Back to Journals » Drug Design, Development and Therapy » Volume 18
The Potential Application of Aloe Barbadensis Mill. as Chinese Medicine for Constipation: Mini-Review
Authors Huang WR, Fang QH, Yu XB, Ge WH, Yu Y
Received 25 October 2023
Accepted for publication 16 January 2024
Published 3 February 2024 Volume 2024:18 Pages 307—324
DOI https://doi.org/10.2147/DDDT.S446563
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Wei-Rui Huang,1,* Quan-Hui Fang,1,* Xiang-Bin Yu,1 Wei-Hong Ge,2,3 Yue Yu1
1School of Pharmacy & Fujian Center for New Drug Safety Evaluation, Fujian Medical University, Fuzhou, 350122, People’s Republic of China; 2Department of Pharmacy, Nanjing Drum Tower Hospital, Nanjing, 210008, People’s Republic of China; 3School of Pharmacy, Macau University of Science and Technology, Macau SAR, 999078, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yue Yu, School of Pharmacy & Fujian Center for New Drug Safety Evaluation, Fujian Medical University, Fuzhou, 350122, People’s Republic of China, Email [email protected] Wei-Hong Ge, Department of Pharmacy, Nanjing Drum Tower Hospital, Nanjing, 210008, People’s Republic of China, Email [email protected]
Abstract: Aloe barbadensis Mill. has a long history of medicinal use in the annals of traditional Chinese medicine, wherein it has garnered considerable renown. Its multifaceted therapeutic properties, characterized by its anti-inflammatory and antibacterial attributes, alongside its established efficacy as a laxative agent, have been extensively documented. This review commences with an exploration of the nomenclature, fundamental characteristics, and principal constituents of Aloe barbadensis Mill. responsible for its laxative effects. Subsequently, we delve into an extensive examination of the molecular mechanisms underlying Aloe barbadensis Mill.’s laxative properties, types of constipation treatments, commercially available preparations, considerations pertaining to toxicity, and its clinical applications. This review aims to serve as a comprehensive reference point for healthcare professionals and researchers, fostering an enhanced understanding of the optimal utilization of Aloe barbadensis Mill. in the treatment of constipation.
Keywords: Aloe barbadensis Mill, constipation, laxative
Graphical Abstract:
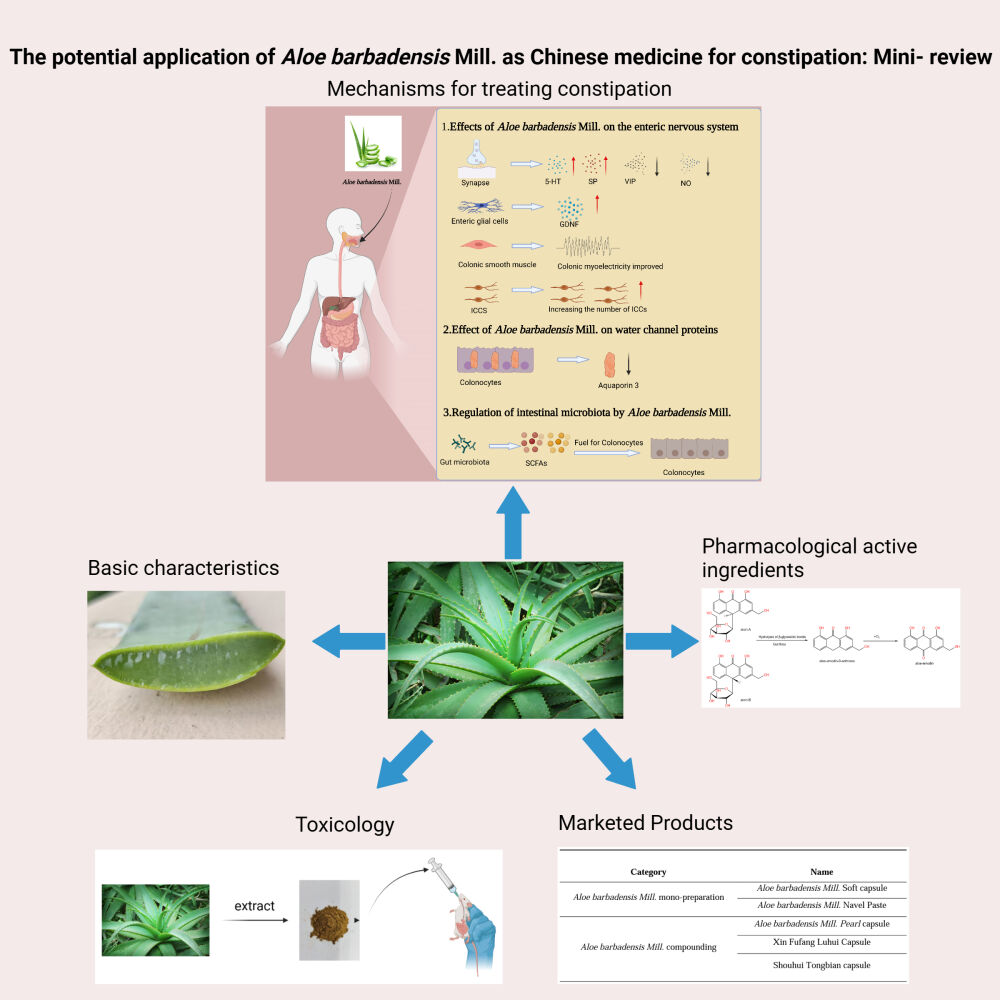
Introduction
Constipation is a prevalent gastrointestinal disorder with a global incidence ranging between 12% and 19%.1 Owing to the impact of occupational circumstances, a substantial proportion of individuals, roughly 70%, in contemporary society are in a suboptimal state of health, characterized by gastrointestinal discomfort, of which constipation is a predominant manifestation.2 The pathophysiology of this disorder is intricate, often arising from a confluence of factors. Although constipation lacks a standardized definition, it is generally typified by clinical indicators encompassing infrequent bowel movements (typically occurring less than three times per week), diminished fecal water content, heightened straining during defecation, and a pervasive sense of incomplete bowel evacuation.3 Prolonged instances of constipation may instigate complications such as rectal prolapse, pubic nerve damage, and hemorrhoids. Furthermore, constipation commonly recurs, and its chronic nature can detrimentally affect the patient’s quality of life, inducing psychological distress and perpetuating a cyclical exacerbation of constipation symptoms.
Aloe barbadensis Mill. is an enduring succulent known for its tenacity in arid environments.4 It has garnered substantial scientific attention due to its historical utilization in traditional medicine.5 Aloe barbadensis Mill. exhibits a multitude of pharmacological activities, such as treatment for constipation, anti-inflammatory, anti-obesity, and blood glucose and cholesterol regulatory effects.6 The origin of its name can be traced back to the Arabic term “alloeh” and the Hebrew term “allal”, both denoting the extraction of bitter sap from the plant’s leaves. Aloe barbadensis Mill., a versatile plant, exhibits a multitude of names, reflecting the diverse geographical and cultural contexts in which it is cultivated. In Latin, French, Portuguese, and German, it is referred to as “Aloe”, while in English, it is commonly known as “Crocodile’s Tongue”. Other regional names include “Jadam” in Malaysia, “Luhui” in China, “Sa’villa” in Spain, “Musabbar” in India, “Ailwa” among Indians, “Sabbar” in Arabic, and “Nassau” in the Philippines.7 This array of nomenclature exemplifies the rich linguistic and cultural heritage associated with Aloe barbadensis Mill.
Aloe barbadensis Mill. has accrued a rich historical legacy as an agent of therapeutic significance, both topically and orally, in the management of constipation across diverse regions worldwide. This review covers the literature between March 1958 and September 2023. With the keywords Aloe barbadensis Mill., aloin, aloe-emodin, laxative, and constipation, the literature is retrieved from electronic databases, such as Web of Science, Elsevier Science Direct, PubMed, CNKI, and others.
The Basic Characteristics of Aloe barbadensis Mill
Structural Composition of Aloe barbadensis Mill
Aloe barbadensis Mill., originating from the Arabian Peninsula, was introduced to the island of Curacao in the 16th century by Spanish colonizers.8 Among the 300 dispersed aloe species worldwide, certain species such as Aloe ferox Mill., Aloe arborescens Mill., and Aloe barbadensis Mill. are recognized as being edible. However, Aloe barbadensis Mill. are distinguished for their medicinal properties and demonstrating the most potent therapeutic value. As a consequence, Aloe barbadensis Mill. has been the subject of extensive research, emerging as the most extensively investigated and promising species of Aloe barbadensis Mill., with a diverse range of applications in the development of food, health products, and cosmetics.9 The leaves of Aloe barbadensis Mill. are long and plump, varying in width, generally triangular or elliptical, with numerous small bumps on the leaf surface, and are covered with a white powder (Figure 1A). Aloe curaçao can be divided into three parts, the outer, middle, and inner layer (Figure 1B). The outer layer, commonly known as the epidermis layer, comprises a robust and rigid cuticularized skin that endows the plant with protective attributes and serves as a site for synthesizing essential biomolecules including carbohydrates and proteins.10 The inner layer (gel layer) is the transparent gel where Aloe barbadensis Mill. stores nutrients and is rich in polysaccharides, minerals, proteins, vitamins (A, B, C, and E), and other active ingredients.11 The middle layer (latex layer) contains yellow latex-like mucilage that acts as a barrier to preserve the inner gel in a sterile state.12
 |
Figure 1 (A) Aloe barbadensis Mill. and (B) its Structure. |
Processing of Aloe barbadensis Mill
Aloe barbadensis Mill., a well-established botanical entity acclaimed for its medicinal attributes, presents itself in four discernible extracted manifestations: gel, dry powder, latex, and whole leaf extract.13 Aloe barbadensis Mill. undergoes various processing methods to derive different products. The first method involves fresh leaf processing, whereby the harvested Aloe barbadensis Mill. leaves are meticulously cleaned, cut, peeled, and de-thorned to yield Aloe barbadensis Mill. gel or fresh juice.14 Alternatively, the second method, dry leaf processing, entails air-drying or arching the harvested leaves, resulting in the production of dry leaves or dry powder.15 The third technique, known as emulsion processing, involves cutting the Aloe barbadensis Mill. leaves to collect the bitter yellow liquid that flows out. Subsequently, this liquid is boiled to obtain a thick paste-like substance, which is then sun-dried, resulting in the formation of a black solid, recognized as the traditional Chinese medicine Aloe barbadensis Mill.16 Finally, in the whole leaf extract processing method, the Aloe barbadensis Mill. leaves are meticulously cleaned, cut, peeled, and de-stemmed, then these processed leaves are then subjected to solvent extraction, yielding the whole leaf extract.17
The Historical and Traditional Use of Aloe barbadensis Mill. for Constipation
Traditional Use as a Natural Remedy for Constipation
The therapeutic potential of Aloe barbadensis Mill. has been meticulously documented throughout history (Figure 2), with its earliest known utilization dating back to 1550 BC as evidenced in the “Ebers Papyrus”, an ancient Egyptian medical manuscript.18 This ancient text notably underscores the medicinal applications of Aloe barbadensis Mill. during that era. Moreover, a captivating legend surrounds Aloe barbadensis Mill., with the plant holding immense significance for the Pharaohs of ancient Egypt due to its perceived capacity to bestow longevity. Funeral customs mandated the inclusion of an aloe staff as a symbol of rebirth, presented to the deceased during the ceremony. Aloe barbadensis Mill. was also extensively cultivated in proximity to the pyramids and along the routes leading to the Valley of the Kings. It was believed to offer sustenance and care to the Pharaoh on their journey to the afterlife. The blossoming of Aloe barbadensis Mill. was considered a favorable omen, signifying the successful transition of the departed soul to the ‘other shore’.19 Over a millennium and a half later, the Greek physician Dioscorides provided the earliest comprehensive description of the pharmacological effects of Aloe barbadensis Mill. in his “De Materia Medica” written in 50 BC.20 Dioscorides emphasized that the juice of Aloe barbadensis Mill., rather than the gel, was the active component for treating constipation. Processed Aloe barbadensis Mill., as dried Aloe barbadensis Mill. juice, was introduced to England in 1300 AD to alleviate gastrointestinal disorders. Eventually, Aloe barbadensis Mill. was recognized as a remedy for constipation in the United States Pharmacopoeia in 1820.21 In the Oriental region, during the late Sui and early Tang dynasties, traders of Arab origin introduced Aloe barbadensis Mill. to China. The first written record of Aloe barbadensis Mill. in China is found in the “Treatise on Medicinal Properties”, written by Zhen Quan during the Tang dynasty, in which he reported Aloe barbadensis Mill. ‘s efficacy in treating “children’s chancre”. During the Song Dynasty, the “Kai Bao Ben Cao”, compiled by Liu Han, documented Aloe barbadensis Mill.’s effectiveness in treating “three insects”, hemorrhoidal fistulas, and croton poisoning. Li Shizhen’s “Compendium of Materia Medica”, written during the Ming Dynasty, specified that Aloe barbadensis Mill. was used to treat children’s spleen chancre.22 “The contemporary National Pharmacopoeia Committee documented the medicinal properties of Aloe barbadensis Mill. in the Chinese Pharmacopoeia, describing it as diaphoretic and treatment for constipation, able to clear liver and fire, kill worms and treat noma”. Above all, we reviewed the utilization of Aloe barbadensis Mill. for ameliorating constipation has been widely documented across Western and Eastern medicinal practices. This historical deployment underscores the enduring acknowledgment of the potential therapeutic advantages associated with Aloe barbadensis Mill.
 |
Figure 2 Historical Texts Documenting the Efficacy of Aloe barbadensis Mill. in Treating Constipation. |
Pharmacological Activity of Aloe Barbadensis Mill
The therapeutic efficacy of Aloe barbadensis Mill. stems from its abundant repertoire of bioactive constituents, primarily sourced from the gel and latex, two anatomically distinct segments of the plant. These specialized constituents encompass a multitude of active compounds, which collectively contribute to the extensive array of biological functionalities displayed by Aloe barbadensis Mill. This part delves into the intricate interplay of these bioactive constituents, shedding light on their individual and synergistic contributions to the plant’s therapeutic properties.
Pharmacological Active Sites of Aloe Barbadensis Mill
Aloe Barbadensis Mill. Gel
The gel residing within the leaves of Aloe barbadensis Mill. encompasses a diverse array of polysaccharides, vitamins, minerals, enzymes, and amino acids, thereby manifesting an extensive range of pharmacological properties including antidiabetic, immunomodulatory, anti-inflammatory, antioxidant, wound-healing promotion, anticancer, hepatoprotective, and antibacterial activities, among others.23 The NMR spectrum of Aloe barbadensis Mill. by Bozzi et al24 clearly exhibits signals corresponding to glucose, malic acid, and the polysaccharide acemannan, which are the three primary natural components found in Aloe barbadensis Mill. gel. A recent investigation has demonstrated the potential of Aloe barbadensis Mill. gel in mitigating the deleterious effects of non-steroidal anti-inflammatory drugs on the small bowel by augmenting the expression of mucin.25 Furthermore, several scholarly inquiries have explored the protective properties of Aloe barbadensis Mill. gel on the intestinal tract in a simulated condition of acetic acid-induced colitis.26 These studies suggest that Aloe barbadensis Mill. gel exerts a safeguarding influence on the intestinal tract through its anti-inflammatory mechanisms, thereby fostering the preservation of gastrointestinal integrity and facilitating treatment for constipation.
Aloe Barbadensis Mill. Latex
In elucidating the composition of latex obtained from Aloe barbadensis Mill., Kim et al27 employed high-performance liquid chromatography, with monitoring conducted at a wavelength of 254 nm. The principal constituents identified in Aloe barbadensis Mill. latex predominantly consist of anthraquinones, specifically aloin A, aloin B, aloenin A, aloenin B, and aloesin. Significantly, these components exhibit varied pharmacological properties, encompassing analgesic, anti-inflammatory, antioxidant, and constipation-treatment effects. This exhaustive analysis, utilizing high-performance liquid chromatography, serves to furnish valuable insights into the chemical composition of Aloe barbadensis Mill. latex,
The Main Treatment for Constipation Pharmacological Active Ingredient —aloin
Molecular Mechanisms of Aloin in the Treatment of Constipation
Aloin (C21H22O9) is the primary active component in this layer of latex and is usually present in Aloe barbadensis Mill. as a pair of non-corresponding isomers, aloin A (barbaloin), and aloin B (iso barbaloin), with aloin A being the predominant conformation. The glycosides in aloin are linked to sugars by β-glycosidic bonds, which are resistant to most bacterial β-glycosidases and can only be hydrolyzed by certain flora in the human and animal intestines. There are large differences in their hydrolysis ability by different species of intestinal flora. Generally, human intestinal flora has the strongest ability to hydrolyze the β-glycosidic bonds of aloe glycosides, followed by rats, and the weakest by mice.28 Upon ingestion, aloin can resist degradation in the upper gastrointestinal tract and reach the large intestine, where it undergoes enzymatic hydrolysis to form glucose and aloe-emodin-9-anthrone. The latter is then reoxidized to aloe-emodin29 (Figure 3), which is absorbed by the intestinal epithelial cells and exerts an inhibitory effect on the Na+/K+-ATPase activity of the superficial epithelial cells in the distal colon.30 This leads to a reduction in water and sodium reabsorption, resulting in water retention in the intestinal lumen and subsequent stool softening. Furthermore, aloe-emodin can directly activate CFTR (Cystic Fibrosis Transmembrane Conductance Regulator) chloride channels, leading to the release of chloride ions into the luminal surface of epithelial cells and an increase in the concentration of chloride ions in the lumen. This results in fluid transport, which further contributes to the softening of stools and facilitates their excretion.
 |
Figure 3 Schematic diagram of the metabolism of aloin. |
Pharmacokinetics of Aloin
Despite being the most potent treatment for constipation compound found in Aloe barbadensis Mill., aloin exhibits limited oral bioavailability. In a study conducted by Niu et al, the investigation focused on assessing the absorption, bioavailability, pharmacokinetic parameters, and distribution and elimination of Aloin-A in rats following oral and intravenous administration. The study revealed that Aloin-A exhibited rapid absorption into the blood circulatory system after oral dosing, as evidenced by the attainment of a maximum plasma concentration (Cmax) of 412.89 ng/mL at 0.25 hours. However, the calculated absolute bioavailability of Aloin-A amounted to a mere 5.79%, thereby indicating suboptimal absorption. This phenomenon could potentially be ascribed to the expedited hydrolysis of anthraquinone glycosides catalyzed by intestinal bacteria. The pharmacokinetic analysis further demonstrated that the AUC(0-t)values after oral (10 mg/kg) and iv (2 mg/kg) administration were calculated to be 391.23 and 1352.56 μg/L*h, respectively, whereas, the AUC(0-∞) values were 421.13 and 1359.86 μg/L*h, respectively. Moreover, Regarding distribution and elimination, the apparent volume of distribution of Aloin-A was significantly greater than the total blood volume in rats, indicating the extensive distribution of Aloin-A in tissues beyond the plasma. The observed half-life (T1/2) values of aloin were determined to be 3.96 hours after oral administration and 2.43 hours following intravenous administration, indicative of a swift elimination process from the blood circulatory system.31 Another investigation conducted by Park et al unveiled the tissue distribution and metabolism of aloin. Tissue distribution analysis revealed the presence of aloin in diverse tissues, with the liver and intestine exhibiting the highest concentrations at 0.5 hours post-administration. Specifically, the liver demonstrated peak levels of 77.15 ± 15.92 ng/g, while the intestine exhibited peak levels of 102.37 ± 14.12 ng/g. However, the levels of aloin in tissues experienced a rapid decline within 90 minutes. In terms of metabolism and excretion, a considerable portion of aloin likely undergoes metabolism or excretion through feces, as evidenced by the relatively low percentage (0.03%) of the initial dose excreted in urine within 24 hours. Significantly, the urinary excretion of aloin A and aloin B collectively constituted 64.97% and 9.7% of the administered dosages, respectively, during the 24-hour period.32 These findings present significant observations regarding the absorption, distribution, metabolism, and excretion profiles of aloin within the human body, thereby establishing a significant basis for advancing the accuracy and efficacy of aloin-based interventions for constipation.
Potential Mechanisms of Aloe barbadensis Mill. as Remedy for Constipation
As reported, the treatment for constipation effect of Aloe barbadensis Mill. was potential by influencing the enteric nervous system, water channel proteins, and gut microbiota. The multifaceted mecha-nisms by which Aloe barbadensis Mill. produces treatment for constipation effects, as illustrated in Figure 4, through which, culminates in the therapeutic aim of alleviating constipation.
 |
Figure 4 Potential mechanisms of Aloe barbadensis Mill. in the treatment of constipation. Created with Biorender.com. |
Effects of Aloe barbadensis Mill. On the Enteric Nervous System
The enteric nervous system (ENS) is a complex neural network composed of neurons and glial cells located within the intestinal wall, which possesses a remarkable degree of autonomy in regulating the intestinal motor, absorptive, and secretory functions, thereby earning the epithet of the “second brain” of the body.33 Dysfunction of the ENS can result from various causes such as neurotransmitter imbalances, structural changes in the ganglia, and neuronal loss, leading to compromised regulatory functions and the eventual onset of constipation. Notably, Aloe barbadensis Mill. possesses therapeutic potential for the management of constipation by ameliorating the pathological condition of ENS.
Neurotransmitters
An imbalance of intestinal neurotransmitters, a class of active small molecule peptides produced in endocrine and nerve cells within the gastrointestinal tract is associated with constipation. The enteric nervous system (ENS) contains two types of neurotransmitters: excitatory neurotransmitters, such as pentraxin (5-HT) and substance P (SP), which promote intestinal smooth muscle contraction and enhance intestinal propulsion; and inhibitory neurotransmitters, such as vasoactive peptide (VIP) and nitric oxide (NO), which relax intestinal smooth muscle and inhibit intestinal motility. Aloe barbadensis Mill. has been observed to modulate the levels of both excitatory and inhibitory neurotransmitters, thereby influencing gastrointestinal motility and intestinal fluid secretion, ultimately manifesting as a therapeutic effect in the treatment of constipation.
Tang et al conducted a study to evaluate the impact of different doses of Aloe barbadensis Mill. on colonic neurotransmitters in mice. The study found increased 5-HT content in mice serum, leading to an accelerated response of the intestinal wall plexus and consequently, faster intestinal peristalsis. Moreover, the intervention resulted in increased SP and decreased VIP content in mice serum, promoting the contraction of intestinal smooth muscle and enhancing intestinal propulsion.34 Beck et al conducted a study that demonstrated the inhibitory effect of nitric oxide (NO) on gastrointestinal motility by reducing intracellular calcium levels and relaxing smooth muscle cells through stimulation of intracellular soluble guanylyl cyclase (sGC).35 Meanwhile, Izzo et al showed that Aloe barbadensis Mill. can enhance smooth muscle contraction in the gastrointestinal tract and promote intestinal peristalsis by inhibiting NO synthesis.36
Glial Cell-Derived Neurotrophic Factor
The Glial cell-derived neurotrophic factor (GDNF) is a neurotrophic factor secreted by glial cells that has demonstrated efficacy in promoting neuronal growth, differentiation, and repair. Within the digestive tract, GDNF is primarily localized to the smooth muscle cells of the gastrointestinal tract, with significantly higher expression levels observed in the colon compared to other regions. The pronounced regional distribution of GDNF within the colon suggests a crucial function in modulating the neuralization processes that govern the muscular layers of this organ.37 In the context of disorders affecting the enteric nervous system, GDNF has demonstrated notable efficacy in attenuating nerve lesions and improving intestinal motility in affected colon segments.
A study has reported that individuals with constipation exhibit significantly lower serum levels of GDNF, potentially compromising the integrity of the intestinal mucosal barrier and hampering the efficiency of mucosal repair processes.38 Li et al have reported that lyophilized Aloe barbadensis Mill. can effectively increase mRNA expression of GDNF, thus conferring therapeutic benefits in murine models of constipation by promoting repair processes within the intestinal intermuscular plexus, nourishing intestinal neurons, and modulating intestinal dynamics.39
Electrophysiological Activity of Colonic Smooth Muscle
The electrophysiological behavior of colonic smooth muscle is primarily characterized by the presence of slow waves and fast waves of an action potential. Under normal physiological conditions, slow waves exhibit a periodic, quasi-sinusoidal electrical activity that persists regardless of whether the intestine is contracted or not, serving as a fundamental component for the initiation of action potentials in colonic smooth muscle. Therefore, modifications in the slow wave characteristics of colonic muscle electrical activity may lead to consequential changes in action potential patterns, ultimately impacting the contractile propulsive function of the colon and thereby affecting the timing of fecal expulsion.
Lu et al investigated the therapeutic effects of Aloe barbadensis Mill. on constipation in mice. Their study revealed an increase in the coefficient of variation for slow wave frequency and amplitude in constipated mice compared to control mice. Subsequently, following the administration of Aloe barbadensis Mill. treatment, a decrease in both coefficients of variation was observed in constipated mice, approaching the levels observed in the control group. These findings suggest that the intake of Aloe barbadensis Mill. could effectively improve abnormal smooth muscle electrophysiological activity in the colon, highlighting its potential as a therapeutic option for constipation.40
Interstitial Cells of Cajal
The interstitial cells of Cajal (ICC) are a type of tubular cells that are widely distributed throughout the smooth muscle layer of the gastrointestinal tract. The ICCs are responsible for regulating the generation of slow waves, transducing neural electrical signals, and modulating the contraction of intestinal smooth muscle.41 The expression of the C-kit gene serves as a specific marker for the identification of ICC, and the signaling pathway that is initiated by the binding of its ligand stem cell factor (SCF) regulates the proliferation and differentiation of ICC, thereby enhancing the contractile and relaxation functions of gastrointestinal smooth muscle and controlling the motility of the gastrointestinal system.42
Tong et al have demonstrated a reduction in the expression of C-kit-positive cells in the gastrointestinal tract of constipated patients.43 This reduction has been shown to elicit changes in the number, volume, and ultrastructure of ICC, leading to alterations in the regularity of slow wave activity and reduced contractile movements of the gastrointestinal smooth muscle. Li’s study found that after administration of aloe capsules to constipated mice, the content of C-kit in the colonic tissue of mice increased to a certain extent compared to the constipation model group, indicating that aloe could enhance the motility of intestinal smooth muscle by increasing the expression of C-kit positive cells and thus the number of ICC in the colon.44
Effect of Aloe barbadensis Mill. on Aquaporins
Aquaporins (AQPs) are a class of membrane proteins that serve as water channel proteins and demonstrate broad expression throughout the gastrointestinal tract. They are involved in critical physiological processes such as cell proliferation, migration, and the modulation of intestinal inflammation, thus highlighting their significance in intestinal health.45 With a molecular weight of approximately 30 kDa,46 AQPs function as osmolarity-driven channels and exhibit selective transport properties for water and other minor solutes. Within the mammalian digestive system, AQPs are extensively distributed in various organs including salivary glands, esophagus, stomach, small and large intestines, liver, gallbladder, bile duct, and pancreas.47 AQPs can be categorized into three groups based on their distinctive functional properties. The first group consists of classical AQPs (AQP0, AQP1, AQP2, AQP4, AQP5, AQP6, and AQP8), which exclusively exhibit selective permeability to water. The second group comprises water-glycerol channel proteins (AQP3, AQP7, AQP9, and AQP10), allowing the permeation of water, glycerol, and urea. Finally, the third group encompasses non-classical AQPs (AQP11 and AQP12), which possess unique characteristics.48 Among these Aquaporins, A comparative analysis revealed diminished AQP3 expression in the colonic tissue of individuals afflicted with diarrhea, while patients experiencing constipation displayed a noteworthy upregulation of AQP3 expression in the colon.49 The underlying mechanism of AQP3 involves the osmotic pressure differential between the colon lumen and blood vessels, facilitating water transport from the intestinal lumen to the vascular side through AQP3. Elevated levels of AQP3 expression result in excessive water depletion from the intestinal contents, leading to the hardening and accumulation of stools, thereby impeding their passage.
Zhu et al conducted an experimental study aiming to investigate the expression levels of AQP3 mRNA and protein in the colon tissue of constipated mice, which demonstrated an upregulation in the expression levels of both AQP3 mRNA and protein. Subsequently, treatment with Aloe barbadensis Mill. led to a significant downregulation in the expression levels of AQP3 mRNA and protein in the colon tissue, ultimately resulting in a decrease in the reabsorption of water in the intestines of the mice.50
Regulation of Intestinal Microbiota by Aloe Barbadensis Mill
The intestinal tract, which is the most densely colonized body part, contains billions of bacteria that strongly influence human health.51 Interactions between the intestinal flora and the human body encompass the regulation of host immune system development and response, as well as the production of metabolites like short-chain fatty acids (SCFAs), methane and bile acids.52 These metabolites exert direct or indirect effects on human tissues or target organs, thereby governing the organism’s pathophysiological state. The symbiotic relationship between the normal microbiota and the host enhances gut digestion and absorption functions, boosts host immunity, inhibits the growth of pathogenic bacteria, safeguards the intestinal mucosa, and preserves gut microenvironmental homeostasis, ultimately contributing to overall health. Conversely, an imbalance in gut microbial composition, manifests as reduced flora abundance and an increased prevalence of pathogenic bacteria, consequently inducing various chronic diseases, including chronic constipation.53 The evaluation of intestinal microbial diversity has been extensively explored through quantitative analyses. This investigation primarily relied on conventional microbial culture techniques and the examination of 16S ribosomal RNA (rRNA) gene sequences.54 Perturbations in the intestinal microbiota of individuals afflicted with constipation manifest as a notable decline in the relative abundance of beneficial bacteria, such as Lactobacillus, Bifidobacterium, and Bacteroides spp., accompanied by an increase in the relative abundance of potentially pathogenic microorganisms.55
In a study conducted by Khalif et al, microbial culture techniques were employed to investigate the gut microbiota of adult patients. The findings revealed a reduced abundance of Bifidobacterium and Lactobacillus in individuals suffering from constipation compared to the control groups, while there was an observed increase in the levels of Enterobacteriaceae and Staphylococcus aureus.56 In a comprehensive exploration of the gastrointestinal microbial composition in human, Mancabelli et al conducted an in-depth analysis of the gastrointestinal microbial composition among individuals with constipation, employing the 16S rRNA sequencing technique alongside whole-genome sequencing methods. The findings unveiled a conspicuous diminishment of Bacteroides, Roseburia, and Coprococcus 3 in the gut microbiota of patients.57 In a parallel cross-sectional pilot study by Zhu et al, employing 16S rRNA gene pyrosequencing, the researchers aimed to elucidate disparities in stool microbial composition between eight constipated individuals and a control group consisting of 14 nonconstipated subjects. The findings from this investigation unveiled a notable decrease in the prevalence of Prevotella, accompanied by an increased representation of various genera within the Firmicutes phylum in constipated patients compared to their nonconstipated counterparts.58 Intestinal flora can influence the propulsion of the gut by affecting the production of SCFAs. The propulsion of gastrointestinal motility can be modulated by the presence of intestinal microbiota, which exerts their influence by regulating the biosynthesis of short-chain fatty acids (SCFAs). These SCFAs, characterized as organic acids consisting of a carbon chain containing fewer than six carbons,59 are synthesized via the anaerobic bacterial metabolic breakdown of carbohydrates within the colonic environment.60 Among the SCFAs, acetate, propionate, and butyrate are the most abundant, with acetate comprising approximately 60% of the total SCFAs content, while propionate and butyrate contribute 20% each.61 Due to the uptake and metabolic processes of SCFAs by colonocytes, especially butyrate, have been primarily associated with colonic functionality, including the regulation of colonic mucosa homeostasis, influence on neuronal excitability, and potential enhancement of the cholinergic-mediated contractile response of the colonic circular muscles ex vivo.62 Therefore, the relationship between gut microbiota and gut transit appears to be bidirectional. In constipation, prolonged colonic transit may facilitate the amplification and colonization of slow-growing species, resulting in significant structural and functional changes throughout the microecology. On the other hand, environmental factors can induce alterations in the gut microbiota, potentially contributing to constipation pathogenesis through microbial metabolic activities.52
The impact of Aloe whole leaf extract on bacterial growth and short-chain fatty acids (SCFAs) synthesis was examined in a study conducted by Pogribna et al. The findings revealed that Aloe whole leaf extract exhibited a stimulating effect on the proliferation of three bacterial strains, namely Bifidobacterium infantis, Bacteroides fragilis, and Eubacterium limosum, within a span of 24 hours. Notably, the extent of bacterial growth promotion displayed a positive correlation with the dosage range of Aloe whole leaf extract. Moreover, the inclusion of Aloe whole leaf extract in the bacterial culture media demonstrated notable alterations in SCFAs profiles. Specifically, the presence of Aloe whole leaf extract led to an augmentation in butyrate production within the Eubacterium limosum medium, while an increase in acetate production was observed in the Bifidobacterium infantis medium.63 Ahluwalla et al investigated to examine the impact of Aloe barbadensis Mill. intervention on individuals diagnosed with irritable bowel syndrome (IBS), a disorder characterized by alternating occurrences of constipation and diarrhea. The study outcomes revealed a noteworthy augmentation in the abundance of Akkermansia muciniphila within the fecal matter, alongside an elevated concentration of butyrate in subjects who demonstrated a favorable response to Aloe barbadensis Mill. treatment.64 These findings suggest the potential of Aloe barbadensis Mill. in enhancing the microbial composition and diversity of the gut, thus promoting the establishment of a balanced intestinal microecology in patients.
Aloe barbadensis Mill. is Used to Treat Various Types of Constipation
Aloe barbadensis Mill. has been acknowledged for its potential therapeutic efficacy in addressing diverse forms of constipation. Its facilitation of intestinal peristalsis serves to expedite the alimentary transit through the digestive tract, concurrently extending the duration of fecal residence in the intestines. Furthermore, the modulation of stool consistency is attributed to Aloe barbadensis Mill.’s augmentation of intestinal water content, thereby ameliorating the ease of fecal passage. Consequently, Aloe barbadensis Mill. emerges as a viable intervention for a spectrum of constipation conditions, encompassing Stroke bed constipation,65 Constipation in heroin addicts,66 Functional constipation in the elderly,67 Granisetron hydrochloride injection causes constipation,68 Stroke constipation,69 Constipation in the large intestine of a horse and mule.70 Detailed categorizations are presented in Table 1, delineating specific constipation subtypes for comprehensive elucidation.
 |
Table 1 Types of Constipation Treated with Aloe Barbadensis Mill |
Aloe barbadensis Mill. Marketed Products
In 2021, the total revenue generated by the sales of treatment for constipation drugs in China amounted to approximately $401 million. Of this, the sales of treatment for constipation drugs containing Aloe barbadensis Mill. as the principal constituent were valued at $126 million, accounting for 31.34% of the overall sales. Due to its recognized gastroprotective and immunoregulatory properties, Aloe barbadensis Mill. has garnered attention as a potential candidate for therapeutic applications. Numerous commercially available products have employed Aloe barbadensis Mill. as a vital ingredient, inspiring researchers to evaluate its efficacy in treating constipation. To gain a deeper understanding of the effectiveness of this botanical remedy, scholars have analyzed commercially available Aloe barbadensis Mill. preparations. Here are a few products that have been marketed for the treatment of constipation with Aloe barbadensis Mill. (Table 2).
 |
Table 2 Composition of Some Aloe Barbadensis Mill. Products and Daily Cost |
Aloe barbadensis Mill. Mono-Preparation
Aloe Barbadensis Mill. Soft Capsule
Aloe barbadensis Mill. soft gels, a traditional mono-preparation, exhibit satisfactory efficacy in treating constipation with a low daily cost of approximately $0.18 for patients. In this study, Hu investigated the treatment for the constipation function of Aloe barbadensis Mill. soft gels by treating constipated mice with varying doses of the capsule’s contents. The results indicated that the 0.800 g/kg dose of Aloe barbadensis Mill. soft capsule significantly increased the rate of small intestine ink advancement, reduced the time to defecate the first black stool, and increased the number of defecations in constipated mice, without inducing diarrhea. Additionally, Hu conducted a clinical trial involving 102 voluntary subjects to assess the efficacy and safety of Aloe barbadensis Mill. soft capsules in treating constipation. Patients in the experimental group received Aloe barbadensis Mill. soft capsules for 7 days, while the control group received a placebo. The results of the study showed that the number of bowel movements in the experimental group increased significantly before and after taking the drug, the stool characteristics were improved, and the total effective rate of taking the drug was 76.47%. Furthermore, the experimental group demonstrated significantly improved bowel movements and stool characteristics compared to the control group after taking the drug, and no adverse reactions or safety concerns were observed in the experimental group.71 These results suggest that Aloe barbadensis Mill. soft capsules may be an effective and safe option for treating constipation.
Aloe Barbadensis Mill. Capsule
Aloe barbadensis Mill. capsule stand as a prominent Chinese herbal therapeutic intervention, celebrated for their notable effectiveness in the mitigation of constipation, accompanied by a cost-effective daily expenditure of merely $0.24 per patient. This herbal remedy has garnered recognition within the realm of traditional Chinese medicine for its commendable outcomes in constipation treatment. Qu et al conducted an investigation involving 60 individuals afflicted with annular mixed hemorrhoids during their postoperative phase. The administration of Aloe barbadensis Mill. capsules yielded substantial improvements in the difficulty of defecation, fecal characteristics, and defecation duration among postoperative mixed hemorrhoid patients. Impressively, the overall treatment efficacy reached 95%.72 In a separate investigation by Ye et al, 50 elderly patients grappling with functional constipation were meticulously examined. The protocol involved the oral intake of 2 Aloe barbadensis Mill. capsules prior to bedtime. The findings indicated notable amelioration in 31 patients, with minimal adverse reactions observed during the treatment course.73 These adverse events were limited to a subset of patients experiencing diarrhea and abdominal discomfort. Importantly, these side effects were effectively managed and resolved promptly through appropriate interventions. These studies collectively underscore the promising therapeutic potential of Aloe barbadensis Mill. capsules in addressing constipation-related issues, serving as a cost-effective and well-tolerated intervention.
Aloe Barbadensis Mill. Navel Paste
Aloe barbadensis Mill. can be administered orally for the treatment of constipation, and it also offers potential for external application in the management of constipation. Because of the rich capillaries distributed on both sides of the navel, the application of Aloe barbadensis Mill. here is conducive to long time storage and penetration of drugs, plus the pungent and warm effect of ethanol, which makes the blood vessels dilate and facilitate the penetration of Aloe barbadensis Mill. into the large intestine, stimulating the wall of the large intestine to cause intestinal contraction and increased secretion of water and fluid to achieve treatment for constipation purposes. Zhang et al carried out a randomized controlled trial on a sample of 118 individuals aged between 19 and 63 years with constipation. Participants were allocated into test and control groups using both self-control and between-group control designs. The test group was administered Aloe barbadensis Mill. soft gels, while the control group was given placebo capsules. After seven consecutive days of the administration, the frequency of bowel movements was measured. The findings revealed that the test group experienced a statistically significant increase in bowel movements in comparison to their pre-intervention levels, and also had a significantly higher number of bowel movements than the control group. Simultaneously, this approach circumvents the potential adverse effects arising from the co-administration of oral treatment for constipation and antipsychotic medications within the gastrointestinal tract, thereby introducing a novel avenue for managing pharmacological constipation.74
Aloe barbadensis Mill. Compounding
Aloe Barbadensis Mill. Pearl Capsule
The aloe pearl capsules consisting of Aloe barbadensis Mill., pearls, and Saussurea costus. Wang et al conducted a study to investigate the effects of the capsule on intestinal peristalsis. The results indicated demonstrated that this particular intervention led to significant enhancements in both the amplitude and frequency of intestinal muscle contractions in rabbits.75 Meanwhile, Liu selected a cohort of 82 patients diagnosed with myocardial infarction and presenting with constipation symptoms. The patients were divided into two groups, a control group consisting of 41 individuals who underwent conventional treatment, and an experimental group of 41 patients who were administered the capsule. The results demonstrated that the treatment efficacy in the experimental group was observed in 39 patients (95.12%), whereas in the control group, it was observed in 28 patients (68.29%). Therefore, the findings of this study suggest that the capsule could be an effective treatment option for patients with myocardial infarction and constipation symptoms.76 In terms of pharmacoeconomics, the capsules in question are deemed cost-effective and readily accessible, with a daily cost of approximately $0.23.
Xin Fufang Luhui Capsule
Xin Fufang Luhui Capsule is composed of Aloe barbadensis Mill., Indigo Naturalis and Amber. Gong’s investigation focused on the potential treatment for constipation properties of the capsules on mice in three distinct constipation models. The study showed that administration of the capsule significantly increased defecation frequency and the number of pellets expelled within 8 hours, while also enhancing small bowel propulsion in mice with gastrointestinal motility disorders. Additionally, a clinical trial was conducted with 224 participants, divided into two groups receiving either the capsule or a placebo, taken twice daily for a week. The study results indicated that the capsules elicited a significant treatment for constipation effect compared to the placebo group, and remained effective for up to one week after discontinuation.77 From a pharmacoeconomic perspective, the capsule presents an advantage in terms of ease of administration and affordability, with a minimal daily treatment expense of $0.26.
Shouhui Tongbian Capsule (SHTC)
The encapsulated therapeutic regimen for ameliorating constipation comprises a proprietary blend of eight traditional Chinese botanical constituents, with particular emphasis on the principal constituent, Aloe barbadensis Mill. and Fallopia multiflora. Lin et al employed loperamide to establish a functional constipation model in rats, characterized by delayed gut transmission. The experimental groups consisted of the normal control group (NC), the Shouhui tongbian capsule group (SH), the loperamide HCl model group (LOP), the bisacodyl enteric-coated tablets positive group (BE), the low Shouhui tongbian capsule dose group (LS), and the high Shouhui tongbian capsule dose group (HS). The findings of this investigation provide evidence supporting the therapeutic effects of SHTC on constipation induced by loperamide. Administration of SHTC demonstrated notable amelioration in key physiological parameters including body weight, gastric emptying rate, and fecal moisture content. Additionally, SHTC treatment exhibited a significant mitigating effect on the histopathological alterations in the small intestine associated with constipation. Notably, SHTC-treated constipated rats displayed a remarkable elevation in the levels of acetic acid and propionic acid, both recognized as short-chain fatty acids (SCFAs), thereby emphasizing the potential biochemical impact of SHTC in the modulation of gut microbiota.78 Meanwhile, Lu et al conducted a study to investigate the efficacy and safety of the capsule in the treatment of functional constipation. The study population consisted of 2396 patients, who were categorized into three age groups: 18–40 years (n = 683), 41–60 years (n = 1018), and 61 years and above (n = 695). The researchers observed improvements in bowel movement frequency, difficulty, and duration in all age groups, indicating the effectiveness of the capsules in treating functional constipation across different age groups. Furthermore, the capsules were found to be safe as no significant adverse effects were observed in any of the groups. Notably, the study results showed that the capsules were more effective in younger patients.79 From a pharmacoeconomic perspective, these capsule exhibits enhanced efficacy but also entails increased expenses for the treatment of constipation, with a daily cost of approximately $7.80.
Toxicology of Aloe barbadensis Mill
Aloe barbadensis Mill. emerges as a comparatively safe category of therapeutic agents for constipation, demonstrating limited documented toxic side effects. Notably, reported clinical instances of toxicity predominantly implicate the kidneys, and the frequency of such events appears correlated with the source of extraction of Aloe barbadensis Mill. Extract. In this study, Florence et al aimed to assess the acute toxicity of Aloe barbadensis Mill. green rind extract and Aloe barbadensis Mill. whole leaf extract in Wistar albino rats via 28 days of gavage administration at 200, 400, and 800 mg/kg dosages. Through examination of photomicrographs, the researchers observed mild necrosis in the kidneys of rats treated with Aloe barbadensis Mill. green rind extract. However, some rats treated with 200 and 800 mg/kg of Aloe barbadensis Mill. whole leaf extract exhibited inflammation of the renal interstitium.80 The above experiments were conducted to investigate the acute toxicity of Aloe barbadensis Mill., and the dose used was relatively high, which inevitably led to toxic side effects, however, such a high dose was not necessary in the use of Aloe barbadensis Mill. for constipation, thus to explore the toxicity of Aloe barbadensis Mill. in the treatment of constipation, Wintola et al administered aqueous extracts of whole leaves of Aloe barbadensis Mill. to rats with loperamide-induced constipation at varying doses of 50, 100 and 200 mg/kg. The effects of the extract on the hematological parameters as well as hepatic and renal functional indices of the rats were evaluated. The results indicate that the extract did not cause any significant adverse effects on the kidney and liver or the indicators of renal function including serum creatinine, uric acid, urea, calcium, and potassium ions levels at all studied doses. Moreover, the extract was able to restore alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) to normal levels in constipated rats after treatment with Aloe barbadensis Mill.81 Based on the available evidence, the study suggests that Aloe barbadensis Mill. is safe for oral administration in the treatment of constipation. Whilst Aloe barbadensis Mill. is generally deemed to be safe, several adverse reactions have been reported in its use as a treatment for constipation. One such reaction is Melanosis coli (MC), which represents a noninflammatory bowel condition characterized by the deposition of brown or black pigment within the colorectal mucosa.82 The incidence rates of MC fall within a range of roughly 0.82% to 1.13%.83 It is typically caused by prolonged use of anthraquinone treatment for constipation, including senna, rhubarb, and Aloe barbadensis Mill. MC tends to occur more frequently in individuals with chronic constipation and is generally a benign and reversible condition that gradually resolves within a few months following the cessation of treatment for constipation use, as the patient’s colon returns to normal.84 Overall, the safety of Aloe barbadensis Mill. as a treatment for constipation is considered to be dependable in the current scientific discourse.
Conclusions
The field of constipation exhibits promising market potential, with historical evidence of Aloe barbadensis Mill. utilization in constipation management tracing back over 4000 years. Capitalizing on recent advancements in scientific and technological research, a prospect pathway emerges for further investigation into the active constituents and molecular mechanisms underlying Aloe barbadensis Mill. ‘s therapeutic effects. By harnessing state-of-The-art techniques, the exploration of this extraordinary herb presents a distinctive prospect to unravel the latent therapeutic capacity in the context of constipation treatment. Simultaneously, the integration of modern technology promises to unveil additional enigmas surrounding Aloe barbadensis Mill., thereby amplifying its intrinsic value in the domain of constipation therapy.
Abbreviations
ALT, Alanine aminotransferase; ALP, Alkaline phosphatase; AQPs, Aquaporins; CFTR, Cystic Fibrosis Transmembrane Conductance Regulator; ENS, Enteric nervous system; GDNF, Glial cell-derived neurotrophic factor; ICC, Interstitial cells of Caja; MC, Melanosis coli; NO, Nitric oxide; SCF, Stem cell factor; SCFAs, Short-chain fatty acids; sCG, soluble guanylyl cyclase; SHTC, Shouhui tongbian Capsule; SP, Substance P; VIP, Vasoactive peptide.
Data Sharing Statement
The data used to support the findings of this study are included in the article.
Acknowledgments
The authors acknowledge the BioRender (www.biorender.com), as Figure 4 and the graphical abstract in this review were created with the BioRender platform.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The Scientific Research Initiation Fund of Fujian Medical University (No. 60000166).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Włodarczyk J, Waśniewska A, Fichna J, Dziki A, Dziki Ł, Włodarczyk M. Current overview on clinical management of chronic constipation. J Clin Med. 2021;10(8):1738. doi:10.3390/jcm10081738
2. Chen L, Zhang J, Suo H, et al. Preventive Effects of Different Fermentation Times of Shuidouchi on Diphenoxylate-Induced Constipation in Mice. Foods. 2019;8(3):86. doi:10.3390/foods8030086
3. Wang J, Wang L, Yu Q, et al. Characteristics of the Gut Microbiome and Serum Metabolome in Patients with Functional Constipation. Nutrients. 2023;15(7):1779. doi:10.3390/nu15071779
4. Chandan B, Saxena A, Shukla S, et al. Hepatoprotective potential of Aloe barbadensis Mill. against carbon tetrachloride induced hepatotoxicity. J Ethnopharmacol. 2007;111(3):560–566. doi:10.1016/j.jep.2007.01.008
5. Comas-Serra F, Martínez-García JJ, Pérez-Alba A, et al. A New Functional Food Ingredient Obtained from Aloe ferox by Spray Drying. Foods. 2023;12(4):850. doi:10.3390/foods12040850
6. Fu S, Dang Y, Xu H, et al. Aloe Vera-Fermented Beverage Ameliorates Obesity and Gut Dysbiosis in High-Fat-Diet Mice. Foods. 2022;11(22):3728. doi:10.3390/foods11223728
7. Parnomo T, Pohan DJ. Test the Effectiveness of Aloe Vera Extract on the Growth of Escherichia coli in vitro. Int J Health Sci Res. 2021;11(8):211–224 doi:10.1016/j.jep.2009.05.037
8. Isager Ahl L, Pedersen HL, Jørgensen B, et al. Exploring the polysaccharide composition of plant cell walls in succulent aloes. Plants, People, Planet. 2023;5(3):335–353. doi:10.1002/ppp3.10361
9. Golmohammadi F. Medical plant of Aloe Vera in desert regions of Iran: greenhouses, economic importance, development, extension, processing and marketing. Black Sea J Agriculture. 2022;5(1):1–15. doi:10.47115/bsagriculture.945710
10. Surjushe A, Vasani R, Saple D. Aloe vera: a short review. Indian j dermatol. 2008;53(4):163. doi:10.4103/0019-5154.44785
11. Martínez-Sánchez A, López-Cañavate ME, Guirao-Martínez J, Roca MJ, Aguayo E. Aloe vera Flowers, a Byproduct with Great Potential and Wide Application, Depending on Maturity Stage. Foods. 2020;9(11):1542. doi:10.3390/foods9111542
12. Mangaiyarkarasi S, Manigandan T, Elumalai M, Cholan PK, Kaur RP. Benefits of Aloe vera in dentistry. J Pharm Bioallied Sci. 2015;7(Suppl 5):S255. doi:10.4103/0975-7406.155943
13. Boudreau MD, Beland FA. An Evaluation of the Biological and Toxicological Properties of Aloe Barbadensis (Miller), Aloe Vera. Journal of Environmental Science and Health, Part C. 2006;24(1):103–154. doi:10.1080/10590500600614303
14. Sadiq U, Gill H, Chandrapala J. Temperature and pH Stability of Anthraquinones from Native Aloe vera Gel, Spray-Dried and Freeze-Dried Aloe vera Powders during Storage. Foods. 2022;11(11):1613. doi:10.3390/foods11111613
15. Ahlawat KS, Khatkar BS. Processing, food applications and safety of aloe vera products: a review. J Food Sci Technol. 2011;48(5):525–533. doi:10.1007/s13197-011-0229-z
16. Wu X, Wan J, Zhong J, Ding W. Research Progress on the Chemical Constituents of Aloe. Chin J Trop Crops. 2015;36(08):1542–1550. doi:10.3969/j.issn.1000-2561.2015.08.030
17. Zhang Y, Bao Z, Ye X, et al. Chemical Investigation of Major Constituents in Aloe vera Leaves and Several Commercial Aloe Juice Powders. J AOAC Int. 2019;101(6):1741–1751. doi:10.5740/jaoacint.18-0122
18. Martínez-Burgos WJ, Serra JL, MarsigliaF RM, et al. Aloe vera: from ancient knowledge to the patent and innovation landscape–A review. S Afr J Bot. 2022. doi:10.1016/j.sajb.2022.02.034
19. Chinchilla N, Carrera C, Durán AG, Macías M, Torres A, Macías FA. Aloe barbadensis: how a miraculous plant becomes reality. Phytochem Rev. 2013;12:581–602. doi:10.1007/s11101-013-9323-3
20. Sánchez-Machado DI, López-Cervantes J, Sendón R, Sanches-Silva A. Aloe vera: ancient knowledge with new frontiers. Trends Food Sci Technol. 2017;61:94–102. doi:10.1016/j.tifs.2016.12.005
21. Ansari M, Meftahizadeh H, Eslami H. Fabrication of multifunctional chitosan-guar-aloe vera gel to promote wound healing. Chem Papers. 2022;76(3):1513–1524. doi:10.1007/s11696-021-01958-4
22. Cheney RH. Aloe drug in human therapy. Quarterly J Crude Drug Res. 1970;10(1):1523–1532. doi:10.3109/13880207009066219
23. Hamman JH. Composition and Applications of Aloe vera Leaf Gel. Molecules. 2008;13(8):1599–1616. doi:10.3390/molecules13081599
24. Bozzi A, Perrin C, Austin S, Vera FA. Quality and authenticity of commercial aloe vera gel powders. Food Chem. 2007;103(1):22–30. doi:10.1016/j.foodchem.2006.05.061
25. Kim MW, Kang J-H, Shin E, et al. Processed Aloe vera gel attenuates non-steroidal anti-inflammatory drug (NSAID)-induced small intestinal injury by enhancing mucin expression. Food Funct. 2019;10(9):6088–6097. doi:10.1039/C9FO01307E
26. Bahrami G, Malekshahi H, Miraghaee S, et al. Protective and Therapeutic Effects of Aloe Vera Gel on Ulcerative Colitis Induced by Acetic Acid in Rats. Clin Nutr Res. 2020;9(3):223–234. doi:10.7762/cnr.2020.9.3.223
27. Rebecca W, Kayser O, Hagels H, Zessin KH, Madundo M, Gamba N. The phytochemical profile and identification of main phenolic compounds from the leaf exudate of Aloe secundiflora by high‐performance liquid chromatography–mass spectroscopy. Phytochem Anal. 2003;14(2):83–86. doi:10.1002/pca.682
28. Van Os F. Some aspects of the pharmacology of anthraquinone drugs. Pharmacology. 1976;14(Suppl. 1):18–29. doi:10.1159/000136683
29. Hattori M, Kanda T, Shu Y-Z, Akao T, Kobashi K, Namba T. Metabolism of barbaloin by intestinal bacteria. Chem Pharm Bull. 1988;36(11):4462–4466. doi:10.1248/cpb.36.4462
30. Ishii Y, Tanizawa H, Takino Y. Studies of Aloe. III.: mechanism of Cathartic Effect.(2). Chem Pharm Bull. 1990;38(1):197–200. doi:10.1248/cpb.38.197
31. Niu C, Ye W, Cui X, et al. UHPLC-MS/MS method for the quantification of aloin-A in rat plasma and its application to a pharmacokinetic study. Journal of Pharmaceutical & Biomedical Analysis. 2020;178:112928. doi:10.1016/j.jpba.2019.112928
32. Park M-Y, Kwon H-J, Sung M-K. Plasma, tissue and urinary levels of aloin in rats after the administration of pure aloin. Nutr Res Pract. 2008;2(1):17–21. doi:10.4162/nrp.2008.2.1.17
33. Avetisyan M, Schill EM, Heuckeroth RO. Building a second brain in the bowel. J Clin Invest. 2015;125(3):899–907. doi:10.1172/JCI76307
34. Tang R, Zhang J, Nan H, et al. Exploring Molecular Mechanisms of Aloe barbadmsis Miller on Diphenoxylate-Induced Constipation in Mice. Evid Based Complement Alternat Med. 2022;2022. doi:10.1155/2022/6225758
35. Beck K, Voussen B, Reigl A, et al. Cell‐specific effects of nitric oxide on the efficiency and frequency of long distance contractions in murine colon. Neurogastroenterol Motil. 2019;31(6):e13589. doi:10.1111/nmo.13589
36. Izzo AA, Sautebin L, Borrelli F, Longo R, Capasso F. The role of nitric oxide in aloe-induced diarrhoea in the rat. Eur. J. Pharmacol. 1999;368(1):43–48. doi:10.1016/S0014-2999(99)00007-2
37. Nosrat C, Tomac A, Hoffer BJ, Olson L. Cellular and developmental patterns of expression of Ret and glial cell line-derived neurotrophic factor receptor alpha mRNAs. Exp Brain Res. 1997;115:410–422. doi:10.1007/PL00005711
38. Chen G, Du Y, Li X, et al. Lower GDNF Serum Level Is a Possible Risk Factor for Constipation in Patients With Parkinson Disease: a Case–Control Study. Front Neurol. 2022;12:777591. doi:10.3389/fneur.2021.777591
39. Li N, Chen XX, Yue WY, et al. Evaluation on laxative efficacy of aloe, senna alone and in combination with Panax quinquefolium. Practical Preventive Med. 2020;27(12):1425–1429. doi:10.3969/j.issn.1006-3110.2020.12.005
40. Lu Z, Jiang H, Ailin D. Effect of Aloe on colon electrical physiology of constipation mice. Chin J Gerontol. 2013;33(05):1068–1069. doi:10.3969/j.issn.1005-9202.2013.05.038
41. Yan S, Yue Y-Z, Wang X-P, et al. Aqueous extracts of Herba Cistanche promoted intestinal motility in loperamide-induced constipation rats by ameliorating the interstitial cells of cajal. Evid Based Complement Alternat Med. 2017;2017. doi:10.1155/2017/6236904
42. Sun Y, Yan C, Jin S, Shi C, Zhao J, Li G. Curative effect and mechanism of guiren runchang granules on morphine-induced slow transit constipation in mice. Evid Based Complement Alternat Med. 2020;2020. doi:10.1155/2020/5493192
43. Tong W-D, Liu B-H, Zhang L-Y, Xiong R-P, Liu P, Zhang S-B. Expression of c-kit messenger ribonucleic acid and c-kit protein in sigmoid colon of patients with slow transit constipation. In Colorectal Dis. 2005;20:363–367. doi:10.1007/s00384-004-0679-0
44. Li XD. Clinical Observation and Mechanism of Xiahuang Granule in the Treatment of Opioid Induced Constipation. Tianjin University of Traditional Chinese Medicine, Chinese Master’s Theses Full-text Database; 2020.
45. Lv H, Li Y, Xue C, Dong N, Bi C, Shan A. Aquaporin: targets for dietary nutrients to regulate intestinal health. J Animal Physiol Animal Nutr. 2022;106(1):167–180. doi:10.1111/jpn.13539
46. Ma T, Verkman A. Aquaporin water channels in gastrointestinal physiology. J Physiol. 1999;517(2):317–326. doi:10.1111/j.1469-7793.1999.0317t.x
47. Zhu C, Chen Z, Jiang Z. Expression, Distribution and Role of Aquaporin Water Channels in Human and Animal Stomach and Intestines. Int J Mol Sci. 2016;17(9):1399. doi:10.1186/s40064-016-2821-1
48. Ikarashi N, Kon R, Sugiyama K. Aquaporins in the Colon as a New Therapeutic Target in Diarrhea and Constipation. Int J Mol Sci. 2016;17(7):1172. doi:10.1002/ppp3.10361
49. Yde J, Keely SJ, Moeller HB. Expression, regulation and function of Aquaporin-3 in colonic epithelial cells. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2021;1863:183619. doi:10.1016/j.bbamem.2021.183619
50. Zhu YL, Zhang JJ, Tang RY, Wang MY, Liu JL, Wang LY. Effects of compatibility of Aloe and Cannabis Fructus on constipated mice induced by compound diphenoxylate. China J Tradition Chinese Med Pharm. 2021;36(08):4986–4990.
51. Wei X, Yu L, Zhang C, et al. Genetic-Phenotype Analysis of Bifidobacterium bifidum and Its Glycoside Hydrolase Gene Distribution at Different Age Groups. Foods. 2023;12(5):922. doi:10.3390/foods12050922
52. Zhang S, Wang R, Li D, Zhao L, Zhu L. Role of gut microbiota in functional constipation. Gastroenterology Report. 2021;9(5):392–401. doi:10.1093/gastro/goab035
53. Xu Z, Liu T, Zhou Q, Chen J, Yuan J, Yang Z. Roles of Chinese Medicine and Gut Microbiota in Chronic Constipation. Evid Based Complement Alternat Med. 2019;2019:9372563. doi:10.1155/2019/9372563
54. Gerritsen J, Smidt H, Rijkers GT, de Vos WM. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209–240. doi:10.1007/s12263-011-0229-7
55. Zhao Y, Yu Y-B. Intestinal microbiota and chronic constipation. Springerplus. 2016;5(1):1–8. doi:10.1186/s40064-016-2821-1
56. Khalif I, Quigley E, Konovitch E, Maximova I. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Digestive Liver Dis. 2005;37(11):838–849. doi:10.1016/j.dld.2005.06.008
57. Mancabelli L, Milani C, Lugli GA, et al. Unveiling the gut microbiota composition and functionality associated with constipation through metagenomic analyses. Sci Rep. 2017;7(1):9879. doi:10.1038/s41598-017-10663-w
58. Zhu L, Liu W, Alkhouri R, et al. Structural changes in the gut microbiome of constipated patients. Physiol Genom. 2014;46(18):679–686. doi:10.1152/physiolgenomics.00082.2014
59. Mortensen PB, Clausen MR. Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand J Gastroenterol. 1996;31(sup216):132–148. doi:10.3109/00365529609094568
60. McNeil N. The contribution of the large intestine to energy supplies in man. The American Journal of Clinical Nutrition. 1984;39(2):338–342. doi:10.1093/ajcn/39.2.338
61. Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001. doi:10.1152/physrev.2001.81.3.1031
62. Wang JK, Yao SK. Roles of Gut Microbiota and Metabolites in Pathogenesis of Functional Constipation. Evid Based Complement Alternat Med. 2021;2021:5560310. doi:10.1155/2021/5560310
63. Pogribna M, Freeman J, Paine D, Boudreau M. Effect of Aloe vera whole leaf extract on short chain fatty acids production by Bacteroides fragilis, Bifidobacterium infantis and Eubacterium limosum. Lett Appl Microbiol. 2008;46(5):575–580. doi:10.1111/j.1472-765X.2008.02346.x
64. Ahluwalia B, Magnusson MK, Böhn L, et al. Randomized clinical trial: effects of Aloe barbadensis Mill. extract on symptoms, fecal microbiota and fecal metabolite profiles in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2020;32(8):e13860. doi:10.1111/nmo.13860
65. Zhen L, Xining Y, Jie W, Quanzhen H, Lanxiang C. Application of aloe vera honey suppository Meridian flow injection in patients with stroke and bedridden constipation. Mod J Integr Traditional Chinese and Western Med. 2023;32(10):1431–1434 doi:DOI:10.3969/j.issn.1008-8849.2023.10.023
66. Hongguo S, Xile D. Clinical observation of compound aloe vera capsule in the treatment of constipation in heroin-dependent patients. Chine J Drug Abuse Prevention and Treatment. 2014;20(04):222–223 doi:10.3969/j.issn.1006-902X.2014.04.013.
67. Chenli J, Min Z, Yufang G, et al. Clinical study of Angelica sinensis aloe vera capsule in the treatment of functional constipation in the elderly. Modern Traditional Chinese Med. 2018;38(05):72–75 doi:10.13424/j.cnki.mtcm.2018.05.025
68. Junkai Z, Guoqiang K, Xiuli B, Jiandong L, Fulan G. The efficacy of compound aloe vera capsule in the prevention and treatment of constipation caused by granisetron hydrochloride injection was observed in 106 cases. Hebei J Trad Chin Med. 2009;31(10):1506 doi:10.3969/j.issn.1002-2619.2009.10.072
69. Jing L. Aloe vera solution enema treated 50 patients with stroke and constipation. China’s Naturopathy. 2003;(01):32–33 doi:10.3969/j.issn.1007-5798.2003.01.052
70. Yanbiao M. Observations on the efficacy of aloe vera on constipation of the large intestine in horse mules. Contemporary Animal Husbandry. 2002;(4):22.
71. Hu CS. Study on Facilitating Feces Excretion Function of Aloe Soft Capsule. Practical Preventive Med. 2009;16(03):891–894. doi:10.3969/j.issn.1006-3110.2009.03.113
72. Yin Q, De Z, Wei Y, Yafeng L, Zhijun Z, Shifang L. Clinical observation on the prevention and treatment of postoperative constipation of circumferential mixed hemorrhoids by Huang Xing Lun Intestinal Tablets. Chine Traditional Patent Med. 2016;38(6):1429–1432 doi:10.3969/j.issn.1001-1528.2016.06.052
73. Ye J, Fan Q, Fu Z. Clinical effect observation of Shumi capsule combined with external application of traditional Chinese medicine on functional constipation in the elderly. Chinese Community Doctors. 2020;36(18):108–109 doi:10.3969/j.issn.1007-614x.2020.18.064
74. Zhao-cui Z, Hui-zhi G. Therapeutic effects of aloe-paste in the treatment of antipsychotic agents induced constipation. Journal of Qilu Nursing. 2004;06:419–420. doi:10.3969/j.issn.1006-7256.2004.06.013
75. Wang YJ, Sun YL, Tang W, Xu H, Xu LX. Experimental Study of Ease-constipation Action of Capsulae Aloes. Special Wild Economic Animal Plant Res. 2007;29(2):36–38. doi:10.3969/j.issn.1001-4721.2007.02.014
76. Liu ZY. Therapeutic Effect of Pearl Aloe Capsule on Myocardial Infarction and Constipation and Its Comprehensive Nursing Intervention. Drugs Clinic. 2016;13(16):51–53. doi:10.3969/j.issn.1672-2809.2016.16.016
77. Gong LB. Evaluation on the Clinical Application of St.John’s Xin Fufang Luhui Jiaonang. Drug Eval. 2018;15(06):7–13. doi:10.3969/j.issn.1672-2809.2018.06.002
78. Lin Q, Liu M, Erhunmwunsee F, et al. Traditional Chinese Patent Medicine Shouhui Tongbian Capsule Attenuated Loperamide-Induced Constipation Through Modulating the Gut Microbiota in Rat. Available at SSRN. 2012;4071673. doi:10.2139/ssrn.4071673
79. Lu Y, Zhang HX, Han B, Zhang ZY, Yu YD, Tian ZG. Study on the Clinical Efficacy of Shouhui Tongbian Capsule in The Treatment of Functional Constipation. World Chinese Medicine. 2020;15(22):3434–3438 doi:10.3969/j.issn.1673-7202.2020.22.013
80. Nalimu F, Oloro J, Peter EL, Ogwang PE. Acute and sub-acute oral toxicity of aqueous whole leaf and green rind extracts of Aloe vera in Wistar rats. BMC Complement Med Therap. 2022;22(1):16. doi:10.1186/s12906-021-03470-4
81. Wintola O, Sunmonu T, Afolayan A. Toxicological evaluation of aqueous extract of Aloe ferox Mill. in loperamide-induced constipated rats. Hum Exp Toxicol. 2011;30(5):425–431. doi:10.1177/0960327110372647
82. Yang N, Ruan M, Jin S. Melanosis coli: a comprehensive review. Gastroenterología y Hepatología. 2020;43(5):266–272. doi:10.1016/j.gastrohep.2020.01.002
83. Wittoesch JH, Jackman RJ, McDonald JR. Melanosis coli: general review and a study of 887 cases. Dis Colon Rectum. 1958;1(3):172–180. doi:10.1007/BF02616828
84. Mellouki I, Meyiz H. Melanosis coli: a rarity in digestive endoscopy. Pan Afr Med J. 2014;16(1). doi:10.11604/pamj.2013.16.86.3331
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
