Dockter Z, Bleeker A, et al. iStent inject trabecular microbypass stent implantation with cataract extraction in open-angle glaucoma: early clinical experience. Eye Vis (Lond). 2020;7(1):28. doi:10.1186/s40662-020-00194-3
27. Salimi A, Watt H, Harasymowycz P. three-year outcomes of second-generation trabecular micro-bypass stents (iStent inject) with phacoemulsification in various glaucoma subtypes and severities. J Glaucoma. 2020. PMID: 33105306. doi:10.1097/IJG.0000000000001716.
28. Clement C, Howes F, Ioannidis AS, et al. Two-year multicenter outcomes of istent inject trabecular micro-bypass stents combined with phacoemulsification in various types of glaucoma and ocular hypertension. Clin Ophthalmol. 2020;14:3507–3517. doi:10.2147/OPTH.S271646
29. Neuhann R, Neuhann T. Second-generation trabecular micro-bypass stent implantation: retrospective analysis after 12- and 24-month follow-up. Eye Vis (Lond). 2020;7(1):1. doi:10.1186/s40662-019-0169-7
30. Hengerer FH, Auffarth GU, Riffel C, Conrad-Hengerer I. Prospective, non-randomized, 36-month study of second-generation trabecular micro-bypass stents with phacoemulsification in various types of glaucoma. Ophthalmol Ther. 2018;7(2):405–415. doi:10.1007/s40123-018-0152-8
31. Hengerer FH, Auffarth GU, Riffel C, Conrad-Hengerer I. Second-generation trabecular micro-bypass stents as standalone treatment for glaucoma: a 36-month prospective Study. Adv Ther. 2019;36(7):1606–1617. doi:10.1007/s12325-019-00984-9
32. Fea AM, Belda JI, Rekas M, et al. Prospective unmasked randomized evaluation of the iStent inject versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophthalmol. 2014;8:875–882.
33. Lindstrom R, Sarkisian SR, Lewis R, Hovanesian J, Voskanyan L. Four-Year Outcomes of Two Second-Generation Trabecular Micro-Bypass Stents in Patients with Open-Angle Glaucoma on One Medication. Clin Ophthalmol. 2020;14:71–80. doi:10.2147/OPTH.S235293
34. Salimi A, Clement C, Shiu M, Harasymowycz P. Second-generation trabecularmicro-bypass (istent inject) with cataract surgery in eyes with normal-tension glaucoma: one-year outcomes of a multi-centre study [published online ahead of print, 2020 Jul 1]. Ophthalmol Ther. 2020. doi:10.1007/s40123-020-00266-6.
35. Salimi A, Abu-Nada M, Harasymowycz P. Matched cohort study of Cataract surgery with and without trabecular micro-bypass stent implantation in primary angle-closure glaucoma. Am J Ophthalmol. 2021;224:310–320. doi:10.1016/j.ajo.2020.12.032. PMID: 33428885.
36. Guedes RAP, Gravina DM, Lake JC, Guedes VMP, Chaoubah A. One-year comparative evaluation of istent or istent inject implantation combined with cataract surgery in a single center. Adv Ther. 2019;36(10):2797–2810. doi:10.1007/s12325-019-01067-5
37. Manning D. Real-world Case series of istent or istent inject trabecular micro-bypass stents combined with cataract surgery. Ophthalmol Ther. 2019;8(4):549–561. doi:10.1007/s40123-019-00208-x
38. Klabe K Minimally invasive glaucoma surgery: 5 years – results with the iStent inject.
39. Samuelson TW, Singh IP, Williamson BK, et al. Quality of life in primary open-angle glaucoma and cataract: an analysis of VFQ-25 and OSDI from the iStent inject® Pivotal Trial. Am J Ophthalmol. 2021;229:220–229. doi:10.1016/j.ajo.2021.03.007. PMID: 33737036.
40. Shingleton BJ, Pasternack JJ, Hung JW, O’Donoghue MW. Three and five year changes in intraocular pressures after clear corneal phacoemulsification in open angle glaucoma patients, glaucoma suspects, and normal patients. J Glaucoma. 2006;15(6):494–498. doi:10.1097/01.ijg.0000212294.31411.92
41. Poley BJ, Lindstrom RL, Samuelson TW. Long-term effects of phacoemulsification with intraocular lens implantation in normotensive and ocular hypertensive eyes. J Cataract Refract Surg. 2008;34(5):735–742. doi:10.1016/j.jcrs.2007.12.045
42. Poley BJ, Lindstrom RL, Samuelson TW, Jr SR. Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes: evaluation of a causal relationship between the natural lens and open-angle glaucoma. J Cataract Refract Surg. 2009;35(11):1946–1955. doi:10.1016/j.jcrs.2009.05.061
43. Mathalone N, Hyams M, Neiman S, Buckman G, Hod Y, Geyer O. Long-term intraocular pressure control after clear corneal phacoemulsification in glaucoma patients. J Cataract Refract Surg. 2005;31(3):479–483. doi:10.1016/j.jcrs.2004.06.046
44. Rothschild P, Komzak K, Hooshmand J, Allen P, Vote B, Toh T. Predictors of success of iStent and iStent inject, when combined with cataract surgery [published online ahead of print, 2020 May 4]. Clin Exp Ophthalmol. 2020. doi:10.1111/ceo.13777.
45. Guedes VM, Chaoubah A. Factors Associated with Unqualified Success After Trabecular Bypass Surgery. A Case-Control Study [published online ahead of print, 2020 Aug 5]. J Glaucoma. 2020. doi:10.1097/IJG.0000000000001626.
46. Hunter K, Fjield T, Heitzmann H, Shandas R, Kahook M. Characterization of micro- invasive trabecular bypass stents by ex vivo perfusion and computational flow modeling. Clin Ophthalmol. 2014;8:499–506.
47. Bahler C, Hann C, Fjield T, Haffner D, Heitzmann H, Fautsch MP. Second-generation trabecular meshwork bypass stent (iStent inject) increases outflow facility in cultured human anterior segments. Am J Ophthalmol. 2012;153(6):1206–1213. doi:10.1016/j.ajo.2011.12.017
48. Huang AS, Penteado RC, Papoyan V, Voskanyan L, Weinreb RN. Aqueous angiographic outflow improvement after trabecular micro-bypass in glaucoma patients. Ophthalmol Glaucoma. 2019;2(1):11–21. doi:10.1016/j.ogla.2018.11.010
49. Samuelson TW, Chang DF, Marquis R, et al.; HORIZON Investigators. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: the HORIZON Study. Ophthalmology. 2019;126(1):29e37. doi:10.1016/j.ophtha.2018.05.012.
50. Lai JSM, Tham CCY, Lam DSC. The efficacy and safety of combined phacoemulsification, intraocular lens implantation, and limited goniosynechialysis, followed by diode laser peripheral iridoplasty, in the treatment of cataract and chronic angle-closure glaucoma. J Glaucoma. 2001;10(4):309–315. doi:10.1097/00061198-200108000-00011
51. Tham CC, Kwong YY, Leung DY, et al. Phacoemulsification versus combined phacotrabeculectomy in medically controlled chronic angle closure glaucoma with cataract. Ophthalmology. 2008;115(12):2167–2173.e2. doi:10.1016/j.ophtha.2008.06.016
52. Konopińska J, Kozera M, Kraśnicki P, Mariak Z, Rekas M. The effectiveness of first-generation istent microbypass implantation depends on initial intraocular pressure: 24-month follow-up—prospective clinical Trial. J Ophthalmol. 2020;2020:1–8. doi:10.1155/2020/8164703. Article ID 8164703.
53. Chansangpetch S, Ittarat M, Yang S, et al. Comparison of 1-Year Effectiveness of Trabecular Microbypass Stent implantation (iStent) in Conjunction With Phacoemulsification Among Mild, Moderate, and Severe Primary Open-angle Glaucoma Patients [published online ahead of print, 2020 May 19]. J Glaucoma. 2020;29(7):542–549. doi:10.1097/IJG.0000000000001542
54. Broadway D, Hitchings R, Grierson I. Topical antiglaucomatous therapy: adverse effects on the conjunctiva and implications for filtration surgery. J Glaucoma. 1995;4(2):136. doi:10.1097/00061198-199504000-00012
55. Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153(1):1–9.e2. doi:10.1016/j.ajo.2011.05.033
56. Fechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29(6):618–621. doi:10.1097/ICO.0b013e3181c325b2
57. Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122(7):1308–1316. doi:10.1016/j.ophtha.2015.03.026
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

