Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 15
Treatment of Vitamin K Deficiency in Hemodialysis Patients – A Pilot Study Comparing Menaquinone-7 Tablets and a Vitamin K Rich Diet
Authors Lentz KA, Vahlgren J, Hansen D, Plebani M , Fusaro M, Rasmussen LM, Jakobsen J, Sloth JJ, Post Hansen H, Andersen JR
Received 11 March 2022
Accepted for publication 29 September 2022
Published 17 October 2022 Volume 2022:15 Pages 267—276
DOI https://doi.org/10.2147/IJNRD.S365912
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Katrine Aagaard Lentz,1 Julie Vahlgren,2 Ditte Hansen,2,3 Mario Plebani,4 Maria Fusaro,4,5 Lars Melholt Rasmussen,6 Jette Jakobsen,7 Jens Jørgen Sloth,7 Henrik Post Hansen,3 Jens Rikardt Andersen1
1Department of Nutrition, Exercise and Sports, University of Copenhagen, Copenhagen, Denmark; 2Department of Nephrology, Herlev-Gentofte Hospital, University of Copenhagen, Herlev, Denmark; 3Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark; 4Department of Medicine, University of Padova, Padova, Italy; 5National Research Council, Institute of Clinical Physiology, Pisa, Italy; 6Department of Clinical Biochemistry and Pharmacology, Odense University Hospital, Odense, Denmark; 7National Food Institute, Technical University of Denmark, Lyngby, Denmark
Correspondence: Ditte Hansen, Email [email protected]
Purpose: Vitamin K deficiency and hence a high level of plasma dephosphorylated undercarboxylated matrix Gla protein (dp-ucMGP) is frequent in patients on hemodialysis. This group is recommended to restrict their potassium intake which often leads to restriction of vitamin K rich foods. A menaquinone-7 (MK-7) supplement has been shown to decrease dp-ucMGP, but it has yet to be examined if a vitamin K rich diet could be equally effective.
Patients and Methods: A prospective randomized crossover intervention trial with two arms; 6 weeks of 360 μg MK-7 tablet/day and 6 weeks of a vitamin K rich diet with a 3-week washout period in between. Participants were 10 patients in hemodialysis and the primary outcome measures were changes in dp-ucMGP, total MGP (tMGP), and undercarboxylated osteocalcin (ucOC). Furthermore, the level of potassium and phylloquinone in broccoli was determined after different durations of boiling.
Results: During the MK-7 intervention the dp-ucMGP and ucOC decreased significantly compared to baseline (− 0.42 [− 0.93; − 0.22] nmol/L (p=< 0.01) and − 1.85 [− 2.91; − 1.30] nmol/L (p< 0.01)), while these were unchanged during the dietary intervention (0.03 [− 0.64; 0.37] nmol/L (p=1.00) and 0.30 [− 1.71; 1.41] nmol/L (p=0.77)). Between the two interventions there was a greater decrease in ucOC (p=0.02) during the MK-7 compared to the dietary period. No significant changes in the total MGP levels were found in any of the periods. The retention of potassium following boiling for 2 minutes and 8 minutes was 76% and 49%, respectively, while for phylloquinone the retention was 92%, and independent of duration of boiling.
Conclusion: A daily MK-7 supplement for 6 weeks lowered dp-ucMGP and ucOC significantly, while a vitamin K rich diet was not able to induce any significant effect. Boiled broccoli maintains a reasonable content of phylloquinone while potassium is extracted and is a reasonable source of phylloquinone for patients on hemodialysis.
Keywords: hemodialysis, phylloquinone, menaquinone, nutrition
Introduction
Chronic kidney disease has become a global health problem with a significant mortality that increases with the progressive decline in estimated glomerular filtration rate (eGFR).1 Patients with end stage kidney disease have been shown to have low levels of vitamin K,2–4 which responds well to vitamin K supplementation.5–7 A low vitamin K intake is associated with increased risk of cardiovascular disease and vascular calcifications2,8 as well as an increased risk of bone fractures.9,10
Vitamin K is a fat-soluble vitamin that is found in three forms – phylloquinone (K1), menaquinone (K2), and menadione (K3).11 Phylloquinone is the main source of vitamin K in the diet and is present in green vegetables and certain oils, while menaquinone is found in cheeses and fermented foods as well as synthesized by bacteria in the large intestine.12 Menaquinone distinguishes itself by its number of isoprene units varying from 1–14. Menadione is a synthetic analog, but also an intermediate in the metabolism of phylloquinone to menaquinone-4 (MK4).13
Vitamin K is a coenzyme in the γ-carboxylation (activation) of proteins containing the amino acid glutamine (Glu) into γ-carboxyglutamate (Gla).14 In extrahepatic tissue vitamin K activates osteocalcin (OC) involved in bone mineralization15 and matrix Gla protein (MGP) which is considered an important inhibitor of vascular calcification.16–18 Dephosporylated-undercarboxylated MGP (dp-ucMGP) and undercarboxylated OC (ucOC) can be used as an indirect measure of vitamin K deficiency.6,19 Total MGP can also be used as an indicator of vitamin K status and includes the different isoforms of MGP (carboxylated, undercarboxylated, phosphorylated, and dephosphorylated).20,21
The cause of vitamin K deficiency amongst patients in dialysis was examined in a previous study of ours22 and found not to be caused by an overall decreased absorption in the gut or washout during dialysis. Low dietary intake of vitamin K has been indicated by previous studies.2,6 Patients in hemodialysis are often recommended to restrict their potassium and phosphate intake, consequently also limiting foods rich in vitamin K.2,23 Dysbiosis due to the uremic milieu may reduce the intestinal production of vitamin K24 and phosphate-binders may bind vitamin K in the gut and reduce the available vitamin K.25
The objective of this study was to compare the effect of a vitamin K rich diet to a daily MK-7 supplement on the vitamin K status measured by the plasma concentration of dp-ucMGP, total MGP, and ucOC in patients on hemodialysis. Furthermore, to investigate if vegetables rich in vitamin K conserve the content of vitamin K while extracting potassium during boiling.
Materials and Methods
Trial Design and Study Participants
A prospective randomized two-arm crossover intervention study. Patients included were ≥18 years old and in chronic hemodialysis treatment (≥3 months). Exclusion criteria were warfarin treatment, malabsorptive disease, and intake of vitamin K supplements. All patients provided informed consent according to the Declaration of Helsinki and the Municipal Regional Committee gave ethics approval for the investigation (no. H-17036789) and the study was approved by the Danish Authorities for Data Protection.
Interventions
Participants were allocated by randomization to 6 weeks of 360 μg MK-7/day or 6 weeks of a vitamin K rich diet as the initial intervention. This was followed by a 3-week washout period and afterwards they were crossed over to the opposite intervention. The order of the interventions was randomized by consecutive opening of sealed envelopes. The envelopes were packed by a third person not involved in the clinical study, according to a computer-generated randomization list. Blood samples were obtained at day 1 of the forthcoming intervention (baseline) and after 6 weeks for both interventions. During the MK-7 intervention the participants received a daily tablet of 360 μg MK-7 for 6 consecutive weeks. The tablets were provided by ORKLA AS, Ishøj, Denmark. The tablets contained synthetic MK-7 (K2VITAL®Delta) produced by Kappa BioScience AS, Oslo.26 Patients were instructed to take the tablet together with foods high in fat to ensure optimal absorption. For the diet intervention participants were given a list of vitamin K rich foods, and they were incited to increase their intake of these foods for the 6 weeks (Table 1). The intake of phosphorous binders were unchanged during the whole study period. The focus was on boiled vegetables, oils, cheese, and meat high in fat, and adjusting the habitual diet of a person in dialysis. The recommendations were individually modified with a dietician to consider their dietary restrictions. Plasma (p)-phosphate and p-potassium levels were monitored after 3 and 6 weeks of intervention to disclose any increase in p-phosphate or hyperkalemia.
 |
Table 1 List of Vitamin K Rich Foods Given to Participants |
The participants filled out a questionnaire regarding the degree of inconvenience of the two interventions. They were asked after each intervention to assess whether it had been easy, slightly difficult, difficult, very difficult, or even so difficult that they could not follow the protocol completely. No food frequency questionnaire was applied.
Blood samples were obtained before the dialysis session. Citrated plasma was prepared after standard centrifugation and stored at −80°C. The content of dp-ucMGP was measured in EDTA plasma at Odense University Hospital using IDS-iSYS inaKtif MGP analysis. The reference measure was 0.75 nmol/L.28 The content of total MGP and ucOC was measured at the Department of Medicine, University of Padova, Italy. The quantitative determination of MGP was performed using the Human MGP–Matrix GLA Protein kit (Cusabio-Pantec, Italy), a competitive ELISA method. For quantitative determination of the undercarboxylated osteocalcin, we used the Glu-OC EIA Kit MK118 (Takara Bio Inc., Otsu, Shiga, Japan), a solid phase EIA based on a sandwich method that utilizes two mouse monoclonal anti-ucOC antibodies to detect uc-OC by a two-step procedure.
P-25-OH-vitamin D and International Normalized Ratio (INR) derived from prothrombin time was measured by routine analyses at the central laboratory of Herlev Hospital (Atellica IM and MediRox Owrens PT).
The effect of the duration of boiling on the content of phylloquinone and potassium in cooked broccoli and the boiled water was investigated. A total of 100 g broccoli head divided into pieces of 50 millimeters was boiled in 500 mL water for 2 and 8 minutes. Each treatment was performed in triplicate, and raw broccoli was analyzed to calculate true retention. Samples of broccoli and boiled water were freeze-dried, homogenized, and vacuum packed, and stored at −20°C until analysis. The content of phylloquinone in the broccoli and water was determined using LC-APCI-MS/MS27 and the content of potassium using ICP-MS at the Technical University of Denmark (DTU).
Statistical Analysis
Sample size was estimated based on the changes in dp-ucMGP found by Westenfeld et al (α=0.05; β=0.20).29 In total, 18 patients were included. Data was presented as the mean and standard deviation (SD), median and interquartile range (IQR), or percentages, as appropriate. Differences between baseline values and 6 weeks as well as differences between the two interventions were assessed with the paired Wilcoxon Signed Rank Tests. The association between changes in plasma markers of vitamin K and level of 25-OH-vitamin D were explored by Pearson correlation. A p-value <0.05 was considered statistically significant. All statistical analyses were conducted using SPSS (v. 25, IBM, SPSS Statistics).
Results
Baseline Characteristics
A total of 12 participants were enrolled in the study from April 1, 2018 to June 31, 2018. Among them six participants were randomly allocated to start with the MK-7 intervention and six participants to start with the dietary intervention. In total 10 participants completed the MK-7 intervention and nine completed both interventions (Figure 1). The baseline demographic and clinical characteristics of the 10 participants completing the periods are listed in Table 2. Four people had a history of cardiovascular disease, including two cases of coronary heart disease, one case of cerebrovascular disease, two cases of peripheral artery disease, and all four had heart failure.
 |
Table 2 Characteristics of the 10 Participants |
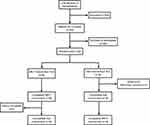 |
Figure 1 Flowchart of participants. A total of 12 patients were enrolled, whereas 10 participants completed the MK-7 intervention and nine completed both interventions. |
Effect of MK-7 Supplement and Vitamin K Rich Diet on Dp-ucMGP, Total MGP, and p-ucOC
The concentrations of dp-ucMGP, total MGP, and p-uc-OC at baseline and after 6 weeks are shown in Table 3. The baseline dp-ucMGP was more than 2-fold higher than the reference value of 0.75 nmol/L before both interventions.28 After 6 weeks of diet intervention there was no significant change in dp-ucMGP (p=1.00), total MGP (p=0.26), or ucOC (p=0.77). After 6 weeks of MK-7 supplementation there was no significant change in total MGP (p=0.24), but there was a significant decrease in dp-ucMGP (p=<0.01) and ucOC (p=<0.01). There was no difference in the changes of dp-ucMGP (p=0.14) or tMGP (p=0.68) between the interventions, but there was a greater decrease in ucOC (p=0.02) during the MK-7 period compared to the dietary period (Figure 2). The participant who only completed the MK-7 intervention was not included in the comparison between the two treatments. To examine if the washout period of 3 weeks was sufficient to prevent carry-over effects, the baseline values before both interventions were compared and showed no statistical difference for dp-ucMGP (p=0.59), total MGP (p=0.14), or ucOC (p=0.21).
 |
Table 3 Changes in Markers of Vitamin K Status |
Effect of MK-7 Supplement and Vitamin K Rich Diet on p-25-OH-Vitamin D and INR
To examine the effect of increased vitamin K intake on absorption of other fat-soluble vitamins, p-25-OH-vitamin D was measured. Baseline and post-intervention values were 96 [90; 127] nmol/L and 97 [77; 97] nmol/L (p=0.37) for the dietary period and 112 [87; 135] nmol/L and 81 [74–112] nmol/L (p=0.07) for the MK-7 period. There was no significant difference between the two interventions (−11 [−27; 3] nmol/L vs −16 [−35; −1] nmol/L, p=0.51). There was no correlation between changes in plasma dp-ucMGP (r=0.235; p=0.576), total MGP (r=0.443; p=0.272), or ucOC (r=−0.216; p=0.602) and changes in 25-OH-vitamin D.
As a safety measurement, INR was measured at baseline and after the two interventions to examine a potential change in blood coagulation due to increased vitamin K intake. Baseline and post-intervention values were 0.9 [0.9; 1] and 0.9 [0.9; 0.9] (p=0.08) for diet and 0.9 [0.9; 0.9] and 0.9 [0.9; 0.9] (p=1.00) for MK-7. There was no significant difference between the two interventions (0 [−0.1; 0] vs 0 [−0.1; 0.1], p=0.53).
Assessment of Difficulty of the Intervention and Safety Monitoring
Participants were given a questionnaire to assess the difficulty of the intervention after completion and 80% rated the MK-7 intervention as easy versus only 10% for the dietary intervention. In total, 40% of participants rated the dietary intervention as difficult or very difficult versus 0% in the MK-7 intervention period. The dietary intervention was found so difficult by 30% of the participants that it could not be followed completely, compared to 0% of the participants reported to be unable to follow the MK-7 intervention.
No harmful increase in p-potassium or p-phosphate was observed throughout the diet period (data not shown).
The Effect of Boiling on the Level of Phylloquinone and Potassium in Broccoli
Potassium and phylloquinone content in broccoli and water after boiling is shown in Table 4. The concentration of phylloquinone and of potassium was lower in boiled broccoli, and for potassium even lower after 8 minutes than 2 minutes of boiling. During boiling the broccoli absorbed water. For both micronutrients the true retention in the boiled broccoli was significantly different from 100% (p<0.01 for phylloquinone; p<0.0001). Phylloquinone in boiled water was negligible (below limit of quantification), whereas potassium diluted in the water during boiling, and a concentration of potassium of up to 33% of the content in broccoli after 8 minutes boiling was observed.
 |
Table 4 Phylloquinone and Potassium Content in Broccoli and Boiled Water After 2 and 8 Minutes of Boiling |
Discussion
This pilot study compared the influence of a vitamin K rich diet or supplementation with tablets containing 360 µg of vitamin K2 on the indirect markers of vitamin K status in a randomized 6-week cross-over trial in patients on hemodialysis. The main findings were a significant decrease in dp-ucMGP and ucOC after 6 weeks of daily MK-7 supplementation, while a diet rich in vitamin K had no significant effect on these parameters. The effect of boiling on the content of vitamin K in broccoli was explored. While potassium was washed out during boiling, vitamin K was preserved in the broccoli.
In the intervention study, the baseline levels of dp-ucMGP were within the same range as previously found amongst patients on hemodialysis.5,20,30 This is more than 2-times higher than the levels found in the general population28 and, as such, the participants were severely vitamin K deficient. After 6 weeks of MK-7 intervention there was a significant decrease in dp-ucMGP, which is consistent with previous studies,5,6 while no significant change was detected during the dietary intervention, indicating a higher effectiveness of the tablet supplementation than the dietary intervention. The dose of MK-7 used seemed appropriate as dp-ucMGP became close to the levels found in the general population after the 6 weeks of supplementation. The recommended diet consisted of food items both containing phylloquinone and menaquinones. These foods items mostly contained phylloquinone. Dietary intervention with food rich in menaquinone, such as the Japanese natto,31 may have had a more pronounced effect on the vitamin K status. However, such a dietary recommendation was considered impossible in a Danish population, as this is not a part of the usual Danish diet.
The median ucOC at baseline was lower than previously described in patients on hemodialysis, but higher than in the healthy population.2,21 There was a decrease in ucOC after 6 weeks for both the dietary and the MK-7 intervention, though, only statistically significant for the MK-7 intervention. There was also a significantly larger decrease in ucOC during the MK-7 intervention compared to the dietary intervention. This also points towards a higher effectiveness of MK-7 supplementation. Vitamin K intake is negatively correlated with ucOC32 and ucOC is used as an indirect indicator of vitamin K status. However, this value reflects a recent vitamin K intake rather than long-term status and it is affected by both PTH and vitamin D which are often severely changed in terms of hyperparathyroidism and deficiency of active vitamin D in patients on dialysis.33,34 As such, the level of ucOC could have been affected by other parameters than Vitamin K.
The level of t-MGP has previously been found to be significantly higher amongst dialysis patients compared to healthy controls and found to be associated to cardiovascular disease.21,35 It is proposed that the increased levels of t-MGP represent increased levels of inactive dp-ucMGP.35 Our study showed no significant effect of neither intervention on t-MGP and no significant difference between the interventions. As t-MGP reflects the total amount of dp-uc MGP and carboxylated MGP (cMGP) the present findings probably reflect a shift from dp-ucMGP towards active carboxylated MGP during the MK-7 intervention, but no change in the total amount of circulating MGP. One study with only a 2-week intervention period found a significant decrease in t-MGP in young healthy males given a diet rich in corn oil, perhaps due to a larger number of participants.36 To our knowledge this is the first study to investigate the effect of vitamin K supplementation on t-MGP amongst patients on hemodialysis treatment.
Vitamin K is a fat-soluble vitamin and could have a competing effect on the absorption of other fat-soluble vitamins such as vitamin D. Although there was no significant change in 25-OH-vitamin D during either intervention the MK-7 supplementation period did result in a decrease from 112 to 81 nmol/L (p=0.07) in the levels of 25-OH-vitamin D. Our sample size is very small and possibly skewed and a link between vitamin K and D should be investigated in a larger study. Furthermore, the influence of vitamin K supplementation on the absorption of vitamin D should be explored in studies with a longer follow-up as the half-life of 25-OH vitamin D is around 21 days. Therefore, steady state in the level of 25-OH-vitamin D may not have been reached during 6 weeks of intervention. All participants had 25-OH-vitamin D values above the reference value of >50 nmol/L37 at both baseline and after intervention, probably due to 50% of the participants receiving native vitamin D. Therefore, it was not due to low vitamin D status that no significant change was detected. In future larger studies the vitamin D status should be monitored during longer periods of MK-7 supplementation.
The results from the boiling of broccoli confirms that potassium is washed out during boiling while phylloquinone shows a high true retention of 92±5%, which is independent of boiling time. The degree of washout of potassium depend on boiling time. It has previously been shown that boiling is effective in potassium removal in vegetables,39,40 our results differ as the cooking procedure was intended to simulate gently and over-boiled broccoli. And to our knowledge the phylloquinone content and the retention after boiling has not previously been examined.
The study has a pragmatic design, making it possible to conduct in a routine practical setting, but entails several limitations. The main limitation was the underpower of the study. A larger sample size may have detected significant changes in the vitamin K status during the dietary intervention as well as found during the MK-7 intervention. Another limitation was the lack of an untreated control group to examine the natural change of dp-ucMGP and ucOC over time. However, the cross-over design eliminated interindividual variation as the participants were their own controls. The participants found the dietary intervention difficult and the tablet intake easy, which may limit the applicability of a vitamin K rich diet at least in the Danish dialysis population, or populations with similar dietary habits. The lacking effect of the diet intervention could preferences when preparing the meals. A dislike towards strongly boiled vegetables is common. There are probably many gastronomic possibilities for a better compliance to longer cooking times, although this potentially could cause new problems with the content of water-soluble vitamins. MK-7 supplementation was chosen due to the longer half-life and increased bioavailability compared to phylloquinone.38 The results may have differed if K1 supplementation had been provided instead of MK-7. A final limitation was the lack of assessment of the exact amount of vitamin K intake during the dietary intervention. The study aimed to examine feasibility of diet intervention compared to MK-7 tablets. Consequently, a comparison was made between unspecified doses of multiple forms of vitamin K in the diet and a specified dose of MK-7. In a future study precise diet registration should be made before and during such intervention. Furthermore, circulating levels of the different vitamin K forms should be measured throughout to determine if participants actually increase their intake of vitamin K and to confirm that the MK-7 supplement is consumed.
Conclusion
This pilot study suggested a significant effect of MK-7 supplementation on vitamin K deficiency for patients on hemodialysis, while dietary advice and instruction of a vitamin K rich dietary intake did not have the same effect on the vitamin K deficiency. Boiling vegetables such as broccoli for a longer period is a possible source of phylloquinone for patients on dialysis respecting the potassium restrictions.
Institutional Review Board Statement
All patients provided informed consent according to the Declaration of Helsinki and the Municipal Regional Committee gave ethics approval for the investigation (no. H-17036789) and the study was approved by the Danish Authorities for Data Protection.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgment
This paper was presented at the 41st ESPEN Congress (August 8 to September 9, 2019) as an abstract with interim findings. The poster’s abstract was published in “Poster Abstracts” in Clinical Nutrition 2019;38:S228.
Funding
This research was funded by The A.P. Møller Foundation for the Advancement of Medical Science (grant no. 17-L-0237) as well as a Student Grant from the Department of Nutrition, Exercise and Sports, University of Copenhagen (grant no. JRA-17-98). Orkla Care© provided the tablets, with no restrictions on the study design, execution, data analyses, or presentation.
Disclosure
The authors declare no conflict of interest.
References
1. Go AS, Chertow GM, Fan D, Mcculloch CE, Hsu C-Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi:10.1056/NEJMoa041031
2. Cranenburg ECM, Schurgers LJ, Uiterwijk HH, et al. Vitamin K intake and status are low in hemodialysis patients. Kidney Int. 2012;82(5):605–610. doi:10.1038/ki.2012.191
3. Cranenburg ECM, Koos R, Schurgers LJ, et al. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost. 2010;104(4):811–822. doi:10.1160/TH09-11-0786
4. Fusaro M, D’Alessandro C, Noale M, et al. Low vitamin K1 intake in haemodialysis patients. Clin Nutr. 2017;36(2):601–607. doi:10.1016/j.clnu.2016.04.024
5. Caluwé R, Vandecasteele S, Van Vlem B, Vermeer C, De Vriese AS. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29(7):1385–1390. doi:10.1093/ndt/gft464
6. Aoun M, Makki M, Azar H, Matta H, Chelala DN. High Dephosphorylated-Uncarboxylated MGP in Hemodialysis patients: risk factors and response to vitamin K2, A pre-post intervention clinical trial. BMC Nephrol. 2017;18(1). doi:10.1186/s12882-017-0609-3
7. Kaesler N, Magdeleyns E, Herfs M, et al. Impaired vitamin K recycling in uremia is rescued by vitamin K supplementation. Kidney Int. 2014;86(2):286–293. doi:10.1038/ki.2013.530
8. Geleijnse JM, Vermeer C, Grobbee DE, et al. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr. 2004;134(11):3100–3105. doi:10.1093/jn/134.11.3100
9. Apalset EM, Gjesdal CG, Eide GE, Tell GS. Intake of vitamin K1 and K2 and risk of Hip fractures: the Hordaland Health Study. Bone. 2011;49(5):990–995. doi:10.1016/j.bone.2011.07.035
10. Nakano T, Tsugawa N, Kuwabara A, Kamao M, Tanaka K, Okano T. High prevalence of hypovitaminosis D and K in patients with Hip fracture. Asia Pac J Clin Nutr. 2011;20(1):56–61.
11. Coombs GF. The Vitamins: Fundamental Aspects in Nutrition and Health.
12. Shearer MJ, Bach A, Kohlmeier M. Chemistry, nutritional sources, tissue distribution and metabolism of vitamin K with special reference to bone health. J Nutr. 1996;126(suppl_4):1181S–1186S. doi:10.1093/jn/126.suppl_4.1181S
13. Hirota Y, Tsugawa N, Nakagawa K, et al. Menadione (vitamin K3) is a catabolic product of oral phylloquinone (vitamin K1) in the intestine and a circulating precursor of tissue menaquinone-4 (vitamin K2) in rats. J Biol Chem. 2013;288(46):33071–33080. doi:10.1074/jbc.M113.477356
14. Vermeer C. γ -carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase. Biochem J. 1990;266(3):625–636. doi:10.1042/bj2660625
15. Ducy P, Desbois C, Boyce B, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382(6590):448–452. doi:10.1038/382448a0
16. Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protien. Nature. 1997;386(6620):78–81. doi:10.1038/386078a0
17. Gast GCM, de Roos NM, Sluijs I, et al. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metabol Cardiovasc Dis. 2009;19(7):504–510. doi:10.1016/j.numecd.2008.10.004
18. Cozzolino M, Fusaro M, Ciceri P, Gasperoni L, Cianciolo G. The Role of Vitamin K in Vascular Calcification. Adv Chronic Kidney Dis. 2019;26:437–444. doi:10.1053/j.ackd.2019.10.005
19. Fusaro M, Crepaldi G, Maggi S, et al. Vitamin K, bone fractures, and vascular calcifications in chronic kidney disease: an important but poorly studied relationship. J Endocrinol Invest. 2011;34(4):317–323. doi:10.1007/BF03347093
20. Delanaye P, Krzesinski J-M, Warling X, et al. Dephosphorylated-uncarboxylated Matrix Gla protein concentration is predictive of vitamin K status and is correlated with vascular calcification in a cohort of hemodialysis patients. BMC Nephrology. 2014;15(1). doi:10.1186/1471-2369-15-145
21. Fusaro M, Noale M, Viola V, et al. Vitamin K, vertebral fractures, vascular calcifications, and mortality: vitamin K Italian (VIKI) Dialysis Study. J Bone Miner Res. 2012;27(11):2271–2278. doi:10.1002/jbmr.1677
22. Wikstrøm S, Lentz KA, Hansen D, et al. Causes of vitamin K deficiency in patients on haemodialysis. Nutrients. 2020;12(9):1–8. doi:10.3390/nu12092513
23. Beto JA, Bansal VK. Medical nutrition therapy in chronic kidney failure: integrating clinical practice guidelines. J Am Diet Assoc. 2004;104(3):404–409. doi:10.1016/j.jada.2003.12.028
24. Evenepoel P, Dejongh S, Verbeke K, Meijers B. The role of gut dysbiosis in the bone-vascular axis in chronic kidney disease. Toxins. 2020;12(5):285. doi:10.3390/toxins12050285
25. Jansz TT, Neradova A, Van Ballegooijen AJ, et al. The role of kidney transplantation and phosphate binder use in vitamin K status. PLoS One. 2018;13(8):e0203157. doi:10.1371/journal.pone.0203157
26. Levy-Schousboe K, Frimodt-Møller M, Hansen D, et al. Vitamin K supplementation and arterial calcification in dialysis: results of the double-blind, randomised, placebo-controlled RenaKvit trial. Clin Kidney J. 2021;14(9):2114–2123. doi:10.1093/ckj/sfab017
27. Jäpelt RB, Jakobsen J. Analysis of vitamin K1 in fruits and vegetables using accelerated solvent extraction and liquid chromatography tandem mass spectrometry with atmospheric pressure chemical ionization. Food Chem. 2016;192:402–408. doi:10.1016/j.foodchem.2015.06.111
28. Usermanual. IDS. InaKtif MGP (dp-ucMGP) Immunoassay. IDS-iSYS InaKtif MGP (dp-ucMGP); 2017 [cited June 4, 2018]. Available from: https://www.idsplc.com/products/inaktif-mgp-dp-ucmgp-2/.
29. Westenfeld R, Krueger T, Schlieper G, et al. Effect of vitamin K 2 supplementation on functional vitamin K deficiency in hemodialysis patients: a randomized trial. Ame J Kidney Dis. 2012;59(2):186–195. doi:10.1053/j.ajkd.2011.10.041
30. Kurnatowska I, Grzelak P, Masajtis-Zagajewska A, et al. Plasma desphospho-uncarboxylated matrix gla protein as a marker of kidney damage and cardiovascular risk in advanced stage of chronic kidney disease. Kidney Blood Press Res. 2016;41:231–239. doi:10.1159/000443426
31. Tsukamoto Y, Ichise H, Kakuda H, Yamaguchi M. Intake of fermented soybean (natto) increases circulating vitamin K2 (menaquinone-7) and γ-carboxylated osteocalcin concentration in normal individuals. J Bone Miner Metab. 2000;18(4):216–222. doi:10.1007/s007740070023
32. Yamauchi M, Yamaguchi T, Nawata K, Takaoka S, Sugimoto T. Relationships between undercarboxylated osteocalcin and vitamin K intakes, bone turnover, and bone mineral density in healthy women. Clin Nutr. 2010;29(6):761–765. doi:10.1016/j.clnu.2010.02.010
33. Fusaro M, Gallieni M, Rizzo MA, et al. Vitamin K plasma levels determination in human health. Clin Chem Lab Med. 2017;55:789–799. doi:10.1515/cclm-2016-0783
34. Szulc P, Chapuy MC, Meunier PJ, Delmas PD. Serum undercarboxylated osteocalcin is a marker of the risk of Hip fracture in elderly women. J Clin Invest. 1993;91(4):1769–1774. doi:10.1172/JCI116387
35. Mizuiri S, Nishizawa Y, Yamashita K, et al. Relationship of matrix Gla protein and vitamin K with vascular calcification in hemodialysis patients. Ren Fail. 2019;41(1):770–777. doi:10.1080/0886022X.2019.1650065
36. Schurgers LJ, Shearer MJ, Soute BAM, et al. Novel effects of diets enriched with corn oil or with an olive oil/sunflower oil mixture on vitamin K metabolism and vitamin K-dependent proteins in young men Novel ef-fects of diets enriched with corn oil or with an olive oil/sunflower oil mixture on vitamin K metabolism and vitamin K-dependent proteins in young men Supplementary key words vitamin E • vegetable oils • gammacarboxy glutamate • blood coagulation • healthy volunteers. J Lipid Res. 2002;43(6):878–884.
37. Official JOurnal Of the internatiOnal SOciety Of nephrOlOgy KDIGO. Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2012;2013(3):1–50.
38. Schurgers LJ, Teunissen KJF, Hamulyák K, Knapen MHJ, Vik H, Vermeer C. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. 2007;109(8):3279–3283. doi:10.1182/blood-2006-08-040709
39. Kimura M, Itokawa Y. Cooking losses of minerals in foods and its nutritional significance. J Nutr Sci Vitaminol. 1990;36:S25–S33.
40. Cupisti A, Kovesdy CP, Kalantar-Zadeh K, Kalantar-Zadeh K. Dietary approach to recurrent or chronic hyperkalaemia in patients with decreased kidney function. Nutrients. 2018;10(3):261. doi:10.3390/nu10030261
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

