Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 16
Time to Diabetic Nephropathy and its Predictors Among Diabetic Patients Treated in Wolaita and Dawuro Zone Hospitals, Ethiopia: A Retrospective Cohort Study
Authors Tekalign T, Guta MT, Awoke N , Chichiabellu TY , Meskele M , Anteneh G, Tura TS , Workie SB
Received 2 December 2022
Accepted for publication 19 May 2023
Published 12 June 2023 Volume 2023:16 Pages 163—172
DOI https://doi.org/10.2147/IJNRD.S396574
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Tiwabwork Tekalign,1 Mistire Teshome Guta,2 Nefsu Awoke,2 Tesfaye Yitna Chichiabellu,2 Mengistu Meskele,3 Gubay Anteneh,4 Tilahun Saol Tura,2 Shimelash Bitew Workie3
1School of Nursing, College of Medicine and Health Science, ArbaMinch University, ArbaMinch, Ethiopia; 2School of Nursing, College of Health Science and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia; 3School of Public health, College of Health Science and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia; 4School of Medicine, College of Health Science and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
Correspondence: Tiwabwork Tekalign, Email [email protected]
Background: Diabetic kidney disease (DKD) develops in nearly half of patients with type 2 diabetes mellitus (DM) and one-third of those with type 1 DM during their lifetime. The incidence of DKD as a cause of end stage renal disease is increasing each year. So this study aimed to assess the time to develop diabetic nephropathy and predictors among diabetic patients treated in Wolaita zone hospitals.
Methodology: A ten-year retrospective cohort study had conducted among 614 diabetic patients using systematic random sampling in Wolaita and Dawuro zone hospitals. Bivariable and multivariable Cox proportional hazards regression had used to identify the possible associations between variables. Those variables with a p-value of less than 0.25 in bivariable analysis exported to multivariable Cox regression analysis. Finally, variables with p-value less than 0.05 at multivariable Cox regression were considered significantly significant. The Cox-proportional hazard model assumption had checked using the Schoenfeld residual test.
Results: Of the total participants, 93 (15.3%; 95% CI = 12.45– 18.14) patients had developed nephropathy in 820,048 people year observation. A mean time to diabetic nephropathy in this study was 189.63 (95% CI, 185.01, 194.25) months. Being illiterate (AHR: 2.21, 95% CI: 1.34– 3.66), being hypertensive (AHR: 5.76, 95% CI: 3.39– 9.59), and being urban dwellers (AHR: 2.25, 95% CI: 1.34– 3.77) increases the hazard of nephropathy.
Conclusion: According to this follow-up study, the overall incidence rate is substantially high over ten year follow-up period. The mean time to develop diabetic nephropathy was sixteen years. Educational status, place of residence, and being hypertensive were the predictors. So stakeholders should work on complication reduction measures and awareness creation of the impact of comorbidities.
Keywords: chronic disease, complication, survival analysis, longitudinal study, high blood sugar
Background
Diabetic kidney disease (DKD) is a progressive increase in albuminuria or/and a glomerular filtration rate less than 60 mL/min/1.73 m2. It occurs as common complications in both types of diabetes. Studies show it is the leading cause of end-stage renal disease (ESRD). Diabetes-related hyperglycemia affects kidney function and alters the usual process of removal of waste products and excess fluid from the body.1–3
Kidney disease attributed to diabetes is a major but under-recognized contributor to the global burden of disease. In 2017, the global prevalence of chronic kidney disease (CKD) was greater than 10%, which are approximately 800 million cases.4,5 In Ethiopia according to systematic review and Meta-analysis the prevalence of CKD was 21.71%.6 DKDdevelops in almost half of patients with type 2 diabetes mellitus (DM) and one-third of those with type 1 DM during their lifetime. It will also increase with age in both men and women.7,8
The incidence of DKD as a cause of ESRD is increasing each year. Also, a considerable number of patients who develops ESRD require renal replacement therapies (dialysis and/or transplantation). It is estimated that by the year 2030, more than 70% of patients will be residents of developing countries.9–11
Current findings show that the prevalence of DM is rapidly increasing, as a result, the number of complications that affect patients’ quality of life, health service demand, and economic costs will also increase.12,13
DM is identified as a leading cause of ESRD. Several studies have found that its burden is higher than that of other chronic illnesses because more money is spent on treating and managing its complications.14–16 Therefore, this study is aimed at assessing the time to develop diabetic nephropathy and its predictors among diabetic patients treated in Wolaita and Dawuro zone hospitals.
Specific Objectives
- To determine time to develop diabetic nephropathy among diabetic patients treated in Wolaita and Dawuro zone hospitals
- To identify the predictors of diabetic nephropathy among diabetic patients treated in Wolaita and Dawuro zone hospitals
Materials and Methods
Study Setting and Period
The study was carried out at public hospitals in the Dawuro and Wolaita zones. They have located about 328 km and 515 km south of Addis Ababa respectively. There are a total of six hospitals (one governmental teaching and referral hospital and five governmental primary hospitals) and 68 health centers found in the Wolaita zone; and three hospitals (one governmental general hospital, two governmental primary hospitals) and 21 health centers in the Dawuro zone. The study was conducted from January 1–30, 2022.
Study Design
An institution-based retrospective cohort study was conducted and strengthening the Reporting of Observational Studies in Epidemiology (STROBE) is used while reporting the study.
Source Participants
The study participants were all medical records of patients who were attending a diabetic clinic from September 1, 2012 to August 30, 2021.
Study Participants
The study participants were selected medical records of patients who were attending a diabetic clinic from September 1, 2012 to August 30, 2021.
Inclusion and Exclusion Criteria
Medical records of diabetic patients who were in follow-up in two zones from September 1, 2012 to August 30, 2021 were included in this study. While medical records of pregnant women, initially presenting with any renal complication, patient charts with no baseline records or those patient cards with incomplete charts or diagnosis year or month missing and patients who had a history of renal complication from other underlying causes will be excluded.
Operational Definitions
Time to Diabetic Nephropathy
It was the time starting from the patient has confirmed as a diabetic patient to the development of a renal complication.
Censored
Patients who were not experiencing renal complications until the end of the study or died or were lost to follow-up before experiencing renal complications within the study period.
Right Censoring
When a subject’s follow-up time terminates before the outcome of interest is observed.
Left Censoring
Observed when an individual had developed the event of interest before the beginning of the study.
Event
The development of a renal complication.
Diabetic Nephropathy
Defined as an estimated Glomerular Filtration Rate (eGFR) < 60 mL/min/1.73 m2 estimated by the Cockcroft-Gault equation.
Sampling Technique and Sample Size Determination
The sample size was determined by using a double population proportion using the previous study as a reference.17–20 By assuming a confidence level of 95%, a power of 90% and by adding 10% for the non-response rate, the final sample size was 614.
Sampling Technique and Procedures
Medical records of diabetic patient were filtered first from the DM registration book according to their entry time and then the samples were proportionally allocated to each hospital according to their total patients. A systematic random sampling technique was used.
Variables of the Study
Dependent Variable
“Time” (Survival time to develop renal complication in months) and “Status” (eGFR) < 60 mL/min/1.73 m2 = 1, Censored = 0)
The Independent Variables
- Socio-demographic characteristics (Sex, Residence, Age of patients, Marital status, Level of education, Duration of diagnosis)
- Health history (Diabetic types, Family history, Blood pressure, Weight, Height, Fasting Blood Sugar [FBS], hemoglobin A1c, HDL, LDL)
- Comorbidity (Heart disease, Pulmonary disease, Other chronic diseases)
- Behavioral factors (Alcohol use, Tobacco use)
Data Collection
Patients’ follow-up cards, DM registration books and computer files were used to collect the data by using structured data extraction checklists by nurses. To ensure the quality of data, the filled sheet was checked for completeness and consistency by supervisors and the principal investigator. Cross-checking was done while the data entry and clarifying any missing data were also done.
Data Processing and Analysis
The data was collected by ODK application and exported to STATA version 14 for analysis. Descriptive statistics like proportion, percentages, means, and standard deviation was calculated. Kaplan Meier survival curve jointly with the Log rank test was done to estimate the survival curve and for the presence of a difference in survival among independent variables. Bivariable and multivariable Cox proportional hazards regression were used to identify the possible associations between independent and outcome variables. Variables with p-value lower than 0.25 during bivariable analysis were exported to multivariable Cox-regression analysis. Finally, variables with p-value less than 0.05 at multivariable Cox-regression were considered as significant. The model assumption was checked using the Schoenfeld residual test.
Data Quality Assurance
Training was provided to data collectors and the supervisor. The questionnaire was initially prepared in English language, and then translated into Amharic, and back to English to verify its consistency. The collected data were reviewed on-site for completeness and consistency daily. Also pre-test was conducted on 5% of the sample size on patients before the year of study period.
Ethical Clearance
Ethical approval is obtained from the institutional review board (IRB) of Wolaita Sodo University College of Health Science and Medicine (ref. No. CRCSD/27/02/2014) and conducted per the Declaration of Helsinki. The patient data was kept confidential. Informed consent was exempt from institutional review board of Wolaita Sodo university due to the retrospective nature of the study.
Result
Socio-Demographic Characteristics
A total of 614 cards were reviewed and included in the analysis. The mean age of the participants was 44.61 with SD ± 13.64 years. More than half of the study participants (365 [59.4%], 333 [54.2], and 353 [57.5%]) were male, unemployed and rural dwellers, respectively. Most of the diabetic patients (532 [86.4%] and 427 [69.5%]) were ever married and literate (Table 1).
 |
Table 1 Socio-Demographic Characteristics Among Diabetic Patients Treated in Wolaita and Dawuro Zone Hospitals, Ethiopia (n = 614) |
Health History of the Participants
Accordingly, the majority of participants ((581 [94.6%] 561 [91.3%], and 539 [87.7%]) do not have comorbid hypertension, are non-smokers, and do not have an alcoholism history, respectively. More than half (346 [56.4%]), near to half (302 [49.2%]), and more than three-quarters (475 [77.4%]) of patients take oral hypoglycemic agents, are in the normal weight range, and type 2 DM patients, respectively. Diabetic retinopathy and foot ulcers were seen among 402 (65.5%) and 26 (4.2%) patients, respectively. Additionally, the study participants had a mean of = 196.82, SD ±103.57, 131.25, SD ±19.69, and 79.33, SD±10.28 fasting blood glucose, systolic blood pressure, and diastolic blood pressure respectively (Table 2).
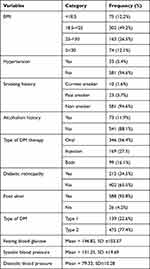 |
Table 2 Health History of Diabetic Patients Treated in Wolaita and Dawuro Zone Hospitals, Ethiopia (n = 614) |
Diabetic Nephropathy Mean Time and Test Equality Among Different Covariates
The Log rank test was performed to test the equality of diabetic nephropathy survival curves for the presence of any significant differences among categorical variables. Accordingly, the mean nephropathy time for female diabetic patients was longer than males (195 months) and it was statistically significant with (p-value = 0.001). Also never married diabetic patients had longer 10-years overall nephropathy-free survival time than ever married patients (199 vs. 187 months, with a P-value of 0.0035). Those who are living in rural areas live longer free of nephropathy than those living in urban areas (199 vs. 187 months with a P-value of 0.001). Those patients having comorbid hypertension had shorter nephropathic-free survival times than their counterparts. This difference was statistically significant p-value = 0.000) (Table 3).
 |
Table 3 Diabetic Nephropathy Overall Survival Rates and Mean Time to Nephropathy Among Diabetic Patients Treated in Wolaita and Dawuro Zone Hospitals, Ethiopia (n = 614) |
Incidence of Diabetic Nephropathy
Of the total participants, 93 (15.3%; 95% CI = 12.45–18.14) developed nephropathy in 820,048 person year-long observation. The incidence rate of diabetic nephropathy was 1.14 per 10,000 people over a 10 year follow-up period. In the present study, diabetes clients were followed up for a total of 120 months, with a mean time to develop nephropathy being 189.63 months (95% CI, 185.01, 194.25) (Figure 1).
 |
Figure 1 KMC mean time to develop nephropathy among diabetic patients treated in Wolaita and Dawuro zone hospitals, Ethiopia (n = 614) June; 2022. |
Predictors of Diabetic Nephropathy
At first, nine variables with a P-value of <0.25 become a candidate for multivariable Cox-regression; then three variables, educational status, residence, and being hypertensive become the predictors of nephropathy. Illiterate patients were at a two times higher risk of diabetic nephropathy as compared to literate (AHR: 2.21, 95% CI: 1.34–3.66). Hypertensive patients were at a six times higher risk of diabetic nephropathy as compared to their counterparts (AHR: 5.76, 95% CI: 3.39–9.59). Patients who were living in urban areas were at a two times higher risk of diabetic nephropathy as compared to rural dwellers (AHR: 2.25, 95% CI: 1.34–3.77) (Table 4).
 |
Table 4 Factors Associated with Diabetic Nephropathy Among Diabetic Patients Treated in Wolaita and Dawuro Zone Hospitals, Ethiopia (n = 614) June; 2022 |
Discussion
According to this cohort study, the overall incidence rate of diabetic nephropathy during 820,048 person-year observation was 1.14 per 10,000 person-years, which is lower than the study conducted in Addis Ababa17 (3.6 per 100), Jimma (2.29 per 1000),21 and Gonder (14 per 10,000).22 This difference is due to sampling size, the demographic nature of the participants, the type of food they consume, and the urbanization level of the cities.
Whereas of the total participants, 15.3% of patients had developed nephropathy over 10 year follow-up period; nearly similar to the Jimma study (15.56%).21 It is lower than a study conducted in Addis Ababa (19.65%),17 this may be due to people living in more urbanized cities being prone to a sedentary way of life and as a result, it predisposes them to early complications of chronic disease.
But higher than the study of Harari (14.5%)23 and Addis Ababa (14.25%),19 this might be the difference in socio-demographic characteristics and socioeconomic characteristics of the study participants.
Accordingly, more than half of the patients were not experiencing diabetic nephropathy until the end of the study. The mean time to develop nephropathy in this study was 189.63 (95% CI, 185.01, 194.25) months. This is different from previous studies, this might be the duration of the study, and the period of the study matters for the development of a long-term complication.
In this study educational status of diabetic patients has an association with the development of a complication. Illiterate patients were two times higher risk of diabetic nephropathy as compared to literate (AHR: 2.21, 95% CI: 1.34–3.66).
A different study finding has indicated that educational status is one risk factor for chronic diseases and their complications.24,25
The multi-morbidity accelerates the occurrence of chronic disease complications and vice-versa. Hypertensive patients were at a six times higher risk of diabetic nephropathy as compared to their counterparts (AHR: 5.76, 95% CI: 3.39–9.59). This is consistent with the findings of the study done in Jimma21 and the Amhara region.26
Hypertension and CKD are closely interlinked patho-physiological states; sustained hypertension can lead to worsening kidney function, and progressive decline in kidney function can conversely lead to worsening blood pressure (BP) control.27,28
Place of residence has a link with the incidence of complications. According to this study Patients who were living in urban areas were at a two times higher risk of diabetic nephropathy as compared to rural dwellers (AHR: 2.25, 95% CI: 1.34–3.77). Different studies also showed that the urbanization level was positively associated with physical inactivity and increases the risk of being overweight; resulting in chronic disease.29,30
Limitations
We face difficulty to generalize the finding because of only hospitals from two zones’ was taken from the region, due to budget constraints and some important blood tests are missed in some hospitals.
Conclusion
According to this follow-up study the overall incidence rate is substantially high over ten years follow up period. The mean time to develop renal complication was 16 years. Educational status, place of residence, and being hypertensive were the predictors. So stake holders should work of complication reduction measures and awareness creation of impact of comorbidities.
Data Sharing Statement
The data sets used during the current study are available from the corresponding author on reasonable request.
Acknowledgment
We would like data collectors, and Wolaita Sodo University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors received funding from Wolaita Sodo University.
Disclosure
All the authors declare that they have no competing interests.
References
1. Idowu AA, Ajose AO, Adedeji AT, Adegoke AO, Jimoh KA. Microalbuminuria, other markers of nephropathy and biochemical derangements in type 2 diabetes mellitus: relationships and determinants. Ghana Med J. 2017;51(2):56–63.
2. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA consensus conference. Diabetes Care. 2014;37(10):2864–2883. doi:10.2337/dc14-1296
3. Hussain S, Jamali MC, Habib A, Hussain MS, Akhtar M, Najmi AK. Diabetic kidney disease: an overview of prevalence, risk factors, and biomarkers. Clin Epidemiology Glob Health. 2021;9:2–6. doi:10.1016/j.cegh.2020.05.016
4. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. doi:10.2215/CJN.11491116
5. Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022;12(1):7–11. doi:10.1016/j.kisu.2021.11.003
6. Animaw Z, Walle Ayehu G, Abdu H. Prevalence of chronic kidney disease and associated factors among patients with chronic illness in Ethiopia: a systematic review and meta-analysis. SAGE Open Med. 2022;10:20503121221089442. doi:10.1177/20503121221089442
7. Hoogeveen EK. The epidemiology of diabetic kidney disease. Kidney Dialysis. 2022;2(3):433–442. doi:10.3390/kidneydial2030038
8. Duan J, Wang C, Liu D, et al. Prevalence and risk factors of chronic kidney disease and diabetic kidney disease in Chinese rural residents: a cross-sectional survey. Sci Rep. 2019;9(1):1. doi:10.1038/s41598-019-46857-7
9. Parving HH. Diabetic nephropathy: prevention and treatment. Kidney Int. 2001;60(5):2041–2055. doi:10.1046/j.1523-1755.2001.00020.x
10. Gheith O, Othman N, Nampoory N, Halimb MA, Al-Otaibi T. Diabetic kidney disease: difference in the prevalence and risk factors worldwide. J Egypt Soc Nephrol Transplant. 2016;16(3):65. doi:10.4103/1110-9165.197379
11. Yirsaw BD. Chronic kidney disease in sub-Saharan Africa: hypothesis for research demand. Ann Afr Med. 2012;11(2):119–120. doi:10.4103/1596-3519.93537
12. Atun R, Davies JI, Gale EA, et al. Diabetes in sub-Saharan Africa: from clinical care to health policy. Lancet Diabetes Endocrinol. 2017;5(8):622–667.
13. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
14. Habebo TT, Pooyan EJ, Mosadeghrad AM, Babore GO, Dessu BK. Prevalence of poor diabetes self-management behaviors among Ethiopian diabetes mellitus patients: a systematic review and meta-analysis. Ethiop J Health Sci. 2020;30:4.
15. World Health Organization. Management of non-communicable diseases; 2021. Available from: https://www.who.int/activities/management-of-noncommunicable-diseases.
16. Choukem SP, Dzudie A, Dehayem M, et al. Comparison of different blood pressure indices for the prediction of prevalent diabetic nephropathy in a sub-Saharan African population with type 2 diabetes. Pan Afr Med J. 2012;11:937–8688.
17. Tamru K, Aga F, Berhanie E, Aynalem YA, Shiferaw WS. Incidence of diabetic nephropathy in patients with type 2 diabetes mellitus at a tertiary healthcare setting in Ethiopia. Diabetes Metab Syndr. 2020;14(5):1077–1083. doi:10.1016/j.dsx.2020.06.028
18. Hintsa S, Dube L, Abay M, Angesom T, Workicho A. Determinants of diabetic nephropathy in Ayder Referral Hospital, Northern Ethiopia: a case-control study. PLoS One. 2017;12(4):e0173566. doi:10.1371/journal.pone.0173566
19. Geletu AH, Teferra AS, Sisay MM, Teshome DF. Incidence and predictors of chronic kidney diseases among type 2 diabetes mellitus patients at St. Paul’s Hospital, Addis Ababa, Ethiopia. BMC Res Notes. 2018;11(1):1–6. doi:10.1186/s13104-018-3618-9
20. Adugna T, Merga H, Gudina EK. Impaired glomerular filtration rate, high grade albuminuria and associated factors among adult patients admitted to tertiary Hospital in Ethiopia. BMC Nephrol. 2018;19(1):1. doi:10.1186/s12882-018-1153-5
21. Debele GR, Hajure M, Wolde HF, Yenit MK. Incidence and predictors of chronic kidney disease among diabetes mellitus patients: a retrospective follow-up study at a tertiary health-care setting of Ethiopia. Diabetes Metab Syndr Obes. 2021;14:4381.
22. Kebede SA, Tusa BS, Weldesenbet AB, Tessema ZT, Ayele TA. Incidence of diabetic nephropathy and its predictors among type 2 diabetes mellitus patients at University of Gondar comprehensive specialized hospital, Northwest Ethiopia. J Nutr Metab. 2021;2021. doi:10.1155/2021/6757916
23. Regassa LD, Gete YK, Mekonnen FA. Time to acute kidney injury and its predictors among newly diagnosed Type 2 diabetic patients at government hospitals in Harari Region, East Ethiopia. PLoS One. 2019;14(5):e0215967. doi:10.1371/journal.pone.0215967
24. Buková A, Chovanová E, Küchelová Z, et al. Association between educational level and physical activity in chronic disease patients of eastern Slovakia. InHealthcare. 2021;9(11):1447. doi:10.3390/healthcare9111447
25. Wu H, Lau ES, Kong AP, et al. Association between educational level and cardiovascular disease and all-cause mortality in patients with type 2 diabetes: a prospective study in the Joint Asia Diabetes Evaluation Program. Clin Epidemiol. 2018;10:1561. doi:10.2147/CLEP.S177437
26. Ahmed MA, Ferede YM, Takele WW. Incidence and predictors of chronic kidney disease in type-II diabetes mellitus patients attending at the Amhara region referral hospitals, Ethiopia: a follow-up study. PLoS One. 2022;17(1):e0263138.
27. Buffet L, Ricchetti C. Chronic kidney disease and hypertension: a destructive combination; 2012.
28. Islam TM, Fox CS, Mann D, Muntner P. Age-related associations of hypertension and diabetes mellitus with chronic kidney disease. BMC Nephrol. 2009;10(1):1–6. doi:10.1186/1471-2369-10-17
29. Allender S, Wickramasinghe K, Goldacre M, Matthews D, Katulanda P. Quantifying urbanization as a risk factor for noncommunicable disease. J Urban Health. 2011;88(5):906–918. doi:10.1007/s11524-011-9586-1
30. Pinchoff J, Mills CW, Balk D. Urbanization and health: the effects of the built environment on chronic disease risk factors among women in Tanzania. PLoS One. 2020;15(11):e0241810. doi:10.1371/journal.pone.0241810
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
