Back to Journals » Research and Reports in Urology » Volume 15
The Use of Prophylactic Ureteric Stents in Major Abdomino-Pelvic Sarcoma Surgery: Risks, Benefits, and Potential Complications
Authors Barns ME, Chau MVHD , Teloken PE, Hodder R
Received 27 September 2023
Accepted for publication 14 December 2023
Published 19 December 2023 Volume 2023:15 Pages 577—585
DOI https://doi.org/10.2147/RRU.S435959
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Panagiotis J Vlachostergios
Mitchell Egerton Barns,1 Matthew Vinh-Hoan Dinh Chau,1 Patrick Ely Teloken,1 Rupert Hodder2
1Sir Charles Gardiner Hospital, Department of Urology, Perth, WA, Australia; 2Sir Charles Gardiner Hospital, Department of General Surgery, Perth, WA, Australia
Correspondence: Mitchell Egerton Barns, Sir Charles Gardiner Hospital, Department of Urology, Perth, WA, 6009, Australia, Tel +61 409 439 006, Email [email protected]
Abstract: Here we present two cases of post-operative obstructive renal failure following major abdomino-pelvic sarcoma surgery. In both cases, prophylactic ureteric stents were inserted to aid the identification and protection of the ureters during resection of these complex retroperitoneal masses. In case one, obstructive renal failure occurred following ureteric stent removal on day 0 post-operatively. In case two, obstructive renal failure developed on day 1 post-operatively despite having a ureteric stent in situ. Here we propose that a combination of reflex anuria/ureteric edema and papillary sloughing led to the obstructive renal failure in both cases. Re-insertion of bilateral ureteric stents in case one, and replacement of a right ureteric stent in case two saw prompt excretion of urine and sloughy debris with rapid improvement of renal function. This article presents these cases in detail and further reviews the use of prophylactic ureteric stents in major abdomino-pelvic surgery along with the current guidelines for their usage.
Keywords: ureteric injury, prophylactic ureteric stenting, endourology, obstructive renal failure, slough papillae
Introduction
Ureteric injury during abdomino-pelvic surgery is rare, with an overall incidence of <2%.1 Avoiding injury to the ureters is paramount, as injury can lead to severe short-term complications like sepsis and can have lasting long-term repercussions for the patient – leading to years of intervention, follow-up, and significant morbidity.2 If these iatrogenic injuries are not recognized and repaired intra-operatively, they often require multiple staged procedures to repair.3 In some cases a nephrectomy is ultimately required.4
General surgical, colorectal surgery, and gynecological procedures are the most common contributors to ureteric injury overall,5,6 with the incidence rising in an era of minimally invasive surgery.1 Distorted anatomy secondary to malignancy, pelvic radiation, or previous surgery increases the risk of iatrogenic injury.7 Pre-operative placement of ureteric stents during major abdominal/pelvic surgery may be beneficial in two ways: 1) intra-operative identification of the ureters and thus prevention of subsequent injury; and 2) early identification of an injury if it does occur. However, routine stenting is associated with prolonged operating time and can lead to stent-related complications.8
The use of ureteric stents in major abdominal/pelvic surgery is often surgeon- and case-specific and can be performed in various ways. Typical 6Fr ureteric stents can be placed cystoscopically and left in-situ up to 12 months. Similarly, ureteric stents can be left attached to their removal strings, which can be secured to the indwelling Foley’s catheter to aid ease of removal at the end of the case. Currently there is no clear consensus on how long these ureteric stents should remain in-situ following major surgery.
This article presents two cases of obstructive renal failure associated with ureteric stenting pre-operatively for elective retroperitoneal sarcoma surgery. Both patients developed anuric renal failure post-operatively which promptly resolved following exchange/re-insertion of ureteric stents. Both patients developed post-obstructive diuresis almost immediately upon exchange or re-insertion of ureteric stents with subsequent return of renal function to baseline within 1 week. We hypothesize that a combination of reflex anuria, ureteric edema, and renal papillary sloughing were responsible in both cases. This article reviews the current use of ureteric stents in major abdomino-pelvic surgery including the guideline recommendations guidelines and rates of stent-related complications.
Case One
A 58-year-old lady with no significant past medical history presents for elective resection of large right pelvic sidewall mass. Pre-operative eGFR was >90. The patient underwent prophylactic ureteric stenting by the urology team with 6Fr multi-length ureteric stents. It was noted intra-operatively that the patient had a complete left sided duplex system requiring two left sided ureteric stents. All three ureteric stents were left on their strings and attached to a Foley’s catheter for ease of removal post-operatively. There were no immediate stent-related complications.
The right pelvic sidewall mass was approached through a lower midline laparotomy. The mass was easily dissected off the pelvic side with the right ureter having been identified and protected throughout the case. The procedure was completed in approximately 2 hours with no significant blood loss. The patient received 1500 mL of crystalloid fluid intravenously during the procedure and produced 250 mL of clear urine. At the end of the case, the Foley’s catheter and ureteric stents were removed without complication. A new 14Fr indwelling Foleys catheter was re-inserted for bladder management and assessment of post-operative fluid balance. In the 24 hours post-operatively the patient was noted to be oligo-anuric, producing roughly 5–10 mL of urine per hour with a reduction in eGFR from >90 to 37. Despite appropriate fluid resuscitation, the patient developed anuric renal failure. A bladder scan revealed 15 mL, which likely reflected the contents of the catheter balloon. Her renal function continued to decline to an eGFR of 18 at 48 hours post-operatively. A subsequent non-contrast Computer Tomography (CT) scan revealed bilateral hydronephrosis/hydroureter down to the level of the pelvic inlet without an obvious cause for obstruction (Figure 1).
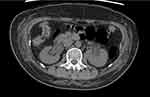 |
Figure 1 An axial section from a non-contrast abdominal CT scan showing severe bilateral hydronephrosis. |
The patient returned to theater for rigid cystoscopy, bilateral retrograde ureteropyelograms, and ureteric stent insertion. Retrograde studies showed large, bilateral mobile filling defects and gross hydroureteronephrosis (Figures 2 and 3). Distal ureteroscopy showed a white sloughy soft tissue mass partially obstructing the left vesicoureteric junction (VUJ) (Figure 4). Upon ureteric stent insertion there was rapid drainage of contrast/urine and sloughy material bilaterally. The patient’s urine output and renal function subsequently returned to normal within 48 hours after ureteric stent insertion. The patient’s ureteric stents were removed using a flexible cystoscope at 4 weeks post-operatively without complication. The patient’s renal function remains at pre-operative baseline with an eGFR >90.
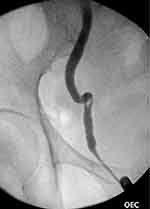 |
Figure 2 A right sided retrograde pyelogram showing severe hydroureter to the level of the vesicoureteric junction with associated filling defect within the distal ureter. |
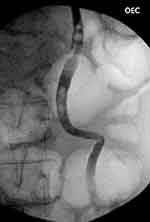 |
Figure 3 A left sided retrograde pyelogram showing severe hydroureter to the level of the vesicoureteric junction with multiple associated filling defects within the distal ureter. |
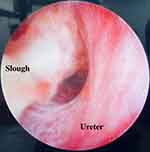 |
Figure 4 Photograph taken during left sided rigid ureteroscopy showing a large white sloughy debris partially occluding the vesicoureteric junction. |
Case Two
A 44-year-old male with biopsy proven large retroperitoneal paraganglioma presented for elective resection. He was otherwise fit and well with no medical co-morbidities and a normal pre-operative hemoglobin count of 150 renal function with eGFR >90. Prophylactic pre-operative ureteric stenting was performed due to the anticipated complexity of the operation and proximity of the left ureter to the mass. Bilateral 6Fr multi-length stents were placed by the urology team, with some left sided upstream hydronephrosis noted intra-operatively likely secondary to extrinsic ureteric compression from the retroperitoneal mass. The ureteric stents were inserted without strings with the plan for outpatient removal after the patient’s recovery from surgery. An indwelling Foley’s catheter was inserted to monitor urine output.
The large left sided retroperitoneal mass was approached via a midline laparotomy. The tumor was noted to be densely adherent to the infra-renal aorta, Inferior Vena Cava (IVC), left kidney, and ureter. The patient was placed on ECMO before the tumor and involved structures were resected en-bloc, including left nephrectomy and low ureteric ligation (including removal of the left ureteric stent). A complex vascular reconstruction of the infra-renal aorta and IVC was performed by the vascular team. The procedure took 12 hours to complete with an estimated blood loss of 4,000 mL. Intra-operatively the patient received 10 units of packed red blood cells and 3,500 mL of crystalloid fluids to maintain mean arterial pressure >65 mmHg. Post-operatively on Day 0, the patient’s haemoglobin was 105 and eGFR had reduced to 58. The patient remained intubated and was transferred to the ICU for supportive care.
On morning review, it was noted that only 10 mL of urine had drained since the patients return from theater despite appropriate intravenous fluid therapy. A bladder scan revealed an empty bladder. The patient was sent for an urgent CT abdomen/pelvis to further investigate. The right kidney showed normal enhancement with an appropriately positioned ureteric stent without evidence of hydronephrosis (Figures 5 and 6). A collapsed urinary bladder was also noted. The patient received further intravenous hydration despite appearing clinically euvolaemic. The patient’s urine output remained at 0 mL by midday with a concurrent worsening of his AKI with eGFR declining to 19.
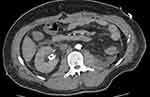 |
Figure 5 An axial section from a non-contrast abdominal CT showing an appropriately positioned right sided ureteric stent without hydronephrosis. |
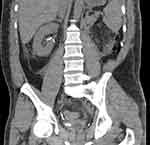 |
Figure 6 A coronal section from a non-contrast abdominal CT showing an appropriately positioned right sided ureteric stent without hydronephrosis. |
The patient was returned to theater for rigid cystoscopy, right retrograde pyelogram, and ureteric stent exchange. Immediately after the old ureteric stent was removed cystoscopically and copious ureteric slough/debris was seen exiting the right ureteric orifice. Subsequent retrograde ureteropyelogram showed moderate hydroureter/hydronephrosis (Figure 7). An 8Fr ureteric stent was re-inserted to further aid the decompression of the right kidney. A new 16Fr indwelling Foley’s catheter was inserted to monitor urine output. The patients urine output immediately increased to 50 mL/hr with concordant improvement in eGFR to 60 within 3 days.
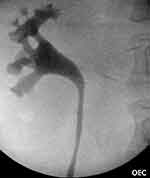 |
Figure 7 A right sided retrograde pyelogram showing mild hydroureter and hydronephrosis. |
Discussion
The Use of Prophylactic Ureteric Stents in Major Abomino-Pelvic Surgery
The European Association of Urology (EAU) guidelines state “visual identification of the ureters and meticulous dissection in their vicinity are mandatory to prevent ureteral trauma during abdominal and pelvic surgery”. The use of ureteric stents are recommended only in “selected cases” based on the risk factors for ureteric injury and the operating surgeons experience.9
A recent large review by Croghan et al documented the indications and, where present, the complications related to prophylactic ureteric stent insertion in elective general surgical cases. Of the 5,968 patients reviewed, the majority underwent colonic resection for diverticular disease (45.38%) or malignancy (33.45%), with the remainder undergoing elective resections for inflammatory bowel disease.10 Surgeon reasoning for prophylactic insertion of ureteric stents included: anticipation of adhesions, complicated diverticular disease, bulky tumors, previous pelvic surgery, anticipation of a difficult surgery, or just surgeon preference.11–13 This study reported higher rates of ureteric injury in the prophylactically stented population regardless of surgical approach. When compared to the non-stented patients, the rate of ureteric injury in prophylactically stented patients was increased by 0.18% in minimally laparoscopic/robotic surgery and 1.08% in open surgery.10 It is important to note that the increased rate of injury in prophylactically stented cases most likely reflects case selection as opposed to the stenting, leading to more injuries.
A retrospective cohort study performed by Hird et al in 2021 evaluated 98,507 patients who underwent major colorectal surgery and compared the incidence of ureteric injury and subsequent stent-related morbidity in patients who did and did not undergo pre-operative stenting. In line with the known estimate that roughly 4% of these major cases requested pre-operative stenting,14 5.1% of this cohorts patients had stents. There was no difference in ureteric injury observed, with no significant stent-related issues recorded either. Conversely, a systematic review performed by Croghan et al in 2019 showed that the rate of ureteric injury was higher in the prophylactically stented patients (1.75% vs 0.17%),10 however this analysis did not take into account case complexity, as more challenging cases were more likely to receive prophylactic ureteric stents. Despite not having been shown to reduce the rate of iatrogenic injury, prophylactic stents have been associated with increased intra-operative identification of ureteric injury which allows for primary repair.15
Potential Complications of Ureteric Stents
Complications related to ureteric stent insertion can occur during the procedure, during early recovery, and in the later stages. Intra-operative complications include hematuria secondary to ureteric/renal trauma from either the insertion wire or the ureteric stent as well as incorrect placement of the stent. Incorrect deployment of the stent, where either the proximal or distal end of the double J stent lie within the ureter, can cause significant patient discomfort and can be challenging to rectify. Lastly, the inability to insert a ureteric stent is also a known complication. This can occur for a multitude of reasons, including the inability to locate the ureteric orifice (secondary to inflamed bladder mucosa or structural abnormalities like ureterocoele), a “fishhook ureter” which does not permit instrumentation, and unrecognized ureteric strictures which can occur for a variety of reasons including radiotherapy or previous ureteric trauma or instrumentation. Unnecessary stenting or prolonged in-situ duration of ureteric stents is associated with significant patient morbidity, repeat presentations to the emergency department, and loss of patient income due to the inability to work.16 Experienced surgeons have higher success and fewer complications of ureteric stent insertion.17
Reflex Anuria and Vesicoureteric Junction Edema
Reflex anuria is a poorly understood phenomenon which has only been described in the form of case presentations. This diagnosis should only be considered once pre-renal, intrinsic, and post-renal causes of failure have been excluded. This rare condition was initially proposed by Sirota and Narins in 1957, who used the term reflex anuria to describe oligo-anuria following ureteric catheterization.18 A post-obstructive reflex mechanism was proposed by Shearlock and Howards in 1976, where angiography was used to display renal arteriolar vasoconstriction in an anuric patient following ureteric stent insertion.19 The term reflex anuria was adapted and broadened by Hull et al in 1980, leading to the definition of ‘Cessation of urine output from both kidneys in response to irritation or trauma to one kidney or its ureter’.20 In the absence of mechanical obstruction or ureteric ligation, reflex anuria is an extremely rare cause of acute renal failure resulting from ureteric manipulation or instrumentation during abdomino-pelvic surgery. Renal parenchymal or ureteric irritation leads to a reno-renal or uretero-renal reflex leading to renal arteriolar vasoconstriction and acute renal failure.
Edema at the vesicoureteric junction (VUJ) following ureteric instrumentation leading to transient obstruction has also been hypothesized as a cause of reflex anuria by various authors.21,22 Currently there is no consensus on how long a ureteric stent should remain in-situ for to allow resolution of edema following ureteric instrumentation. Although there are no comparative human studies, animal studies have shown upper tract obstruction secondary to ureteric edema can occur for up to 96 hours following ureteric instrumentation.23 A recent study by Paul et al compared post-operative outcomes following unilateral ureteroscopy in patients who were left with a ureteric stent for 3 vs 7 days. This study showed an 18% reduction in post-procedure events for patients in which ureteric stents remained for 7 days (as compared to 3 days).24 When compared to ureteric stent insertion alone, endoscopic stone surgery can be associated with significantly more ureteric trauma for a variety of reasons (impacted ureteric stones, ureteric access sheath trauma, and direct endoscope or laser trauma); for these reasons ureteric stents are often placed at the completion of the case. The EAU and AUA outline patient and surgical criteria for stent omission following uncomplicated ureteroscopy; however, no such recommendations exist for prophylactically placed ureteric stents.25 To our knowledge, no studies have investigated the effects of uncomplicated ureteric stent insertion of ureteric mucosal edema.
Renal Papillary Necrosis and Sloughed Papillae
Renal ischemia can occur within a physiologically normotensive range (in adults >90 mmHg systolic) termed normotensive ischemic acute renal injury. Modest reductions in blood pressure, often caused by volume depletion, can significantly reduce renal perfusion pressure, leading to ischemic injury in the absence of overt hypotension.26 Renal autoregulation is largely responsible for maintaining renal perfusion and a glomerular filtration rate with varying mean arterial pressure. Autoregulation is impaired in patients with atherosclerosis, hypertension, or underlying chronic kidney disease, leading to an increased renal susceptibility to ischemic injury.26 Anatomically, the renal medulla and papilla are highly susceptible to ischemic necrosis; whereby any physiological or pathological reduction in renal perfusion can lead to necrosis and sloughing of tissue. Renal papillary necrosis (RPN) can lead to detachment or sloughing of papillae into the collecting system which can manifest with ureteric obstruction, flank pain, or hematuria. The most common risk factors for RPN include Diabetes Mellitus, cadaveric renal transplant recipients, and analgesic induced nephropathies such as phenacetin.27
Clinically, these patients present with signs and symptoms identical to calculus ureteric colic. Sloughed papillae are radiolucent, however, with non-contrast imaging often revealing no clear obstructive cause. Ultrasound and CT imaging are relatively insensitive in diagnosing sloughed papillae, therefore CT intravenous pyelography (CT IVP) or retrograde pyelography are often required to demonstrate calyceal or ureteric filling defects.28 Classically sloughed papillae produce a “ball in tee” or “lobster claw” sign on pyelography which represents the filling defect.29 However, as these patients often present with significant renal impairment, the use of intravenous contrast is often omitted to prevent further renal injury. Much like an obstructing ureteric calculus, if left untreated an obstructing slough papilla can precipitate renal failure or perpetuate sepsis if there is concomitant urinary tract infection. In such cases, decompression of the urinary collecting system either via percutaneous nephrostomy or ureteric stenting is indicated.30
Case Discussion; Causes for Obstructive Uropathy in the above Cases
In case one, bilateral hydroureteronephrosis to the level of the VUJ without a clear obstructive cause on CT KUB could be explained by either mechanism. As the patient’s three ureteric stents were removed at the conclusion of the case, VUJ edema, obstruction from sloughed papillae or a combination of both factors could be causative. During cystoscopy and retrograde pyelogram on the following day, large mobile filling defects were seen (Figures 2 and 3). On distal ureteroscopy these soft tissue masses were directly visualized (Figure 4). Re-insertion of ureteric stents resulted in prompt excretion of urine, contrast, and debris. Although the patient in case one did not have any additional risk factors for RPN and had a stable MAP throughout the case, modest reductions in blood pressure or normotensive ischemic renal injury may still be responsible for sloughed papillae.
Similarly, in case two, mild upstream hydronephrosis in the context of an appropriately positioned ureteric stent may support stent obstruction from VUJ edema or stent occlusion from slough papillae. However, due to the significant blood loss encountered in case two (4,000 mL) and fluctuant MAPs, renal ischemia and subsequent sloughing appears more likely. Additionally, prompt excretion of sloughy debris and urine was seen after replacement of the ureteric stent.
Conclusion
Prevention and early recognition of ureteric injury during major abdomino-pelvic surgery are paramount. The use or omission of prophylactic ureteric stents in major abdomino-pelvic surgery remains at the treating surgeons discretion. A higher rate of iatrogenic ureteric injury in patients with prophylactic ureteric stents is likely confounded by the complexity of the planned surgery, however the intraoperative identification and primary repair of this injuries is significantly improved in patients with ureteric stents. Ureteric stents are associated with a wide range of side-effects and complications; however, when inserted by senior staff, these risks can be reduced.
Patients with underlying risk factors for renal ischemia should have invasive blood pressure monitoring intra-operatively to allow thorough assessment and correction of changes in mean arterial pressure. Ureteric stent occlusion from slough papillae or VUJ edema should be considered early in patients who develop oligo-anuric renal failure following major surgery. The lack of hydronephrosis in the early post-operative period does not exclude any obstructive cause for renal failure. Early stent re-insertion or stent replacement should be considered in patients who develop oligo-anuric renal failure post-operatively despite adequate fluid replacement.
Key Points
- Ureteric stenting has not been proven to reduce iatrogenic ureteric injury but does improve early recognition of these injuries.
- Intraoperative optimization of renal blood flow may reduce the risk of papillary necrosis and sloughed papillae causing ureteric obstruction, especially in patients with risk factors for renal ischemia.
- In patients with oligo-anuria post-operatively early stent re-insertion or exchange of ureteric stents should be considered, irrespective of radiological signs of ureteric obstruction.
- The indwelling time of prophylactically inserted stents needs to be individualized.
- Ureteric stents inserted by adequately trained staff likely reduce stent-related complications.
Ethics and Consent
Consent was obtained from all involved patients to participate and publish case data. Institutional approval was not required to publish case details.
Disclosure
The authors do not have any conflicts of interest or financial contributions to disclose.
References
1. Andersen P, Andersen LM, Iversen LH. Iatrogenic ureteral injury in colorectal cancer surgery: a nationwide study comparing laparoscopic and open approaches. Surg Endos. 2015;29:1406. doi:10.1007/s00464-014-3814-1
2. El Abd AS, El-Abd SA, El-Enen MA, et al. Immediate and late management of iatrogenic ureteric injuries: 28 years of experience. Arab J Urology. 2015;13(4):250–257. doi:10.1016/j.aju.2015.07.004
3. Al-Awadi K, Kehinde EO, Al-Hunayan A, Al-Khayat A. Iatrogenic Ureteric Injuries: Incidence, Aetiological Factors and the Effect of Early Management on Subsequent Outcome. Int Urol Nephrol. 2005;37(2):235–241. doi:10.1007/s11255-004-7970-4
4. Nature Reviews Urology. Ureteric injury: a challenging condition to diagnose and manage; 2022. Available from: https://www.nature.com/articles/nrurol.2012.254.
5. Selzman AA, Spirnak JP. Iatrogenic Ureteral Injuries: A 20-Year Experience in Treating 165 Injuries. J Urol. 1996;155(3):878–881. doi:10.1016/S0022-5347(01)66332-8
6. Mahendran HA, Praveen S, Ho C et al. Iatrogenic Ureter Injuries: Eleven Years Experience in A Tertiary Hospital. Med J Malaysia. 2012;67(2):4.
7. Althumairi AA, Efron JE. Genitourinary Considerations in Reoperative and Complex Colorectal Surgery. Clin Colon Rectal Surg. 2016;29(2):145–151. doi:10.1055/s-0036-1580629
8. Hird AE, Nica A, Coburn NG, Kulkarni GS, Nam RK, Gien LT. Does prophylactic ureteric stenting at the time of colorectal surgery reduce the risk of ureteric injury? A systematic review and meta-analysis. Colorectal Dis. 2021;23(5):1060–1070. doi:10.1111/codi.15498
9. Summerton DJ, Lumen N, Serafetinides E Guidelines On Urological Trauma. Eur Ass Urology. 2014;2014:17.
10. Croghan SM, Zaborowski A, Mohan HM, et al. The sentinel stent? A systematic review of the role of prophylactic ureteric stenting prior to colorectal resections. Int J Colorectal Dis. 2019;34(7):1161–1178. doi:10.1007/s00384-019-03314-1
11. Colorectal Surgery | JAMA Surgery | JAMA Network. Prophylactic Ureteral Stent Placement vs No Ureteral Stent Placement During Open Colectomy; 2023. Available from: https://jamanetwork.com/journals/jamasurgery/article-abstract/2654946.
12. Andersen P, Andersen LM, Iversen LH. Iatrogenic ureteral injury in colorectal cancer surgery: a nationwide study comparing laparoscopic and open approaches. J Laparoendosc Surg. 2015;29:1406. doi:10.1007/s00464-014-3814-1.
13. Luks VL, Merola J, Arnold BN, Ibarra C, Pei KY. Prophylactic Ureteral Stenting in Laparoscopic Colectomy: Revisiting Traditional Practice. J Surg Res. 2019;234:161–166. doi:10.1016/j.jss.2018.09.041
14. Speicher PJ, Goldsmith ZG, Nussbaum DP, Turley RS, Peterson AC, Mantyh CR. Ureteral stenting in laparoscopic colorectal surgery. J Surg Res. 2014;190(1):98–103. doi:10.1016/j.jss.2014.02.025
15. Bothwell WN, Bleicher RJ, Dent TL. Prophylactic ureteral catheterization in colon surgery: a five-year review. Dis Colon Rect. 1994;37:330. doi:10.1007/BF02053592
16. Staubli SEL, Mordasini L, Engeler DS, Sauter R, Schmid HP, Abt D. Economic Aspects of Morbidity Caused by Ureteral Stents. Urologia Inter. 2016;97(1):91–97. doi:10.1159/000443379
17. Leijte JAP, Oddens JR, Lock TM. Holmium Laser Lithotripsy for Ureteral Calculi: Predictive Factors for Complications and Success. J Endourol. 2008;22(2):257–260. doi:10.1089/end.2007.0299
18. Sirota JH, Narins L. Acute Urinary Suppression after Ureteral Catheterization. N Engl J Med. 1957;257(23):1111–1113. doi:10.1056/NEJM195712052572303
19. Shearlock KT, Howards SS. Post-obstructive Anuria: a Documented Entity. J Urol. 1976;115(2):212–213. doi:10.1016/S0022-5347(17)59136-3
20. Hull JD, Kumar S, Pletka PG. Reflex Anuria from Unilateral Ureteral Obstruction. J Urol. 1980;123(2):265–266. doi:10.1016/S0022-5347(17)55891-7
21. Avni FE, Priso RH. Acute Urinary Tract Obstruction and Urological Emergencies. In: Avni EF, Petit P, editors. Imaging Acute Abdomen in Children. Cham: Springer International Publishing; 2018:267–275. doi:10.1007/978-3-319-63700-6_20
22. Udeaja YZ, Vikram SR. Reflex anuria post-prophylactic bilateral ureteric catheterisation: a rare postoperative complication. BMJ Case Reports CP. 2019;12(5):e227522. doi:10.1136/bcr-2018-227522
23. Boddy SAM, Nimmon CC, Jones S, et al. Acute Ureteric Dilatation for Ureteroscopy An Experimental Study. Br J Urol. 1988;61(1):27–31. doi:10.1111/j.1464-410X.1988.tb09156.x
24. Paul CJ, Brooks NA, Ghareeb GM, Tracy CR. Pilot Study to Determine Optimal Stent Duration Following Ureteroscopy: Three versus Seven days. Current Urology. 2017;11(2):97–102. doi:10.1159/000447201
25. Hiller SC, Daignault-Newton S, Rakic I, et al. Appropriateness Criteria for Ureteral Stent Omission following Ureteroscopy for Urinary Stone Disease. Urol Pract. 2022;9(3):253–263. doi:10.1097/UPJ.0000000000000302
26. Abuelo JG. Normotensive Ischemic Acute Renal Failure. N Engl J Med. 2007;357(8):797–805. doi:10.1056/NEJMra064398
27. Zadeii G, Lohr JW. Renal papillary necrosis in a patient with sickle cell trait. J Am Soc Nephrol. 1997;8(6):1034. doi:10.1681/ASN.V861034
28. Sutariya HC, Pandya VK. Renal Papillary Necrosis: Role of Radiology. J Clin Diagn Res. 2016;10(1):TD10–2. doi:10.7860/JCDR/2016/15092.7091
29. Xiang H, Han J, Ridley WE, Ridley LJ. Lobster claw sign: Renal papillary necrosis. J Med Imaging Radiat Oncol, 2018;62(Suppl1):90. doi:10.1111/1754-9485.37_12784
30. Pan HH, Luo YJ, Zhu QG, Ye LF. Renal papillary necrosis with urinary tract obstruction: A case report. World J Clin Cases. 2022;10(16):5400–5405. doi:10.12998/wjcc.v10.i16.5400
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
