Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 15
The Positive Impact of Foods Support on Loss to Follow Up Among Children and Adolescents on HIV Antiretroviral Therapy in a District Hospital in East Cameroon
Authors Yumo H , Ndenkeh J Jr, Beissner M
Received 19 April 2023
Accepted for publication 4 September 2023
Published 10 November 2023 Volume 2023:15 Pages 663—670
DOI https://doi.org/10.2147/HIV.S417852
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Habakkuk Yumo,1,2 Jackson Jr Ndenkeh,1,2 Marcus Beissner2
1R4D International Foundation, Yaounde, Cameroon; 2Center for International Health, Ludwig-Maximilians-University of Munich, Munich, Germany
Correspondence: Habakkuk Yumo, Email [email protected]
Introduction: The pediatric HIV treatment coverage in Cameroon remains low at 35%. The high loss to follow up (LTFU) remains a major factor to this dismal performance which is related to the lack of implementation of effective interventions to improve retention in care. This study assessed the impact of foods support (FS) on LTFU among children and adolescents in a rural district hospital in eastern Cameroon.
Methods: This was a retro-prospective study conducted in Abong Mbang District Hospital (ADH) in the East Region of Cameroon. We provided foods kits to children and adolescents initiated on antiretroviral therapy (ART) in this facility during the study and followed them up prospectively (prospective phase). On the other hand, using medical records, we collected retrospectively data for children and adolescents who enrolled on ART in the hospital prior to the study (retrospective phase). We then compared the proportions of children and adolescents LTFU before (no FS) and after (with FS) the study, using the Fisher’s exact test, logistic regression, Kaplan–Meier survival curves and Cox proportional-hazards model at 5% significant level.
Results: We found that with FS, the proportion of children and adolescents LTFU was 11 times lower (2.4% vs 26.7%, p=0.014), the mean time of retention in care was 30% higher (17 months vs 12 months, p< 0.001) and children and adolescents who did not receive FS were 10 times more likely to be LTFU [aHR=10.3 (4.0– 26.2), p< 0.001)].
Conclusion: Foods support is an effective intervention in reducing LTFU among children and adolescents on ART. This intervention should be adequately funded to enable a large-scale implementation in the field. This could help to improve the outcome of pediatric ART coverage in resource-limited settings.
Keywords: HIV care, foods assistance, foods support, loss to follow up, retention, children, adolescents
Introduction
Despite a considerate global scale-up in pediatric HIV/AIDS care and treatment, there is still disparity in HIV morbidity and mortality among children in sub-Saharan Africa as compared to high-income countries.1–3 One of the major setbacks to HIV management in sub-Saharan Africa is the low retention in HIV care known to negatively impact the treatment outcome.2,4 Hence, for every diagnosed HIV-infected child linked to HIV care, there is an absolute necessity to retain that child in care to maximize treatment outcome.5 Retention in care is thus critical in optimizing viral suppression, slowing disease progression and eventual death as well as in preventing HIV transmission, treatment failure and possible resistance to antiretroviral drugs.1,5–7 The rapid expansion of antiretroviral therapy (ART) with the event of the WHO test and treat policy remains a dilemma as retention in care becomes a serious threat to the success of antiretroviral treatment.2,8
Previous studies in Africa have documented loss to follow up (LTFU) rates ranging from 5% up to as high as 40% after 12 months of care, and even higher in the subsequent years of care.1,4,9–11 According to UNAIDS, in 2021 only 35% of children aged less than 15 years eligible for ART were effectively on treatment in Cameroon as compared to 81% of those aged 15 years and above.12 Furthermore, in 2016, Billong et al reported that the 12 months ART retention was 60.4% and 58.6% among adults and among children and adolescents, respectively.6 A wide range of clinical, social, economic, systems and policy-related factors have a great negative influence on retention to HIV care. For example, poverty, low income and low education are associated with poor adherence to HIV treatment and virological failure.13,14 Poverty is highly correlated to food insecurity with the latter having the potential of leading to poor adherence to HIV treatment, missed drug refill appointments and possible LTFU.7,15 More so, malnutrition (especially severe wasting) is associated with poor ART outcome, especially a higher mortality in children.16–18 Thus, it is important to ensure food security among all HIV patients when scaling up ART. Indeed, the positive impact of foods support services on retention of children has been reported in other contexts.19,20 However, there is paucity of evidence on the effect of this intervention in the rural context of Cameroon. This study aimed at bridging this information gap by assessing the impact of foods support on loss to follow up among children and adolescents in HIV care in a district hospital of Cameroon.
Methods
Study Design and Setting
This was a quasi-experimental retro-prospective study conducted in Abong Mbang District Hospital (ADH) in the East Region of Cameroon. It was a part of the “Active Search for Pediatric HIV/AIDS” (ASPA) study conducted to compare the acceptability, feasibility and effectiveness of two HIV testing approaches of provider-initiated testing and counseling (PTIC) in Cameroon.21 ADH is a health facility providing comprehensive health care services to the catchment population (mostly rural), including the management of HIV/AIDS.
Study Period and Population
The population was all consecutive children and adolescents enrolled on ART at the hospital over the study periods. The retrospective phase (control group) was from June 2014 through May 2016 while the prospective phase was conducted from June 2016 through March 2018 (intervention group).
Intervention, Enrollment and Data Collection
Intervention
The intervention was foods support provided to HIV-infected children/adolescents during the prospective phase (this intervention was not available in the hospital before the study). This foods support consisted of the provision of foods kits to all children/adolescents on ART during that period. The foods kits provided were the same for all participants, and were composed of 1 liter of cooking oil, 1 kg of rice, 1 kg of powdered sugar and 125 g (5 sachets: 20 g/sachet) of powdered milk.
Data Collection – Prospective Phase
During the prospective phase, all HIV-infected children/adolescents on ART coming to the hospital for their monthly ARV drug refills or newly enrolled on ART were counseled by a trained data clerk for participation in the study. All children of consenting parents/guardians were recruited into the study (intervention group). Assent was sought for adolescents above 11 years of age prior to recruitment. At study enrollment, using a standardized questionnaire, we collected from each participant their socio-demographic data (sex, age) and anthropometric parameters (weight and height). In addition, during each drug refill visit, the parents/child couple received the routine adherence counseling after which a foods kit was supplied to the child and the next appointment for drug refill made. Appointment visits of all participants were aligned to the hospital routine ARV drug refill practice. Thus, children (accompanied by their parents/guardians) were to report to the hospital on monthly basis for follow up (drugs refill and clinical monitoring). Children/adolescents were thus followed up within a period of 22 months.
Data Collection – Retrospective Phase
This phase consisted only of data extraction from HIV medical records. Using the hospital’s HIV management registers, we enumerated all children/adolescents (same age group as above) enrolled on ART in the hospital within the retrospective timeframe (control group). Then, using a standardized form, we extracted from these registers the socio-demographic data and anthropometric parameters indicated above.
Study End Point, Definition and Calculation Parameters
The study end point was loss to follow up (LTFU) at 3, 6, 12 and 18 months from enrollment on ART. A participant was considered as LTFU if he/she missed the drugs refill visit for at least 3 consecutive months during the study period. The LTFU rate for each time point in the study was calculated as a proportion, having as the numerator the number of participants LTFU and as the denominator the number of participants on ART at the beginning of the cohort of interest and who were eligible for that follow up visit.
To be able to appreciate the effect of the foods support on the nutritional wellbeing of the prospective participants, their body mass indices (BMIs) were calculated from weights and heights measured during each of their follow up visits. For those who had BMI measurement pairs for their initial and the final follow up visits, their change in BMI was calculated (formula: ΔBMI=BMIM18 follow visit–BMIM3 follow up visit).
Data Management and Analysis
De-identified data from the questionnaires were entered into a data entry spreadsheet in Epi Info v7.2 (CDC, Atlanta, GA, USA), extracted in an Excel 2010 spreadsheet (Microsoft) for cleaning and then analyzed using SPSS v23 (IBM Corporation). Socio-demographic information was presented as proportions in cross-tabulation with foods support status. The proportions of LTFU were presented and compared amongst children at 3, 6, 12 and 18 months of follow up with respect to foods support status using the Fisher’s exact test. The odds of LTFU at 3, 6, 12 and 18 months were calculated and compared with respect to foods support status using logistic regression while adjusting for age at enrollment and sex. The unadjusted time on ART (before LTFU) was presented with respect to foods support status using the Kaplan–Meier survival curve and the observed difference in survival time was compared using the Mantel–Cox test. Their hazards of being LTFU were also calculated and compared between both groups using Cox regression while adjusting for age at enrollment and sex. All the above tests were conducted at a 5% significance rate.
Ethical Considerations
The ASPA study complies with the Declaration of Helsinki. The participation in the prospective phase was voluntary. Children/adolescents were enrolled in the study only upon signed informed consent from parents/guardians and assent obtained from children above 11 years of age. The study received ethical clearances from the Ludwig Maximilian University of Munich Ethics Commission (Germany), from the Cameroon National Ethics Committee (N° 631–29.15/AAR/MINSANTE/SG/DROS/NTF du 18 juin 2015), and an Administrative Authorization from the Cameroon Ministry of Public Health.
Results
Socio-Demographic and Anthropometric Characteristics of Children
Overall, 152 children/adolescents were recruited in the study among which 80 (52.6%) and 72 (47.4%) were of the retrospective and prospective phases, respectively. 77% of participants were female and the age ranged from 4 months to 19 years with a median age of 10 years. The anthropometric data (weight and height) were unavailable for participants in the retrospective phase. Among the 72 children and adolescents who received foods support during the prospective phase, BMI data were available only for 68 of them. In this group, at months 3, 6, 12 and 18, mean BMIs were 17.0±3.6 kg/m2, 17.2±4.0 kg/m2, 18.1±3.7 kg/m2 and 19.1±3.1 kg/m2 respectively (Figure 1).
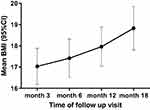 |
Figure 1 Evolution of BMI in children receiving foods support. |
Furthermore, 36 of the above participants had weight and height measurements at the initial and the final follow up visits, thus permitting the calculation of BMI change (ΔBMI). Of those, 86.1% (31/36) had an increase in their BMI during the follow up period with a median ΔBMI of 1.6 kg/m2 (IQR; 0.4–4.0 kg/m2), and the mean ΔBMI was 2.11±2.84 kg/m2.
Impact of Foods Support on Loss to Follow Up of Children and Adolescents on ART
The proportions of children and adolescents lost to follow up at 3, 6, 12 and 18 months in the retrospective phase (non-foods support period) compared to the prospective phase (foods support period) were: [28.8% vs 0%]; [17.6% vs 5.6%]; [13.5% vs 0.0%], and [26.7% vs 2.4%]. These differences were all statistically significant (Table 1).
 |
Table 1 LTFU at 3, 6, 12 and 18 Months in Children and adolescents with Respect to Foods Support |
When adjusted for sex and age at enrollment, the odds of LTFU among children/adolescents who did not receive foods support (compared to those who received foods support) were: [12.8 (4.2-39.1)], [18.3 (5.9-56.1)], and [30.9 (9.7-99.7)] at 6, 12 and 18 months, respectively (Table 2).
 |
Table 2 Odds of LTFU at 6, 12 and 18 Months with Respect to Foods Support Status Adjusted for Age at Enrollment and Sex at Birth |
The mean time on ART in the intervention group (foods support) was 17 months (95% CI; 16.3–17.9) against 12 months (95% CI; 9.8–14.7) in the control group (no foods support). This difference was statistically significant with log-rank (Mantel–Cox) p <0.001 (Figure 2).
 |
Figure 2 Kaplan–Meier survival curve of time in care until LTFU by nutritional support status. |
When the hazards of LTFU given the foods support status were adjusted for sex and age, participants who did not receive foods support were 10.2 (4.0–26.1) times more likely to be LTFU as compared to those who did receive foods support (Table 3).
 |
Table 3 Hazard Ratio of LTFU with Respect to Age, Sex and Foods Support Status |
Discussion
There is growing evidence of household economic strengthening interventions on retention in HIV care, ART adherence, morbidity and HIV-related mortality.7 This study is a contribution to that discourse.
Our results show that children and adolescents who did not receive foods kits were 10, 18 and 30 times more likely to be LTFU at 6, 12 and 18 months compared to those who received. The proportions of children/adolescents LTFU were significantly lower among children and adolescents in the intervention group. For example, the 12 months LTFU percentage was 0% among children and adolescents who received foods kits against 13.5% (p=0.006) among those who did not receive this intervention. This corresponds to a retention rate of 100% at 12 months in the foods support group. This proportion was higher compared to the retention rate of 60.4% reported by Billong et al in 2016 in the general population in Cameroon.6 Further, according to the Kaplan–Meier curve, the mean time on ART care was 17 months in the intervention group against 12 months in the control group. Moreover, the hazard (risk) of being LTFU was 10 times higher in the control group compared to the intervention group. These results are compelling evidence of the association between foods support and retention of children/adolescents on ART. They also concur with previous studies showing the positive outcome of foods services in HIV retention (including among children and adolescents) in other countries.15,19
It is noteworthy that the LTFU hazards were not significantly associated with age (or gender). This finding is not in line with previous studies showing younger children to be less likely to be retained in HIV care as their retention is dependent on caregivers amongst other reasons.1,22 This indicates that foods support could improve retention on ART even in children as young as 4 months of age.
Available studies on the impact of foods support on HIV treatment outcome vary widely in terms of quality and context. A review by Swann in 2018 reported that a good number of studies in Africa showed food assistance to be positively associated to self-reported adherence, medication pick up and appointment.7 On the contrary, a study in Cambodia showed no association between food assistance and treatment adherence even though the inconsistency of food assistance was noted here.23 Thus, the delivery modalities of the foods kits to the beneficiaries could explain the difference in outcome of this intervention. Our intervention could serve as an example of foods support delivery model for children and adolescents in HIV care.
Policy Implications
This study adds to the available body of evidence on the positive impact of foods support in HIV retention among people living with HIV including children and adolescents. Despite this knowledge, foods support services are either limited or inexistent in ART clinics in Cameroon, likewise in other sub-Saharan African countries. So far, the recommendations of international organizations such as the WHO, the Joint United Nations Programme on HIV/AIDS, the World Food Program (WFP) and the US President’s Emergency Plan for AIDS Relief (PEPFAR) to integrate foods support services into HIV/AIDS treatment and care programs have not been translated into practice. This situation is mainly due to funding gaps for this intervention, especially in non-PEPFAR funded countries.24,25 Retention into care being essential in achieving the elimination of HIV/AIDS by 2030, there is an urgent need for HIV global community to re-strategize HIV funding allocations to commit adequate resources for foods support services in high HIV/AIDS burden resource-constrained countries.
Strengths and Limitations
The strength of this study lies with the acquisition of scarce primary data on the impact of foods support among children and adolescents at district level and in a rural environment in Cameroon. To the best of our knowledge, this study is first of its kind in this country. However, the results cannot be generalized as the data are from one site, not randomly selected. Additionally, because of the quasi-experimental design, the validity of our results may have been affected by confounding variables (seasonality when using before and after design) and other potential biases inherent to non-randomized trials and pre–post design studies.26 That notwithstanding, the comparison of the retrospective and prospective groups provided needed evidence on the feasibility and the impact of foods support on ART retention in children and adolescents in this rural facility. Moreover, our study has provided new knowledge that could inform the implementation of foods support among children and adolescents on HIV care. Furthermore, our results could guide the design of further robust research on the impact of foods support services in reducing LTFU and improving retention in HIV care among neonates, children and adolescents in Cameroon and beyond.
Conclusions
Foods support is an effective intervention in improving HIV retention among children and adolescents. Despite this fact, this intervention is not yet readily available for patients and this is due to a funding gap. The HIV global community should re-strategize and allocate adequate funding to enable the implementation of this intervention. This could help in improving HIV retention among children and contribute to HIV/AIDS elimination among this sub-population by 2030.
Acknowledgments
This study constitutes a part of the PhD Medical Research-International Health dissertation of Dr. Habakkuk Yumo (corresponding author) at the Center for International Health (CIH)–Ludwig Maximilian Universität in Muenchen (Germany). He is thankful to Prof. Thomas Loescher and Christopher Kuaban, PhD Advisors and all the lecturers of the PhD Medical Research-International Health for guidance and support. The authors are very thankful to all of the parents and children who participated in this study. They thank all of the health personnel of the Abong-Mbang for their collaboration. The authors also appreciate the role of Dr. Titus Sabi (Camfomedics e.V.) for his support in the grant management. Likewise, their gratitude goes to the ASPA Study Central Coordination Team at R4D International Foundation (Yaoundé) as well as the coordinators and research officers/data clerks.
Funding
This study was funded the Else Kroener-Fresenius-Stiftung (Bad Homburg, Germany). Camformedics e.V. (Essen, Germany) coordinated the management of the study funds between the Else Kroener-Fresenius-Stiftung and R4D International Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. McNairy ML, Lamb MR, Carter RJ, et al. Retention of HIV-infected children on antiretroviral treatment in HIV care and treatment programs in Kenya, Mozambique, Rwanda, and Tanzania. J Acquir Immune Defic Syndr. 2013;62(3):e70–81. doi:10.1097/QAI.0b013e318278bcb0
2. Boyles TH, Wilkinson LS, Leisegang R, Maartens G, Wilkinson RJ. Factors influencing retention in care after starting antiretroviral therapy in a rural South African programme. PLoS One. 2011;6(5):e19201. doi:10.1371/journal.pone.0019201
3. Phelps BR, Ahmed S, Amzel A, et al. Linkage, initiation and retention of children in the antiretroviral therapy cascade: an overview. Aids. 2013;27(Suppl 2):S207–13. doi:10.1097/QAD.0000000000000095
4. Scott CA, Iyer H, Bwalya DL, et al. Retention in care and outpatient costs for children receiving antiretroviral therapy in Zambia: a retrospective cohort analysis. PLoS One. 2013;8(6):e67910. doi:10.1371/journal.pone.0067910
5. Ugwu O. Impact of a child -friendly clinic on retention of HIV-infected children in care: an intervention study. Niger J Paediatr. 2017;44(4):175–179. doi:10.4314/njp.v44i4.1
6. Billong SC, Fokam J, Penda CI, et al. Predictors of poor retention on antiretroviral therapy as a major HIV drug resistance early warning indicator in Cameroon: results from a nationwide systematic random sampling. BMC Infect Dis. 2016;16(1):678. doi:10.1186/s12879-016-1991-3
7. Swann M. Economic strengthening for retention in HIV care and adherence to antiretroviral therapy: a review of the evidence. AIDS Care. 2018;30(sup3):99–125. doi:10.1080/09540121.2018.1479030
8. Mugglin C, Haas AD, van Oosterhout JJ, et al. Long-term retention on antiretroviral therapy among infants, children, adolescents and adults in Malawi: a cohort study. PLoS One. 2019;14(11):e0224837. doi:10.1371/journal.pone.0224837
9. Ramdas N, Meyer JC, Cameron D. Factors associated with retention in HIV care at sediba hope medical centre. South Afr J HIV Med. 2015;16(1):347. doi:10.4102/sajhivmed.v16i1.347
10. Janssen S, Wieten RW, Stolp S, et al. Factors Associated with retention to care in an HIV clinic in Gabon, Central Africa. PLoS One. 2015;10(10):e0140746. doi:10.1371/journal.pone.0140746
11. Abuogi LL, Smith C, McFarland EJ, Okulicz JF. Retention of HIV-infected children in the first 12 months of anti-retroviral therapy and predictors of attrition in resource limited settings: a systematic review. PLoS One. 2016;11(6):e0156506. doi:10.1371/journal.pone.0156506
12. UNAIDS. Country fact sheet Cameroon. Geneva: UNAIDS; 2018. Available from: www.unaids.org/en/regionscountries/countries/cameroon.
13. Burch LS, Smith CJ, Anderson J, et al. Socioeconomic status and treatment outcomes for individuals with HIV on antiretroviral treatment in the UK: cross-sectional and longitudinal analyses. Lancet Public Health. 2016;1(1):e26–e36. doi:10.1016/S2468-2667(16)30002-0
14. Tran BX, Hwang J, Nguyen LH, et al. Impact of socioeconomic inequality on access, adherence, and outcomes of antiretroviral treatment services for people living with HIV/AIDS in Vietnam. PLoS One. 2016;11(12):e0168687. doi:10.1371/journal.pone.0168687
15. Young S, Wheeler AW, McCoy SI, Weiser SD. A review of the role of food insecurity in adherence to care and treatment among adult and pediatric populations living with HIV and AIDS. AIDS Behav. 2014;18(S5):S505–S15. doi:10.1007/s10461-013-0547-4
16. Jesson J, Leroy V. Challenges of malnutrition care among HIV-infected children on antiretroviral treatment in Africa. Med Mal Infect. 2015;45(5):149–156. doi:10.1016/j.medmal.2015.03.002
17. Paton NI, Sangeetha S, Earnest A, Bellamy R. The impact of malnutrition on survival and the CD4 count response in HIV-infected patients starting antiretroviral therapy. HIV Med. 2006;7(5):323–330. doi:10.1111/j.1468-1293.2006.00383.x
18. Taye B, Shiferaw S, Enquselassie F. The impact of malnutrition in survival of HIV infected children after initiation of antiretroviral treatment (ART). Ethiop Med J. 2010;48(1):1.
19. Adjorlolo-Johnson G, Uheling AW, Ramachandran S, et al. Scaling up pediatric HIV care and treatment in Africa: clinical site characteristics associated with favorable service utilization. J Acquir Immune Defic Syndr. 2013;62(1):e7–e13. doi:10.1097/QAI.0b013e3182706401
20. Rachlis B, Bakoyannis G, Easterbrook P, et al. Facility-level factors influencing retention of patients in HIV care in East Africa. PLoS One. 2016;11(8):e0159994. doi:10.1371/journal.pone.0159994
21. Yumo HA, Kuaban C, Ajeh RA, et al. Active case finding: comparison of the acceptability, feasibility and effectiveness of targeted versus blanket provider-initiated-testing and counseling of HIV among children and adolescents in Cameroon. BMC Pediatr. 2018;18(1):309. doi:10.1186/s12887-018-1276-7
22. Sikhondze N, Mahomed OH. Retention of children under 18 months testing HIV positive in care in Swaziland: a retrospective study. Pan Afr Med J. 2017;28:316. doi:10.11604/pamj.2017.28.316.13857
23. Daigle GT, Jolly PE, Chamot EAM, et al. System-level factors as predictors of adherence to clinical appointment schedules in antiretroviral therapy in Cambodia. AIDS Care. 2015;27(7):836–843. doi:10.1080/09540121.2015.1024098
24. Ivers LC, Cullen KA, Freedberg KA, Block S, Coates J, Webb P. HIV/AIDS, undernutrition, and food insecurity. Clin Infect Dis. 2009;49(7):1096–1102. doi:10.1086/605573
25. Anema A, Zhang W, Wu Y, et al. Availability of nutritional support services in HIV care and treatment sites in sub-Saharan African countries. Public Health Nutr. 2012;15(5):938–947. doi:10.1017/S136898001100125X
26. Cindy T. Threats to validity in retrospective studies. J Adv Pract Oncol. 2012;3(3):181–183. pmid: 25031944.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
