Back to Journals » Drug, Healthcare and Patient Safety » Volume 15
The Prevalence and Severity of Potential Drug–Drug Interactions in Internal Medicine Ward at Soba Teaching Hospital
Authors Hamadouk RM , Alshareif EM, Hamad HM, Yousef BA
Received 12 September 2023
Accepted for publication 27 October 2023
Published 1 November 2023 Volume 2023:15 Pages 149—157
DOI https://doi.org/10.2147/DHPS.S436458
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Hemalkumar B Mehta
Riham M Hamadouk,1,2 Einass Mahmoud Alshareif,1 Huda M Hamad,1 Bashir A Yousef3
1Department of Clinical Pharmacy, Faculty of Pharmacy, University of Khartoum, Khartoum, Sudan; 2Department of Clinical Pharmacy and Pharmacy Practice, Faculty of Pharmacy, Almughtaribeen University, Khartoum, Sudan; 3Department of Pharmacology, Faculty of Pharmacy, University of Khartoum, Khartoum, Sudan
Correspondence: Bashir A Yousef, Department of Pharmacology, Faculty of Pharmacy, University of Khartoum, Al-Qasr Ave, Khartoum, 11111, Sudan, Tel +249155662037, Fax +249183780696, Email [email protected]
Background: Multiple drug therapies are commonly used to achieve a desired therapeutic goal, especially in hospitalized patients. However, drug–drug interactions might occur and threaten the patients’ safety.
Objective: This study aims to assess the prevalence and severity of potential drug–drug interactions (PDDIs) in the internal medicine ward at Soba Teaching Hospital.
Methods: A retrospective cross-sectional hospital-based study was carried out in the internal medicine ward at Soba Teaching Hospital from June 2021 to December 2021. The data was collected from patients’ medical records. PDDIs were identified using Lexicomp® drug interaction software.
Results: A total of 377 patients were included in this study, and overall prevalence of PDDIs was 62.9%. We have identified 989 potential DDIs and 345 pairs of interacting drugs, the mean of the PDDIs per patient was 4.17 ± 4.079. Among 345 PDDIs most were of moderate interactions 70.1% (n=242) followed by Minor interactions 19.1% (n=66). The most common type of interaction was of category C representing 63.5% (n=219). A significant association was observed between the occurrence of PDDIs with patients’ age, presence of chronic diseases, length of hospital stay, and number of medications received by the patients.
Conclusion: Drug–drug interactions were highly prevalent in the internal medicine ward. Therefore, certain attempts are required to increase the awareness of the physicians about these interactions and minimize their occurrence.
Keywords: potential drug–drug interactions, prevalence, severity, patient safety
Introduction
Patient safety is considered one of the most important principles of health care According to studies, 10% of the annual worldwide receivers of health care suffer from adverse events, either from medication or surgical errors.1 An adverse drug event (ADE) is defined as the physical or mental harm or even loss of function that could arise from the use of medications.2 There are many identified causes of ADE, but the most common cause of them is drug interactions. International reports showed that around 21% of the adverse drug event-related hospital admissions were results of drug interactions.3 A drug interaction happens when the patient’s response to a drug is changed by nutritional supplements, environmental factors, formulation excipients, food, other drugs, or disease.4
Drug–drug interactions (DDIs) are defined as changes in the drug’s efficacy or toxicity due to the concomitant administration of another drug.5 They may occur due to pharmacokinetic (PK) mechanisms, ie when one drug (the perpetrator) alters the concentration of another drug (the object), or due to pharmacodynamic (PD) mechanisms, ie, when the two drugs act on the same or interrelated target resulting in additive or opposing effects.6 Pharmacodynamic drug–drug interactions can be predicted by most physicians, on the other hand, pharmacokinetic drug–drug interactions are more difficult to predict and understand, and both of them can cause significant adverse reactions if not taken into consideration.7 Drug interactions are either real drug interactions that can be established in clinical practice or potential drug–drug interactions (PDDIs) in which a potentially harmful combination occurs.8
The consequences of these DDIs vary, from minor and undetectable to severe enough that can affect the patient’s health, increase the treatment costs, or even can lead to death.9 There are many factors associated with the occurrence of DDIs, and one of them is the use of multiple drug treatments, which might be necessary to achieve the required therapeutic goal.5
DDIs are highly prevalent in hospitalized patients,10 because of multiple and severe illnesses, multiple-drug treatments, and chronic therapeutic regimens.11 Krahenbuhl-Melcher et al suggest that DDIs account for 17% of all ADEs during hospitalization.12 Similarly, a study in Switzerland, concluded that 56.2% of patients were exposed to at least one major or moderate PDDI in the internal medicine ward.13 Also, a study was undertaken in two hospitals in Pakistan to investigate potential DDIs in internal medicine wards. Out of 400 patients, 53% patients had at least one potential DDI, and their potential adverse effects included damage to the liver, bleeding, ototoxicity, poisoning of the kidneys, and low or high blood sugar.14
Although globally several studies have evaluated the prevalence and severity of DDIs, very few studies have addressed drug–drug interactions and their prevalence in Sudan.15,16 Hence, the present study aims to review retrospectively hospitalized patients to determine the prevalence and severity of PDDIs in the internal medicine ward at Soba Teaching Hospital.
Methods
Study Design and Settings
A retrospective observational cross-sectional hospital-based study through the use of patients’ records as the source of data. The study was conducted at Soba Teaching Hospital targeting patients who were admitted to the internal medicine ward during the period from June 2021 to December 2021. At the time of the study, the internal medicine ward at Soba Teaching Hospital has a capacity of 24 beds for male patients and 18 beds for female patients.
Patient Selection
In this study, a total coverage sampling technique was applied. All inpatients at the internal medicine ward at Soba Teaching Hospital, who were 18 years of age and above were included in the study, whereas patients whose medical records were incomplete or included less than two drugs were excluded from the study. The total number of patients included in the study was 377 patients.
Data Collection
The reporting data of the current study conform to the STROBE guideline.17 The data were collected over 2 months, between April 2022 and June 2022, by the research team using data collection sheets designed for the study. The collection tool was verified by a pilot study using 15 data collection sheets before the study. The following information was collected: demographic information (age and sex), date of admission, date of discharge, chronic diseases, chronic medications, length of hospital stay, main diagnosis, number of medications provided in the hospital, details of medication therapy prescribed in the hospital including regular and PRN (pro- re-nata, which means as required). Lexicomp® drug interaction software was used to screen patients’ records for PDDIs. All drugs in each patient’s record were entered one by one into the interaction checker software.
Classification of DDIs
As mentioned above, DDIs were classified as PK and PD, another classification of DDIs is according to the risk rate that indicates the level of urgency and how to respond to the interaction. Lexicomp® classified the interactions into five categories: A means no known interactions (no evidence to support pharmacodynamic or pharmacokinetic interactions), B means no action needed (evidence demonstrates that two drugs may interact with each other but there is little to no clinical data to support it), C means to monitor therapy (evidence suggests that the two drugs may interact with each other in a clinically significant manner), D means considering therapy modification (evidence suggests that the two medications may interact with each other in a clinically significant manner, as results specific actions must be taken to minimize the toxicity resulting from the concomitant use of the medications), and X means avoid combination.18
Also, DDIs classified according to the severity of interaction, which indicated the magnitude of an interaction outcome. This classifies DDI into major if the interaction is possibly life-threatening or might cause permanent damage, moderate if the interaction may cause deterioration in the patient’s condition, or additional therapy or hospitalization may be required, and minor if the interaction is inconvenient but not medically significant.19
Statistical Analysis
The data were analyzed using the Statistical Package for Social Sciences software, version 23.0 (IBM SPSS Inc., Chicago, IL.) Descriptive statistics were performed to analyze continuous and categorical variables and the results were represented as percentage and frequency tables. The chi-square test was applied to identify whether there is a significant association between the occurrence of DDIs and sociodemographic and clinical characteristics of patients such as the age of the patients, hospital stay, number of prescribed drugs, and presence of chronic diseases, with a P value < 0.05 was considered statistically significant.
Ethical Considerations
The study was conducted agreeing with the recommendations of the Declaration of Helsinki, and the ethical approval to conduct the study was obtained from the Ethical Committee of the Faculty of Pharmacy, University of Khartoum (FPEC-28-2021). Additional approval was obtained from Soba Teaching Hospital to check the patient’s medical records at the hospital. Due to the retrospective nature of the study, the ethics committee specifically waived the requirement for informed consent for this study, and collected data were also assured of strict privacy and confidentiality, and the names and other personal identifiers of patients were not being registered.
Results
General Characteristics
After reviewing the medical records of patients hospitalized in the internal medicine ward during the period from June 2021 to December 2021, a total of 377 patients were found to fulfill the inclusion criteria. Among them, 194 (51.5%) were males and most of the patients 73 (19.4%) aged between 61 and 70 years, 69 (18.3%) were between 51 and 60 years, while 66 (17.5%) patients were between 18 and 30 years. Regarding the length of hospital stay, majority of the patients (45.9%) were hospitalized for 5–10 days, and 123 (32.6%) patients had at least one chronic disease. The number of medications administered to the patients at the hospital ranged from 2 to 18 drugs (mean administered drugs 6.67 ± 3.36), 124 (32.9%) patients received more than seven drugs, while 188 (49.9%) received from four to seven drugs. After analysis of DDIs using Lexicomp® software, PDDIs were detected in 237 (62.9%) patients who had at least one PDDI.
A total of 989 PDDIs and 345 types of interacting drug combinations were detected in this study. The PDDIs ranged from 1 to 24, and the mean of the PDDIs per patient was 4.17 ± 4.079. The number of PDDIs per patient was categorized as follows: less than 3 which was detected in 110 (46.4%) patients, from 3 to 6 in 78 (32.9%) patients, and more than 6 in 49 (20.7%) patients. More details are in Table 1.
 |
Table 1 Sociodemographic and Clinical Characteristics of All Patients Included in the Study (N=377) |
Among patients with PDDIs, the most common diagnosis was cardiovascular diseases 57 (24.1%) followed by infectious diseases 35 (14.8%), gastrointestinal diseases 34 (14.3%), and renal diseases 22 (9.3%). Some patients had multiple diagnoses, 13 (5.5%) patients had cardiovascular and infectious diseases and 12 (5.1%) patients had renal and infectious diseases. The rest of the patients’ distribution according to their diagnosis is in Table 2.
 |
Table 2 Distribution of Patients with Potential Drug–Drug Interactions According to Their Diagnosis (N=237) |
Types of PDDIs in the Internal Medicine Ward
Based on the level of severity, the most prevalent PDDIs among the 345 pairs of interacting drugs were moderate interactions 70.1% followed by Minor interactions 19.1%. Based on risk rating, the commonest kind of interaction was type C 63.5% followed by type B 20.3%, while type X represented 3.8% of all the interactions. More information is presented in Table 3. Furthermore, some of the most frequently occurring potential drug–drug interactions of moderate and major severity, and their risk rating category in addition to their potential adverse outcome are presented in Table 4.
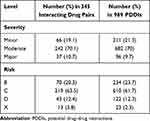 |
Table 3 The Severity and Risk Rating of the Identified Potential Drug–Drug Interactions |
 |
Table 4 The Most Common Interacting Drug Combinations of Major and Moderate Interactions and Their Risk Rating Categories |
The chi-square test showed that the incidence of PDDIs was significantly associated with the age of the patient (P = 0.001), the length of hospital stay (P = 0.001), the presence of chronic diseases (P = 0.000), and the number of medications received by the patients (P = 0.001). No significant association was found between the occurrence of DDIs and the gender of the patient (Table 5).
 |
Table 5 Relationship Between Patients’ Demographic and Clinical Characteristics and the Incidence of Potential Drug–Drug Interactions (N=377) |
Discussion
The improper use of drugs can change their intended role from therapeutic tools to harmful agents that may affect the quality of the patient’s life, increase morbidity and might cause mortality.20 Drug–drug interaction is one of the improper drug use that imposes dangerous clinical consequences and huge economic burdens.21
The present study is conducted to determine the prevalence and severity of PDDIs in the internal medicine ward at Soba Teaching Hospital. The overall prevalence of PDDIs was 62.9%, which came in accordance with several studies, where the prevalence of PDDIs was also high. Bhagavathula et al have found that the prevalence of PDDIs among patients in the internal medicine wards at an Ethiopian hospital was 78%.11 Also, in another study from Ethiopia, Teka et al found an overall prevalence of 62.2% among hospitalized patients.22 Further, in a study from Pakistan, the prevalence of PDDIs was reported to be 91.6%.9 Similar findings were also observed in studies from Iran,23 India,24 and Mexico.25 Although these studies were different in their study design and the characteristics of the study population yet, all of them identified that the increase in the number of medications is a predictor for the occurrence of PDDI in the patients, which coincide with what found in our study.
In contrast, some studies have reported a low prevalence of PDDIs. In a Canadian study, the prevalence of PDDIs among hospitalized patients was 18.8%,26 and in another study from Iran, the prevalence was 20.3% among hospitalized patients.27 Moreover, in a study conducted at a University Hospital in Thailand, the reported prevalence of PDDIs was 27.9%,28 while in a Brazilian hospital, it was found to be 37%.29
In this study, a total of 345 pairs of interacting combinations were identified, and the majority of the PDDIs (70.1%) were moderate interactions, these interactions have considerable potential to deteriorate the patient’s condition and may require medical treatment. Major interactions which may result in serious negative outcomes and may lead to death contributed to (10.7%), of the total PDDIs. While the rest (19.1%) contributed to minor interactions, which have limited or no clinical effects. These results are consistent with the results of other studies by Riechelmann et al, who found that moderate interactions attributed to 77% of the total identified PDDIs, while minor and major interactions represented 14% and 9%, respectively.30
In a study from a Swiss hospital, of the total identified PDDIs, 70.1% were moderate, 17.7% were minor, and 12.2% were major interactions.31 Furthermore, a study conducted in Ethiopia showed that of the detected PDDIs: 61.2% were moderate, 26% were minor, and 12.8% were major interactions.11 In contrast to our results, major interactions were the most prevalent interactions in the study by Murtaza et al, which found that 86.3% of the PDDIs were major interactions.9 Also, Teka et al have found that 32.9% of the patients had as a minimum one major interaction.22
Regarding risk rating, type C interaction was the most prevalent type among all interactions accounting for 63.5% fortunately, these interactions are not expected to cause serious or fatal consequences, and an appropriate monitoring plan will be effective in preventing any negative outcomes. On the other hand, the higher risk rating type D and type X, which necessitate taking specific actions to minimize the toxicity in the first and to avoid combination in the second together account for 16.2% of all the interactions. These results are comparable to the findings of other studies that reported the risk rating of PDDIs.10,32–34
It has been documented in the literature that many factors are associated with the occurrence of DDIs, including advanced age, certain diseases such as renal failures and cirrhosis, concomitant administration of a large number of drugs, and duration of therapy.35,36 In our study, we found some factors significantly associated with the occurrence of PDDIs that include patients’ age, presence of chronic diseases, length of hospital stay, and number of medications received by the patients. Significant associations between various factors and PDDIs have also been reported in different other studies. In a study conducted in a university hospital in Thailand, the results showed old age and polypharmacy were significantly associated with the presence of DDIs.28 In another study that aimed to assess PDDIs in hospitalized cardiac patients,9 a significant association was found between the incidence of PDDIs and the age of 60 years or above, longer hospital stay, and a higher number of medications prescribed to patients. Further, a study conducted in Pakistan14 also found associations between the presence of PDDIs with advanced age, increased length of hospitalization, and increased number of prescribed medications.
In addition, two Ethiopian studies have found a significant association between PDDIs and an increased number of drugs, but both of them did not find a significant association with gender or length of hospital stay.11,21 Apart from the type and severity of the identified PDDIs, the current study has recorded a high prevalence of PDDIs in the internal medicine ward at Soba Teaching Hospital. Many of these interactions can be minimized or even prevented by using alternative medications that are not related to the drug interactions. Even though polypharmacy may be necessary for specific conditions,37 careful selection and balancing in using multiple drugs is important to avoid the harmful consequences of drug interactions.
This study has some limitations. First, our study is a retrospective observational study, and as there is no direct interaction with the patients, the identified DDIs may not actually occur. This study did not measure the actual adverse clinical effects of the identified PDDIs on the patients. Also, because the study is medical record-based, nonprescription medications were not taken into account. Furthermore, the contribution of factors such as the type and number of chronic diseases, a specific diagnosis, and the use of a specific class of drugs were not investigated in this study.
Conclusion
From our study, it can be concluded that there is a high prevalence of PDDIs in the internal medicine ward. The majority of the identified PDDIs had moderate severity and belonged to risk category C. A considerable number of major PDDIs were also recorded. This study also depicted that there is a significant association between patients’ age, hospital stay, the number of prescribed medications, and the presence of chronic diseases. To avoid PDDIs certain attempts must be made. The use of software screening tools to detect PDDIs by health-care professionals must be encouraged. Continuous medical education sessions must be conducted, emphasizing on drug-drug interactions. Also, assigning clinical pharmacists with their proper role may result in avoiding these PDDIs from the beginning.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Makkaoui N, Halaoui A, Atoui Z, et al. Knowledge, attitudes, and practices regarding drug interactions among community pharmacists. J Public Heal. 2021;29(6):1357–1363. doi:10.1007/s10389-020-01252-9
2. Tsui V, Thomas D, Tian S, et al. Adverse Drug Events, Medication Errors, and Drug Interactions. In: Clinical Pharmacy Education, Practice and Research. Elsevier; 2019:227–245.
3. Gebretsadik Z, Gebrehans M, Getnet D, Gebrie D, Alema T, Belay YB. Assessment of drug-drug interaction in ayder comprehensive specialized Hospital, Mekelle, Northern Ethiopia: a Retrospective Study. Biomed Res Int. 2017;2017:9792363. doi:10.1155/2017/9792363
4. Snyder BD, Polasek TM, Doogue MP. Drug interactions: principles and practice. Aust Prescr. 2012;35(3):85–88. doi:10.18773/austprescr.2012.037
5. Zheng WY, Richardson LC, Li L, Day RO, Westbrook JI, Baysari MT. Drug-drug interactions and their harmful effects in hospitalised patients: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2018;74(1):15–27. doi:10.1007/s00228-017-2357-5
6. Shetty V, Chowta MN, Chowta KN, Shenoy A, Kamath A, Kamath P. Evaluation of potential drug-drug interactions with medications prescribed to geriatric patients in a tertiary care hospital. J Aging Res. 2018;2018:5728957. doi:10.1155/2018/5728957
7. Georgiev KD, Hvarchanova N, Stoychev E, Kanazirev B. Prevalence of polypharmacy and risk of potential drug-drug interactions among hospitalized patients with emphasis on the pharmacokinetics. Sci Prog. 2022;105(1):003685042110701. doi:10.1177/00368504211070183
8. Korucu FC, Senyigit E, Köstek O, et al. A retrospective study on potential drug interactions: a single center experience. J Oncol Sci. 2018;4(2):80–84. doi:10.1016/j.jons.2018.06.001
9. Murtaza G, Yasir M, Khan G, Azhar S, Ali S, Khan TM. Assessment of potential drug – drug interactions and its associated factors in the hospitalized cardiac patients. Saudi Pharm J. 2016;24(2):220–225. doi:10.1016/j.jsps.2015.03.009
10. Nusair MB, Al-azzam SI, Arabyat RM, Amawi HA, Alzoubi KH, Rabah AA. The prevalence and severity of potential drug-drug interactions among adult polypharmacy patients at outpatient clinics in Jordan. Saudi Pharm J. 2020;28(2):155–160. doi:10.1016/j.jsps.2019.11.009
11. Bhagavathula AS, Berhanie A, Tigistu H, et al. Prevalence of potential drug-drug interactions among internal medicine ward in University of Gondar Teaching Hospital, Ethiopia. Asian Pac J Trop Biomed. 2014;4(Suppl 1):S204–8. doi:10.12980/APJTB.4.2014C1172
12. Krähenbühl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krähenbühl S. Drug-related problems in hospitals: a review of the recent literature. Drug Saf. 2007;30(5):379–407. doi:10.2165/00002018-200730050-00003
13. Vonbach P, Dubied A, Krähenbühl S, Beer JH. Prevalence of drug-drug interactions at hospital entry and during hospital stay of patients in internal medicine. Eur J Intern Med. 2008;19(6):413–420. doi:10.1016/j.ejim.2007.12.002
14. Ismail M, Iqbal Z, Khattak MB, et al. Potential drug-drug interactions in internal medicine wards in hospital setting in Pakistan. Int J Clin Pharm. 2013;35(3):455–462. doi:10.1007/s11096-013-9764-1
15. Osman MA, Abdalla MA, Mohamed AA, Yousef BA. Assessment of drug–drug interactions between chemotherapeutic and chronically used medications at Khartoum oncology hospital. Matrix Sci Medica. 2020;4(3):79–84. doi:10.4103/MTSM.MTSM_17_20
16. Hamadouk RM, Albashair ED, Mohammed FM, Yousef BA. The practice of the community pharmacists in managing potential drug-drug interactions: a simulated patient visits. Integr Pharm Res Pract. 2022;11:71–84. doi:10.2147/IPRP.S355675
17. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):1623–1627. doi:10.1371/journal.pmed.0040296
18. Lexicomp® Drug Interact. Wolters Kluwer. Available from: https://online.lexi.com/loc/action/home.
19. Van LRWF, Swart EL, Boven E, Boom FA, Schuitenmaker MG, Hugtenburg JG. Potential drug interactions in cancer therapy: a prevalence study using an advanced screening method. Ann Oncol. 2011;22(10):2334–2341. doi:10.1093/annonc/mdq761
20. Abunahlah N, Elawaisi A, Velibeyoglu FM, Sancar M. Drug related problems identified by clinical pharmacist at the internal medicine Ward in Turkey. Int J Clin Pharm. 2018;40(2):360–367. doi:10.1007/s11096-017-0585-5
21. World Health Organization. Safety of Medicines: A Guide to Detecting and Reporting Adverse Drug Reactions: Why Health Professionals Need to Take Action. Geneva: World Health Organization; 2002.
22. Teka F, Teklay G, Ayalew E, Teshome T. Potential drug-drug interactions among elderly patients admitted to medical ward of ayder referral hospital, Northern Ethiopia: a cross sectional study. BMC Res Notes. 2016;9(1):431. doi:10.1186/s13104-016-2238-5
23. Mousavi S, Ghanbari G. Potential drug-drug interactions among hospitalized patients in a developing country. Casp J Intern Med. 2017;8(4):282–288.
24. Kulkarni V, Bora SS, Sirisha S, Saji M, Sundaran S. A study on drug–drug interactions through prescription analysis in a South Indian teaching hospital. Ther Adv Drug Saf. 2013;4(4):141–146. doi:10.1177/2042098613490009
25. Doubova SV, Reyes-Morales H, Torres-Arreola LDP, Suárez-Ortega M. Potential drug-drug and drug-disease interactions in prescriptions for ambulatory patients over 50 years of age in family medicine clinics in Mexico City. BMC Health Serv Res. 2007;7(147). doi:10.1186/1472-6963-7-147
26. Reimche L, Forster AJ, Van Walraven C. Incidence and contributors to potential drug-drug interactions in hospitalized patients. J Clin Pharmacol. 2011;51(7):1043–1050. doi:10.1177/0091270010378858
27. Sepehri G, Khazaelli P, Dahooie FA, Sepehri E, Dehghani MR. Prevalence of potential drug interactions in an Iranian general hospital. Indian J Pharm Sci. 2012;74(1):75–79. doi:10.4103/0250-474X.102548
28. Janchawee B, Wongpoowarak W, Owatranporn T, Chongsuvivatwong V. Pharmacoepidemiologic study of potential drug interactions in outpatients of a university hospital in Thailand. J Clin Pharm Ther. 2005;30(1):13–20. doi:10.1111/j.1365-2710.2004.00598.x
29. Moura C, Acurcio F, Belo N. Drug-drug interactions associated with length of stay and cost of hospitalization. J Pharm Pharm Sci. 2009;12(3):266–272. doi:10.18433/J35C7Z
30. Riechelmann RP, Tannock IF, Wang L, Saad ED, Taback NA, Krzyzanowska MK. Potential drug interactions and duplicate prescriptions among cancer patients. J Natl Cancer Inst. 2007;99(8):592–600. doi:10.1093/jnci/djk130
31. Egger SS, Drewe J, Schlienger RG. Potential drug-drug interactions in the medication of medical patients at hospital discharge. Eur J Clin Pharmacol. 2003;58(11):773–778. doi:10.1007/s00228-002-0557-z
32. Andersson ML, Böttiger Y, Kockum H, Eiermann B. High prevalence of drug–drug interactions in primary health care is caused by prescriptions from other healthcare units. Basic Clin Pharmacol Toxicol. 2018;122(5):512–516. doi:10.1111/bcpt.12939
33. Jazbar J, Locatelli I, Horvat N, Kos M. Clinically relevant potential drug–drug interactions among outpatients: a nationwide database study. Res Soc Adm Pharm. 2018;14(6):572–580. doi:10.1016/j.sapharm.2017.07.004
34. Dirin MM, Mousavi S, Afshari AR, Tabrizian K, Ashrafi MH. Potential drug ‑ drug interactions in prescriptions dispensed in community and hospital pharmacies in East of Iran. J Res Pharm Pract. 2014;3(3). doi:10.4103/2279-042X.141118
35. Hasnain H, Ali H, Zafar F, et al. Drug-drug interaction; facts and comparisons with national and international bench marks. A threat more than a challenge for patient safety in clinical and economic scenario. Prof Med J. 2017;24(03):357–365.
36. Riaz MK. Potential drug-drug interactions and strategies for their detection and prevention. Farmacia. 2019;67(4):572–579. doi:10.31925/farmacia.2019.4.3
37. Bojuwoye AO, Suleman F, Perumal-Pillay VA. Polypharmacy and the occurrence of potential drug–drug interactions among geriatric patients at the outpatient pharmacy department of a regional hospital in Durban, South Africa. J Pharm Policy Pract. 2022;15(1):1–12. doi:10.1186/s40545-021-00401-z
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
