Back to Journals » Journal of Inflammation Research » Volume 17
Therapeutic Plasma Exchange in AChR-Ab Positive Generalized Myasthenia Gravis: A Real World Study About Its Early Response
Authors Chen J , Feng L, Li S, Wang H, Huang X, Shen C, Feng H
Received 15 December 2023
Accepted for publication 6 April 2024
Published 16 April 2024 Volume 2024:17 Pages 2299—2308
DOI https://doi.org/10.2147/JIR.S455104
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Jiaxin Chen,1,2 Li Feng,1,2 Shiyin Li,2 Haiyan Wang,1,2 Xin Huang,1,2 Cunzhou Shen,1,2 Huiyu Feng1,2
1Department of Neurology and Neurointensive Care Unit, the First Affiliated Hospital, Sun Yat-sen University, Guangzhou, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Diagnosis and Treatment of Major Neurological Diseases, National Key Clinical Department and Key Discipline of Neurology, Guangzhou, People’s Republic of China
Correspondence: Huiyu Feng, Department of Neurology and Neurointensive Care Unit, the First Affiliated Hospital of Sun Yat-sen University, No. 58 Zhongshan Road 2, Guangzhou, 510080, People’s Republic of China, Tel +86-020-87755766, Email [email protected]
Background: Since there is no clear priority or selection principle in the guidelines for myasthenia crisis, therapeutic plasma exchange (TPE) and intravenous immunoglobulin are often administered randomly. However, it should be more prudent in taking TPE due to its higher cost and risk. Studying its early response factors is crucial for managing myasthenia crisis and can improve medical and economic benefits.
Methods: A prospective observational study was conducted, and patients classified as having “impending myasthenia crisis” or experiencing a myasthenia crisis and treated by TPE were included. The primary endpoint was the response after TPE. Univariate logistic regression analysis and repeated measurement were performed to analyze factors related to TPE efficacy.
Results: A total of 30 patients who treated with TPE as their fast-acting treatments were enrolled. After TPE, those whose QMGs and/or MGCs decreased by ≥ 5 points or ≥ 30% of the baseline were judged as “response group”, accounting for 66.67% (20/30). Respiratory symptoms had a response rate of 72.00% (18/25), showing the most remarkable improvement. Meanwhile, extraocular symptoms were the least sensitive, with only 8.00% (2/25) showing efficacy. Thymoma (100.00% vs 50.00%, P=0.002) and a high concentration of AChR-Ab (37.37 nmol/L vs 25.4 nmol/L, P=0.039) were common in the early response group. Repeated measures showed significant changes in AChR-Ab and CD19+ B cells before and after TPE (all with P < 0.05). After treatment, the CD19+ B cells tended to decrease in the response group.
Discussion: These results indicated that, for AChR-Ab positive generalized MG, TPE can quickly improve respiratory symptoms. Thymoma and a high concentration of AChR-Ab before TPE predict an early better response. Additionally, TPE may work by decreasing AChR-Ab levels and inducing immune regulation. Future prospective and randomized controlled studies are needed.
Keywords: plasma exchange, myasthenia gravis, crisis, efficacy, neuroimmunology
Introduction
A myasthenia crisis is the acute exacerbation of myasthenia gravis (MG) that can be life-threatening, leading to a 6% to 30% likelihood of in-hospital mortality worldwide.1–3 International consensus guidance for management of MG recommends therapeutic plasma exchange (TPE) and intravenous immunoglobulin (IVIG) as the first-line treatment for a crisis.4 However, there is no clear priority or selection principle between those two treatments in the guideline. In clinical application, their efficacy is not guaranteed for every patient, only about 55~87% will respond. Therefore, previous studies attempted to determine the efficacy of TPE and IVIG, and which treatment is preferred under specific disease conditions. They found that in muscle-specific kinase antibody positive MG, the response rate of TPE ranges from 50% to 93%, significantly higher than that of IVIG, which is 11% to 61%.5 For MG women during pregnancy, both are equally effective, but IVIG is safer than TPE.6 And TPE has a better response than IVIG in those juvenile MG who younger than 18 years old.7 Moreover, a meta-analysis showed that there was a higher response rate with TPE than IVIG in acute MG patients and patients undergoing thymectomy.8 Above all these findings indicate that TPE and IVIG are not simply juxtaposed without principle in MG patients. In specific populations or disease conditions, they have different priorities. Meanwhile, the decision to treated with TPE or IVIG may also depend on factors such as access and convenience. As known to modulate the immune response, IVIG has many indications.9 But the immunoglobulins, deriving from plasma from healthy donors, always fails to meet clinical demand. While TPE requires special equipment and trained personnel to perform it. Moreover, as an invasive treatment, the cost of TPE is much higher. It not only leads to prolonged hospitalization times and increased physical risks, but also imposes greater psychological and economic burdens.10,11 Thus, it is important to study the early effective factors of TPE and predict suitable patients before treatment, contributing to MG and improving medical and economic benefits.
Materials and Methods
Patients and Subgroups
In this prospective, open label, observational study, we screened MG patients admitted to the Department of Neurology and Neurointensive Care Unit of The First Affiliated Hospital, Sun Yat-sen University from September 1st 2019 to September 30th 2022.
The inclusion criteria were as follows: (1) in an “impending myasthenia crisis” or a myasthenia crisis state according to the international and Chinese guidelines for diagnosis and treatment of MG,4,12 (2) serum acetylcholine receptors antibody (AChR-Ab) level > 0.45 nmol/L (using the enzyme-linked immunosorbent assay [ELISA] method) and muscle-specific kinase antibody- and lipoprotein-related protein 4 antibody-negative (cell-based assay), (3) receiving TPE firstly as acute treatment, (4) informed consent provided by the patient and/or family members. The exclusion criteria were as follows: (1) presence of hyperthyroidism, systemic lupus erythematosus, or other immune diseases, (2) presence of other malignant tumors in addition to thymoma.
The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments and was approved by the local ethics committee for clinical research (Chinese Clinical Trial Registration: ChiCTR2100043920). Informed consent was obtained from all study subjects and no personal information of the participants has been reported in this manuscript.
TPE Protocol and Items Evaluations
TPE was performed using an Aminco Cell Separator. The TPE specifications were (1) volume of blood processed 40–50 mL/kg per TPE procedure; (2) each course of treatment involved 3 times TPE procedures, and the frequency was every 2 or 3 days; and (3) replacement fluids consisted of 4% albumin.
The quantitative MG score (QMGs) and MG composite score (MGCs) were assessed in the morning before the patients took a cholinesterase inhibitor or ≥4 hours after taking a cholinesterase inhibitor. For patients who had been intubated and were on a nasogastric tube nasogastric feeding diet, items such as phonation, vital capacity, and head raising were assigned the maximum score based on their medical history while intubation.
This study compared the clinical characteristics of patients by measuring AChR-Ab concentration using ELISA (RSR, Cardiff, UK). Cytokines concentrations were detected using immunofluorescence (Siemens healthineers, Germany and Cell-genebio, China), and the proportion of lymphocyte subsets was evaluated using Multitest 6-Color TBNK Reagent (BD, USA) by flow cytometry. Blood samples were collected total four times, including before TPE and within 1 hours after each time TPE procedure.
Follow-Up Endpoint and Effective Definition
The primary endpoints was the curative effect after a complete course of TPE treatment, as follow: (1) improved or stable (successfully removed the tracheal intubation; or the QMGs /MGCs decreased by ≥5 points or ≥ 30% of the baseline), (2) unchanged or invalid (still obvious respiratory muscle/throat weakness symptoms, or the QMGs /MGCs decreased by <5 points or 30% of the baseline, sequential IVMP or IVIG treatment was needed), (3) worsen or needed to be hospitalized again after discharge (compared with this time of discharge, the QMGs /MGCs increased by ≥30%; The daily dose of cholinesterase inhibitor is increased by ≥50%; Symptoms of obvious involvement of respiratory muscles). Based on these, (1) was the “response group”, and it would be reviewed and confirmed at the one-month outpatient follow-up by activities of daily living. While (2) and (3) were the “non-response group”. The secondary endpoint was the hospital follow-up time (5 ± 2 days after TPE).
Statistical Analyses
Continuous variables that conform to the normal distribution were displayed by the mean and standard deviation, otherwise, the median and quartile were used. To identify the predictors of clinical outcome, we applied univariate logistic regression analysis to the continuous variables. Fisher’s exact and chi-square tests were used to analyze the categorical variables. Repeated measurement was performed to analyze the related factor with TPE efficacy. The Mauchly’s test of sphericity was conducted, and if conforms (P≥0.05), using the tests of within-subjects effects, otherwise (P<0.05), using multivariate tests. When the results were statistically different, pairwise comparison was made. All statistical analyses were performed using IBM SPSS version 26.0 (SPSS Company, Chicago, IL, USA) and GraphPad Prism 8 version 8.0.2 (GraphPad Software Inc., San Diego, CA, USA) was used to generate the figures.
Results
A total of 59 patients with myasthenia crisis were admitted to the hospital. Among them, 31 patients treated with TPE as their fast-acting treatments were enrolled in this study. One patient was excluded because TPE was discontinued due to a local catheter-related infection. It happened after 2 times of TPE and was characterized by erythema, pain, and purulent secretion within 2 centimetre around the puncture point. Purulent secretion showed Gram-positive bacteria, while blood culture showed no bacteria. A total of 30 patients were finally included. All of them had not received treatment with high-dose intravenous methylprednisolone or IVIG or TPE or rituximab in the past 3 months. From the beginning of TPE to the end of the follow-up, those whose QMGs and/or MGCs decreased by ≥5 points or ≥30% of the baseline were judged as “response group”, accounting for 66.67% (20/30). The non-response group made up 33.33% (10/30), as shown in Figure 1. And those non-responder would take some additional interventions after 3 times TPE, such as additional TPE (till 5~7 times in total), or alternative treatments such as IVIG (0.4g/kg/d, 3 to 5 days) and intravenous methylprednisolone (500mg to 1000 mg, 3 to 5 days). One of them treated with Rituximab (500mg) after TPE and IVIG.
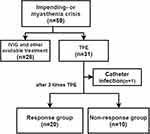 |
Figure 1 Flowchart of the study. Abbreviations: IVIG, intravenous immunoglobulin; TPE, therapeutic plasma exchange. |
Treated with TPE, Respiratory Muscles Were Sensitive, While Extraocular Muscles Were Refractory
The most commonly involved muscle groups in the enrolled cases were extraocular muscles, bulbar muscles, limb muscles and respiratory muscles, as shown in Figure 2. After TPE, respiratory symptoms showed remarkable improvement, and its response rate was 72.00% (18/25), while that of limb muscles and bulbar muscles were 65.38% (17/26) and 68.18% (15/22), respectively. Extraocular symptoms were most frequently involved, but they were least sensitive to TPE, with a response rate of only 8.00% (2/25).
Thymoma and a High Concentration of AChR-Ab Before TPE Predict a Better Response
In our previous studies, we found that these blood indices were related to the severity and activity of MG, including the concentrations of AChR-Ab, IgG and IL-6, the ratio of CD4+/CD8+ T cells, and the proportion of CD19+ B cells.13 Thus, in this study, they were used as part of the observations. Before TPE, there was no statistical difference in sex, age, the disease duration and clinical scores between two groups, as shown in Table 1. However, the proportion of thymoma (100.00% vs 50.00%, P=0.002) and concentration of AChR-Ab (37.37 nmol/L vs 25.4 nmol/L, P=0.039) were higher in the response group.
 |
Table 1 The Indexes Before TPE Between Two Groups |
The Changes of AChR-Ab and CD19+ B Cells Were Related to the Efficacy of TPE
The indices before TPE and immediately after each replacement were analyzed, as shown in Figure 3. As TPE progressed, the serum concentrations of AChR-Ab and IgG decreased significantly, and the proportion of CD19+ B cells increased. Although repeatedly measured, the factors related to the efficacy of TPE were shown in Table 2. Except for CD19+ B cells, all other indices rejected the spherical hypothesis (P all <0.05). The concentration of AChR-Ab and the proportion of CD19+ B cells showed an interaction between time and group (P all <0.05). And they significantly changed before and after TPE treatment as indicated by time effects. The concentration of IgG also changed with time; however, there was no inter-group effect (P all > 0.05).
 |
Table 2 Repeated Measurement to Analysis the Effective Related Indicators in TPE |
 |
Figure 3 The AChR-Ab, IgG, IL-6, CD4+ T cells, CD8+ T cells and CD19+ B cells before TPE and immediately after each replacement. |
TPE May Induce Immune Regulation
Before TPE, there was no statistical difference in T and B cells between the two groups with different treatment effects. After three rounds of TPE, the flow rates of CD4+/CD8+ cells and CD19+ B cells were illustrated in Figure 4. The change trend of lymphocyte subsets in the response group and the non-response group is different. In the response group, the proportion of CD4+ and CD8+ T cells increased, leaning towards T cells, especially the CD4+ T cells, while the proportion of CD19+ B cells remains stable In the non-response group, the proportion of CD19+ B cells increased, and the proportion of CD4+ and CD8+ T cells decreased. However, the sample size is small, and the P value after analysis is >0.05 at present.
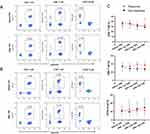 |
Figure 4 The proportions of CD4+, CD8+ T cells and CD19+ B cells of a response patient (A) and a non-response patient (B) before and after TPE. (C) Changes of lymphocytes in two groups. |
Discussion
In the present study, we revealed three major findings. First, TPE proved to be an effective treatment for myasthenia crisis, with a response rate of 65.22%. Respiratory symptoms were sensitive to TPE, while extraocular muscles has the lowest response rate. Second, the presence of thymoma and a high concentration of AChR-Ab before TPE predicted a better response to TPE. Third, TPE played a role in reducing the circulating pathogenic AChR-Ab and inducing the immune regulation. The results of flow cytometry showed that the proportion of CD4+ T cells, CD8+ T cells and CD19+ B cells in the different response groups changed in the opposite direction. And it is worth further study.
Patients with AChR-Ab positive MG can be acutely treated with TPE or IVIG with response rates ranging from 57% to 80% and 55% to 87%, respectively.8,9 However, the efficacy and safety of these two treatments have not been thoroughly characterized across many clinical programs. Previous study showed that, the clinical efficacy was equally comparable in both interventions after 1 month. But TPE was associated with an early response and a reduction of the intensive care unit stay in AChR-associated myasthenia crisis.14 To date, there is no definitive understanding of how to make a fast and efficacious choice.15,16 We aimed to study TPE with MG patients in a real-world setting and identify the related factors of its early efficacy. In our study, symptoms of respiratory muscle, along with thymoma and a high concentration of AChR-Ab showed a better response to TPE. Thymoma is considered a risk factor for MG, indicating refractory and more severe symptoms with a worse prognosis.17 Thymoma associated MG exhibits a more complex micro-environments dedicated to antibodies and the production of many other unknown pathogenic substances, although its etiologic factors are not understood. TPE, by removing plasma indistinguishably, may simultaneously remove those unknown pathogenic substances, resulting in a better response.
Inflammation and auto-antibodies are the characteristics of autoimmune diseases. By rapidly removing circulating AChR-Ab antibodies, TPE can improve myasthenia symptoms and reduce hospitalization mortality.18,19 An existing study involving ten patients with crisis underwent TPE with a median of 6 times (5 to 27 times). At the end of the TPE course, AChR-Ab level decreased by an average of 71% and their symptoms improved. The most significant improvement occurred about one week after completing the TPE course.20 By six weeks after TPE, AChR-Ab had basically returned to the baseline level.21 Similar phenomena were observed in other studies. In a recent retrospective observational study, the serum concentration of AChR-Ab rebounded in 5 MG patients after TPE, reaching its peak after 5 to 6 weeks.22 Although TPE removed the antibodies, newly produced antibodies may be induced to redistribute, leading to an increase in serum concentration, even overshoot (exceeding their baseline levels). And recurrence typically occurs 5 to 6 weeks after TPE, suggesting that antibodies rebound or overshoot may underlie the recurrence. However, the rebound of AChR-Ab may not always lead to the exacerbation, nor does it imply that repetitive TPE is ineffective.
The eighth edition of the guidelines of the American Society for Apheresis (ASFA) in 2019 suggested that refractory MG can be treated with maintenance TPE once a week or once every two weeks (Class II evidence, recommended by Grade 2B), in addition to acute exacerbation.23 Continuous and regular TPE can effectively prevent the rebound of pathogenic AChR-Ab, keeping them at a low level and improving symptoms.24 Now, an emerging approach for the removal of IgG antibodies is targeting neonatal Fc receptor (FcRn), offering higher selectivity but less invasive administration.25,26 FcRn targeting, demonstrating similar degrees of IgG reduction as TPE, has been proven effective in some IgG-mediated diseases.27–29 However, its use in acute or crisis states has not yet been studied. Moreover, though both can remove IgG, compared with FcRn inhibitor, TPE has another clinical advantage, which can also clear other pathogenic circulating substances, and may even regulate lymphocytes. Theoretically, TPE does not affect lymphocytes directly. However, due to the elimination of pathogenic media and induction of immune regulation, changes in lymphocytes and cytokines are observed after TPE.30 Before TPE, the percentages of T and B cells in Guillain-Barré syndrome patients were 53% and 15.5%, respectively, which differed from those in the control group (78% and 8%). After TPE, T cells in patients increased to 71.75%, while B cells decreased to 8.75%. The distribution of T and B cells tended to be consistent with that in the control. Correspondingly, their symptoms improved. By incubating the plasma removed from each patient with control peripheral lymphocytes, and then exposing them to mitogens and tritiated thymidine, researchers found that TPE removed a lymphocyte suppressor factor, which consistently decreased the responsiveness of normal lymphocytes to mitogens.31 TPE may correct the imbalance of Th1/Th2 and play a role in the immune regulation indirectly, after removing the antibodies and other pathogenic substances. In the present study, the level of AChR-Ab and IgG decreased, and T cells and CD19+ B cells tended to be normalize in the better response group after TPE. Antibodies rebound may be caused by the activation of B cells, change of T cells subsets and the weakening of feedback inhibition after TPE.32 Therefore, combining immunotherapy targeting B cells with TPE could achieve a better outcome.
There are some limitations to our study. The sample size is small to make a meaningful interpretation. And the small number of replacements and short follow-up observation time may underestimate the efficacy of TPE. Additionally, as a single-center study, the selection bias also cannot be completely eliminated. Moreover, based on the results of our previous studies, we focus on specific indices, including antibodies, IgG, IL-6, CD4+/ CD8+T cells and CD19+ B cells before and after TPE. This may result in missing other potentially relevant factors. Multi-center studies are needed to validate our findings and establish a reliable predictive model.
Conclusion
For AChR-Ab positive MG, TPE can quickly improve the symptoms of respiratory weakness. Thymoma and a high concentration of AChR-Ab before TPE predicts an early better response. Besides, the decrease in serum AChR-Ab and the proportion of CD19+ B cells through TPE may be the related factors for its efficacy. Future studies are needed.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.
Ethical Statement
The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments, and approved by the ethical committee of The First Affilliated Hospital, Sun Yat-sen University (Chinese Clinical Trial Registration: ChiCTR2100043920).
Acknowledgments
This work was supported by the Guangdong Provincial Key Laboratory of Diagnosis and Treatment of Major Neurological Diseases (2020B1212060017), Guangdong Provincial Clinical Research Center for Neurological Diseases (2020B1111170002), Southern China International Joint Research Center for Early Intervention and Functional Rehabilitation of Neurological Diseases (2015B050501003 and 2020A0505020004), Guangdong Provincial Engineering Center for Major Neurological Disease Treatment, Guangdong Provincial Translational Medicine Innovation Platform for Diagnosis and Treatment of Major Neurological Disease, Guangzhou Clinical Research and Translational Center for Major Neurological Diseases (201604020010).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was granted from the National Natural Science Foundation of China (82301587, 82201554), Guangdong Natural Science Foundation Committee (2022A1515010478) and China Postdoctoral Science Foundation (2022M723601).
Disclosure
Dr Jiaxin Chen reports grants from National Nature Science Foundation of China and China Postdoctoral Science Foundation, during the conduct of the study. Dr Huiyu Feng reports grants from Guangdong Natural Science Foundation Committee, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Liu F, Wang Q, Chen X. Myasthenic crisis treated in a Chinese neurological intensive care unit: clinical features, mortality, outcomes, and predictors of survival. BMC Neurol. 2019;19(1):172. doi:10.1186/s12883-019-1384-5
2. Lv Z, Zhong H, Huan X, et al. Predictive score for in-hospital mortality of myasthenic crisis: a retrospective Chinese cohort study. Eur Neurol. 2019;81(5–6):287–293. doi:10.1159/000503961
3. Hsu CW, Chen NC, Huang WC, et al. Hemogram parameters can predict in-hospital mortality of patients with Myasthenic crisis. BMC Neurol. 2021;21(1):388. doi:10.1186/s12883-021-02412-4
4. Narayanaswami P, Sanders DB, Wolfe G, et al. International consensus guidance for management of myasthenia gravis: 2020 update. Neurology. 2021;96(3):114–122. doi:10.1212/WNL.0000000000011124
5. Morren J, Li Y. Myasthenia gravis with muscle-specific tyrosine kinase antibodies: a narrative review. Muscle Nerve. 2018;58(3):344–358. doi:10.1002/mus.26107
6. Ferrero S, Pretta S, Nicoletti A, Petrera P, Ragni N. Myasthenia gravis: management issues during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2005;121(2):129–138. doi:10.1016/j.ejogrb.2005.01.002
7. Liew WK, Powell CA, Sloan SR, et al. Comparison of plasmapheresis and intravenous immunoglobulin as maintenance therapies for juvenile myasthenia gravis. JAMA Neurol. 2014;71(5):575–580. doi:10.1001/jamaneurol.2014.17
8. Ipe TS, Davis AR, Raval JS. Therapeutic Plasma Exchange in Myasthenia Gravis: a Systematic Literature Review and Meta-Analysis of Comparative Evidence. Front Neurol. 2021;12:662856. doi:10.3389/fneur.2021.662856
9. Morales-Ruiz V, Juárez-Vaquera VH, Rosetti-Sciutto M, Sánchez-Muñoz F, Adalid-Peralta L. Efficacy of intravenous immunoglobulin in autoimmune neurological diseases. Literature systematic review and meta-analysis. Autoimmun Rev. 2022;21(3):103019. doi:10.1016/j.autrev.2021.103019
10. Chen J, Tian DC, Zhang C, et al. Incidence, mortality, and economic burden of myasthenia gravis in China: a nationwide population-based study. Lancet Reg Health West Pac. 2020;5:100063. doi:10.1016/j.lanwpc.2020.100063
11. Landfeldt E, Pogoryelova O, Sejersen T, Zethraeus N, Breiner A, Lochmüller H. Economic costs of myasthenia gravis: a systematic review. Pharmacoeconomics. 2020;38(7):715–728. doi:10.1007/s40273-020-00912-8
12. Neuroimmunology of Chinese Immunology Society. Chinese guidelines for diagnosis and treatment of myasthenia gravis 2020. Chin J Neuroimmunol Neurol. 2021;28(1):1–12. doi:10.3969/j.issn.1006-2963.2021.01.001
13. Chen J, Li S, Feng L, Wang H, Huang X, Feng H. Nomogram for the acute exacerbation of acetylcholine receptor antibody-positive generalized myasthenia gravis. Neurol Sci. 2023;44(3):1049–1057. doi:10.1007/s10072-022-06493-y
14. Wang Y, Huan X, Jiao K, et al. Plasma exchange versus intravenous immunoglobulin in AChR subtype myasthenic crisis: a prospective cohort study. Clin Immunol. 2022;241:109058. doi:10.1016/j.clim.2022.109058
15. Dhawan PS, Goodman BP, Harper CM, et al. IVIG versus PLEX in the treatment of worsening myasthenia gravis: what is the evidence?: a critically appraised topic. Neurologist. 2015;19(5):145–148. doi:10.1097/NRL.0000000000000026
16. Harris L, Allman PH, Sheffield R, Cutter G. Longitudinal analysis of disease burden in refractory and nonrefractory generalized myasthenia gravis in the United States. J Clin Neuromuscul Dis. 2020;22(1):11–21. doi:10.1097/CND.0000000000000301
17. Chen J, Shang W, Chen Y, et al. Thymomatous myasthenia gravis: 10-year experience of a single center. Acta Neurol Scand. 2021;143(1):96–102. doi:10.1111/ane.13332
18. Dogra A, Rana K, Rathod C, Prakash S. Outcome of therapeutic plasma exchange in myasthenia gravis patients. J Family Med Prim Care. 2020;9(12):5971–5975. doi:10.4103/jfmpc.jfmpc_1026_20
19. Rath J, Brunner I, Tomschik M, et al. Frequency and clinical features of treatment-refractory myasthenia gravis. J Neurol. 2020;267(4):1004–1011. doi:10.1007/s00415-019-09667-55
20. Rønager J, Ravnborg M, Hermansen I, Vorstrup S. Immunoglobulin treatment versus plasma exchange in patients with chronic moderate to severe myasthenia gravis. Artif Organs. 2001;25(12):967–973. doi:10.1046/j.1525-1594.2001.06717.x
21. Guptill JT, Juel VC, Massey JM, et al. Effect of therapeutic plasma exchange on immunoglobulins in myasthenia gravis. Autoimmunity. 2016;49(7):472–479. doi:10.1080/08916934.2016.1214823
22. Ching J, Richards D, Lewis RA, Li Y. Myasthenia gravis exacerbation in association with antibody overshoot following plasmapheresis. Muscle Nerve. 2021;64(4):483–487. doi:10.1002/mus.27341
23. Padmanabhan A, Connelly-Smith L, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the writing committee of the American Society for Apheresis: the eighth special issue. J Clin Apher. 2019;34(3):171–354. doi:10.1002/jca.21705
24. Sheckley H, Malhotra K, Katyal N, Narula N, Govindarajan R. Clinical experience with maintenance therapeutic plasma exchange in refractory generalized myasthenia gravis. J Clin Apher. 2021;36(5):727–736. doi:10.1002/jca.21923
25. Pyzik M, Kozicky LK, Gandhi AK, Blumberg RS. The therapeutic age of the neonatal Fc receptor. Nat Rev Immunol. 2023;23(7):415–432. doi:10.1038/s41577-022-00821-1
26. Di Stefano V, Alonge P, Rini N, et al. Efgartigimod beyond myasthenia gravis: the role of FcRn-targeting therapies in stiff-person syndrome. J Neurol. 2024;271(1):254–262. doi:10.1007/s00415-023-11970-1
27. Howard JF, Bril V, Vu T, et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, Phase 3 trial. Lancet Neurol. 2021;20(7):526–536. doi:10.1016/S1474-4422(21)00159-9
28. Maho-Vaillant M, Sips M, Golinski ML, et al. FcRn Antagonism Leads to a Decrease of Desmoglein-Specific B Cells: secondary Analysis of a Phase 2 Study of Efgartigimod in Pemphigus Vulgaris and Pemphigus Foliaceus. Front Immunol. 2022;13:863095. doi:10.3389/fimmu.2022.863095
29. Mina-Osorio P, Tran MH, Habib AA. Therapeutic Plasma Exchange Versus FcRn Inhibition in Autoimmune Disease. Transfus Med Rev. 2024;38(1):150767. doi:10.1016/j.tmrv.2023.150767
30. Soltész P, Aleksza M, Antal-Szalmás P, Lakos G, Szegedi G, Kiss E. Plasmapheresis modulates Th1/Th2 imbalance in patients with systemic lupus erythematosus according to measurement of intracytoplasmic cytokines. Autoimmunity. 2002;35(1):51–56. doi:10.1080/08916930290005909
31. Glassman AB, Bennett CE. Alterations of lymphocyte responsiveness in Guillain-Barré syndrome. effects of plasma exchange. Transfusion. 1983;23(5):369–372. doi:10.1046/j.1537-2995.1983.23584018711.x
32. Bennani HN, Lagrange E, Noble J, et al. Treatment of refractory myasthenia gravis by double-filtration plasmapheresis and rituximab: a case series of nine patients and literature review. J Clin Apher. 2021;36(3):348–363. doi:10.1002/jca.21868
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

