Back to Journals » Open Access Emergency Medicine » Volume 14
The Necessity of Individualized Treatment for Sepsis-Associated Disseminated Intravascular Coagulation by Infected Organ
Authors Kobayashi M , Ehama Y, Hirayama S
Received 20 January 2022
Accepted for publication 22 March 2022
Published 7 April 2022 Volume 2022:14 Pages 133—140
DOI https://doi.org/10.2147/OAEM.S359216
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Hans-Christoph Pape
Makoto Kobayashi,1 Yoshimatsu Ehama,2 Suguru Hirayama2
1Intensive Care Center, Hakodate Goryoukaku Hospital, Hakodate City, Hokkaido, Japan; 2Department of Emergency Medicine, Hakodate Goryoukaku Hospital, Hakodate City, Hokkaido, Japan
Correspondence: Makoto Kobayashi, Hakodate Goryoukaku Hospital, 38-3 Goryoukaku-cho, Hakodate City, Hokkaido, 040-8611, Japan, Tel +81-138-51-2295, Fax +81-138-56-2695, Email [email protected]
Objective: Several studies have shown that anticoagulation can improve survival outcomes in patients with sepsis-associated disseminated intravascular coagulation (DIC). A guideline from Japan in 2020 suggested two therapeutic agents for sepsis-associated DIC treatment: antithrombin (AT) replacement therapy and recombinant thrombomodulin (rTM) preparation. In 2021, our preliminary study proposed that different organs of septic infection might lead to distinct treatment outcomes following different therapies against DIC. In this study, we created a subanalysis on the influence of AT replacement therapy and rTM preparations on overall survival (OS) by comparing two causative organs: biliary and respiratory tract infections.
Patients and Methods: This retrospective cohort study in a single institution involved patients with sepsis-associated DIC treated by either AT or rTM who were categorized based on sources of infection. The two groups defined for this study were biliary (n = 62) and respiratory tract infection (n = 84). To assess the clinical efficacy of AT and rTM, 30-day OS was examined using a stepwise variable selection for a Cox proportional hazards model.
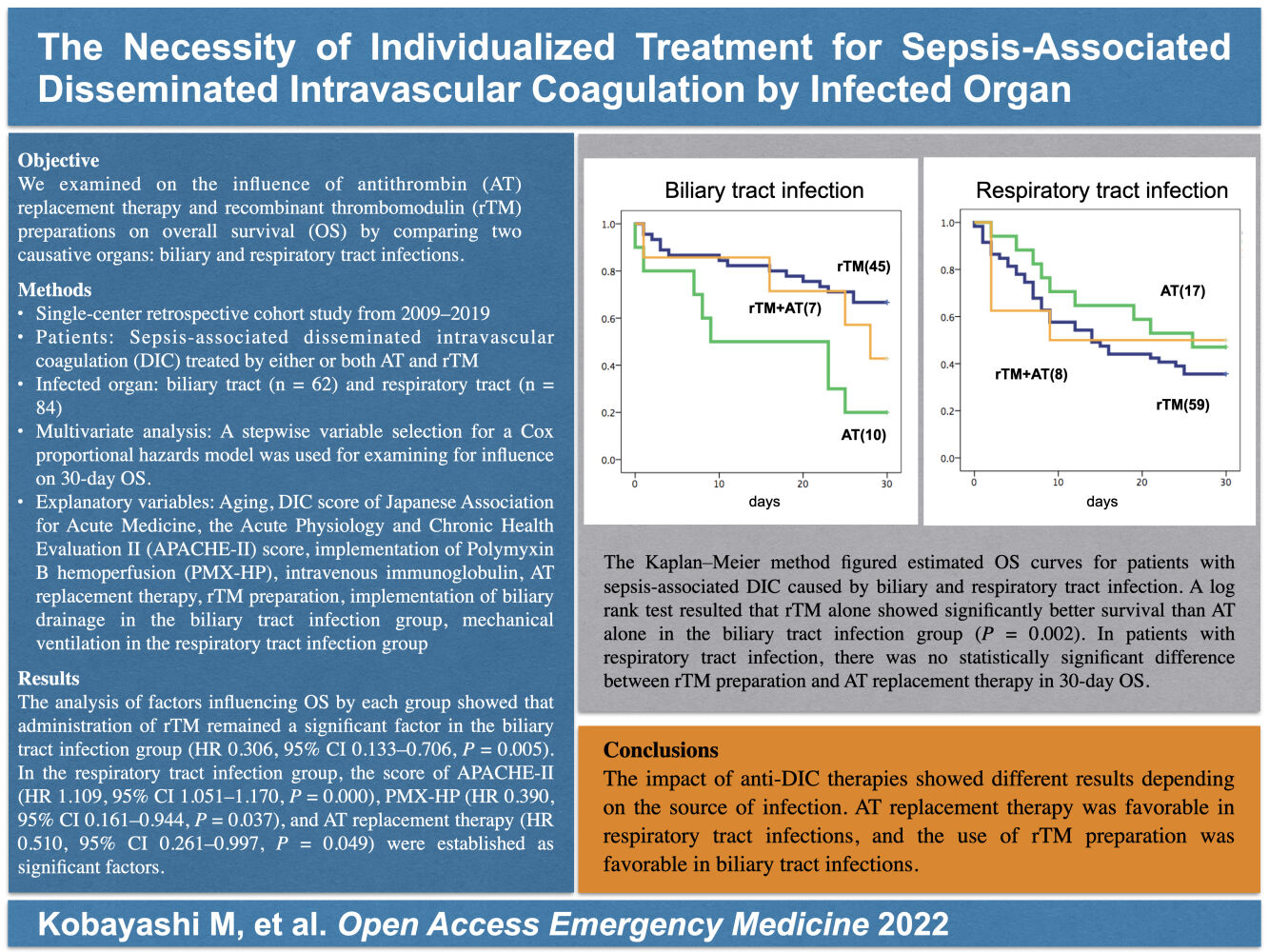
Results: The analysis of factors influencing OS by each group showed that rTM preparation remained a significant factor in the biliary tract infection group (HR 0.306, 95% CI 0.133– 0.706). In the respiratory tract infection group, the score of the Acute Physiology and Chronic Health Evaluation II (HR 1.109, 95% CI 1.051– 1.170), polymyxin B hemoperfusion (HR 0.390, 95% CI 0.161– 0.944), and AT replacement therapy (HR 0.510, 95% CI 0.261– 0.997) were established as significant factors.
Conclusion: This study revealed that the OS of patients with biliary tract and respiratory tract infections differed depending on the DIC therapeutic agent. Based on these results, we could suggest that it is necessary to develop individualized treatment strategies for septic infections, taking into consideration the differences in the infected organs.
Graphal Abstract:
Keywords: antithrombin, recombinant thrombomodulin, overall survival, biliary tract infection, respiratory tract infection
Graphical Abstract:
Introduction
A large Japanese observational study of sepsis patients in 2019 reported a significantly higher mortality rate among sepsis-associated disseminated intravascular coagulation (DIC) patients compared to the overall mortality rate among septic patients.1 In addition, several studies have shown that anticoagulation can improve survival outcomes in patients with DIC.2–4 Based on these results, the Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 20205 suggested two therapeutic agents for DIC treatment: antithrombin (AT) replacement therapy and recombinant thrombomodulin (rTM) preparation. However, different evaluations of these drugs have been reported in overseas clinical trials. In reviewing two Japanese meta-analyses on the prognostic value of AT replacement therapy,6,7 it was determined that these revised results did not change the overall conclusion that the quantity and quality of evidence supporting the use of AT in sepsis patients with DIC was low.8 The SCARLET randomized clinical trial evaluated the effect of rTM on mortality in patients with sepsis-associated coagulopathy, and concluded that administration of rTM did not significantly reduce 28-day all-cause mortality.9 In view of these results, international sepsis treatment guidelines for 2021 do not indicate the need for treatment of DIC,10 with DIC treatment still being controversial. The pathogenesis of sepsis is diverse, and we proposed in 2021 that different organs of infection might lead to different treatment outcomes when following different DIC therapies.11 In this study, we created a subanalysis of the effect of different DIC therapies on overall survival (OS) by comparing two causative organs: infections of the biliary and the respiratory tracts.
Methods
The study protocol was approved by the ethics committee of Hakodate Goryoukaku Hospital (Approval No. 2020–049). Informed consent was waived because of the retrospective study design; however, the committee also verified that the data remained confidential by concealing the privacy of patients and complying with the Declaration of Helsinki.
Patient Selection
This was a retrospective cohort study of patients with sepsis-associated DIC who were admitted to Hakodate Goryoukaku Hospital during the period of May 2008 to December 2019. In total, 297 patients who suffered from sepsis-associated DIC and were treated by either or both AT replacement therapy and rTM preparation were assessed for inclusion. Patients were categorized based on sources of infection, and the two groups defined for this study were biliary (n = 62) and respiratory tract infection (n = 84). Patients were excluded from the study if they suffered from Child–Pugh C hepatic cirrhosis, hematologic diseases such as leukemia, or end-stage cancer.
Definitions
Sepsis was diagnosed based on the Sepsis-3 criteria,12 and DIC was defined as a total score of ≥4 according to the Japanese Association for Acute Medicine (JAAM) DIC scoring system.13 The Acute Physiology and Chronic Health Evaluation II (APACHE-II)14 was used to objectively assess patient clinical severity. Baseline data was collected from electronic medical records and evaluated to confirm diagnosis when DIC treatment had been planned. DIC improvement was defined as when the JAAM DIC score decreased by seven days after starting administration of an anti-DIC drug. All patients were followed-up for 30 days after a diagnosis of sepsis with DIC. The observation period in the survival time analysis was defined as from the start of DIC treatment to 30 days later.
Treatment Management
All participants were first administered antibacterial drugs against each cause of infection, and once a diagnosis of DIC was made, then treated by an anticoagulant therapy. The anticoagulant therapies for DIC available in our hospital were limited to the use of AT replacement therapy or rTM preparations. For AT replacement therapy, the supplementation dose of AT was 1500 IU/day for three days; rTM was administrated at a dose of 380 U/kg, but the dose was reduced to 130 U/kg in patients with renal disorders. Intravenous immunoglobulin was provided intravenously for three consecutive days at 5g/body. In this retrospective study, there were no predefined protocols regarding the definite indications for using AT replacement therapy, rTM preparation, or immunoglobulin. Therefore, these therapeutic modalities were administered at the discretion of the attending physician. Polymyxin B hemoperfusion (PMX-HP) was indicated for patients with septic shock unresponsive to standard fluid resuscitation and cardiovascular agents; however, its enforcement was left to the discretion of the attending physician. A biliary drain was applied for treatment of biliary obstruction as needed in the biliary tract infection group.
Statistical Analysis
Results are presented as median or mean ± standard deviation. OS was defined as the time from DIC diagnosis to any cause death within 30 days. The Kaplan–Meier method was used to estimate survival rates depending on the source of infection, and a comparison between anti-DIC therapies was made using a log rank test. To assess the outcomes of the clinical treatments, 30-day survival was examined using a Cox proportional hazards model, and improvement of DIC state was analyzed by logistic regression models. For both analyses, the variables considered are as follows: aging, JAAM DIC score, APACHE-II score, implementation of PMX-HP, intravenous immunoglobulin, AT replacement therapy, and rTM preparation. Date and time of the data used for statistical variables for the JAAM DIC and APACHE-II scores were from before beginning DIC treatments. As for additional variables for the multivariate analysis, implementation of biliary drainage in the biliary tract infection group and mechanical ventilation in the respiratory tract infection group were included in each group analysis, respectively. Hazard ratios (HRs), odds ratios, and 95% confidence intervals (CIs) were determined for these variables. In all tests, P < 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics for Macintosh, Version 21.0. (IBM, Armonk, NY, USA).
Results
Patient Characteristics
Table 1 shows patients characteristics in each group. All patients in this study suffered from DIC for the first time and were receiving anti-DIC treatment for the first time. All patients were treated in the ICU during the onset of DIC.
 |
Table 1 Baseline Clinical Characteristics of Patient with Sepsis-Associated DIC by Comparing Two Causative Organs |
Influence of DIC Treatment on Improvement of DIC
The improvement rate of DIC was 66% in the biliary tract infection group and 45% in the respiratory tract infection group (P = 0.012, chi-square test). In respiratory tract infection group, the rate of DIC improvement by anti-DIC therapy was 59% (10/17) of AT replacement therapy alone and 42% (25/59) of TM preparation alone (P = 0.231, chi-square test).
In biliary tract infection group, the rate of DIC improvement by anti-DIC treatment was 40% (4/10) of AT replacement therapy alone and 73% (33/45) of TM preparation alone (P = 0.042, chi-square test). Logistic regression analysis showed that the influence of DIC drugs on the rate of DIC improvement was not significant for either drug in each group.
Influence of DIC Treatment on 30-Day Overall Survival
In a study of OS by DIC drugs, rTM alone showed significantly better survival than AT alone in the biliary tract infection group (P = 0.002) (Figure 1). In the respiratory tract infection group, there was no difference in survival rates between different DIC drugs (Figure 2). Cox proportional hazards model analysis of factors influencing OS by group showed that administration of rTM remained a significant factor in the biliary tract infection group (HR 0.306, 95% CI 0.133–0.706, P = 0.005). In the respiratory tract infection group, APACHE-II (HR 1.109, 95% CI 1.051–1.170, P = 0.000), PMX-HP (HR 0.390, 95% CI 0.161–0.944, P = 0.037), and AT replacement therapy (HR 0.510, 95% CI 0.261–0.997, P = 0.049) were selected as significant factors by a stepwise variable selection for a Cox proportional hazards model.
Discussion
Guidelines from various countries on sepsis treatment do not outline specified treatments for different causes of infection. In this study, we investigated the effect of DIC therapies in these two groups: sepsis caused by biliary and respiratory tract infections. The results showed that different outcomes for AT replacement therapy and rTM preparation occurred due to different sites of infection.
There are several reports indicating the usefulness of rTM for respiratory failure in patients with septic DIC.15–18 However, a large retrospective nationwide study in Japan19 demonstrated that there might be little association between the use of rTM and mortality in severe pneumonia patients with sepsis-associated DIC. In contrast, a study on the effect of AT supplementation in pulmonary infections using the same Japanese database concluded that AT treatment may be associated with reduced 28-day mortality in patients with severe pneumonia and sepsis-associated DIC.20 These results suggest that AT replacement therapy may be more effective than other treatments in improving the prognosis of patients with sepsis due to respiratory tract infection.
Initial management of acute biliary infection and acute cholangitis (Tokyo Guidelines 2018)21 stated that the value of anticoagulants for associated DIC in severe cholangitis was still unclear, but the administration of rTM might be considered. Conversely, there is no decisive report indicating the benefits of AT replacement therapy for biliary tract infection associated DIC. A study on the efficacy of AT supplementation in combination with rTM preparation in 2019 reported that the concomitant use of AT with rTM for acute cholangitis-induced DIC may not improve treatment outcomes.22 A multicenter study evaluating the clinical effectiveness of rTM in 284 patients with acute cholangitis and sepsis-induced DIC who underwent biliary drainage23 concluded that after propensity score matching, the 28-day survival rate was significantly higher in the rTM group when compared with the non-rTM group. Thus far, many reports have suggested the usefulness of rTM preparations in patients with septic DIC caused by biliary tract infection.
In the present study, we found differences in outcomes between biliary and respiratory tract infections due to the use of different anti-DIC drugs. In determining how this could occur, the structure and function of blood vessels and how they vary from organ to organ should be considered. Infection-triggered DIC starts with a disorder of the coagulation-fibrinolysis system, primarily on the endothelial surface of blood vessels. Endothelial cell structure is specific for each organ and forms at least three types of capillaries: continuous, fenestrated, and sinusoidal. In particular, the liver is sinusoidal and the lungs are continuous, with obvious structural differences.24–26 On the surface of vascular endothelial cells, there is a structure called the glycocalyx. The glycocalyx is responsible for maintaining blood flow, regulating coagulation, modifying permeability, and controlling inflammatory responses. Its changes are closely related to disorders of vascular functions.27–29 In the case of sepsis, it is known that the glycocalyx of the vascular endothelium decreases from an early stage.30 The thinning of the glycocalyx causes changes in vascular permeability, and the loss of AT and TM contained in the glycocalyx causes an imbalance in the coagulation system.29 The thickness of the glycocalyx structure differs among organs,31 and the sinusoids of the liver have a thinner glycocalyx structure than the continuous type of vessels in the lungs.32 These differences in the capillary structure of the endothelial cells and the glycocalyx thickness of the blood vessels may be responsible for the different outcomes in the efficacy of AT replacement therapy and rTM preparation.
Different organs have unique vascular structures and various defense mechanisms against infection and tissue destruction. This and other factors lead to individual differences in biological responses. In 2019, Singer33 pointed out in his review the problematic nature of guidelines with homogenous management therapies, and the importance of individualized treatment. He commented that guidelines were valuable as an aide in memorization, however, the evidence that strict protocols with homogenized management resulting in outcome benefits was weak at best. Logically, treatment should be tailored to meet an individual’s needs and underlying comorbidities.
Limitations
There were several limitations associated with the present study. Firstly, our study was a single-center, retrospective analysis without randomization, and the number of patients was small, with a long enrollment period for eligible patients. Although the cases were collected over a period of 11 years, no statistically significant influence on treatment outcome by time was found by multivariate analysis. Secondly, the source of infection was classified into two groups, each of which could include various pathological conditions, and a bias could still be present in the form of confounders. Additionally, we made no definite indications for the use of AT replacement therapy or rTM preparation; these therapeutics were administered at the discretion of the attending physician. Also, the decision about the number of days the rTM preparation was administered was left to the discretion of the attending physician, as there is no strict provision for discontinuation of medication in our institution. Lastly, the mean blood AT activity level before the start of treatment in the biliary tract infection group was 49%, which was significantly lower than the mean of 58% in the respiratory tract infection group (P = 0.010, Student-t test). It has been indicated that the Japanese insurance coverage dose of 1500 units/day of the AT supplement may not be sufficiently effective in patients with blood AT activity levels below 50%.34 This may be one of the reasons for the inadequate efficacy of the AT replacement therapy for the biliary tract infection group in this study.
Conclusion
This study revealed that the OS of patients with biliary tract and respiratory tract infections differed depending on the DIC therapeutic agent. Based on these results, we could suggest that it is necessary to develop individualized treatment strategies for septic infections, taking into consideration the differences in the infected organs.
Acknowledgments
The authors would like to thank Enago (Crimson Interactive Japan Co., Ltd, Japan) for the English language review.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gando S, Shiraishi A, Yamakawa K, et al. Role of disseminated intravascular coagulation in severe sepsis. Thromb Res. 2019;178:182–188. doi:10.1016/j.thromres.2019.04.025
2. Umemura Y, Yamakawa K, Ogura H, et al. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta-analysis of randomized controlled trials. J Thromb Haemost. 2016;14(3):518–530. doi:10.1111/jth.13230
3. Yamakawa K, Umemura Y, Hayakawa M, et al. Benefit profile of anticoagulant therapy in sepsis: a nationwide multicentre registry in Japan. Crit Care. 2016;20:229. doi:10.1186/s13054-016-1415-1
4. Hayakawa M. Management of disseminated intravascular coagulation: current insights on antithrombin and thrombomodulin treatments. Open Access Emerg Med. 2018;10:25–29. doi:10.2147/oaem.s135909
5. Egi M, Ogura H, Nishida O, et al. The Japanese clinical practice guidelines for management of sepsis and septic shock 2020 (J-SSCG 2020). J Intensive Care. 2021;9:53. doi:10.1186/s40560-021-00555-7
6. Tagami T. Antithrombin concentrate use in sepsis-associated disseminated intravascular coagulation: re-evaluation of a “pendulum effect” drug using a nationwide database. J Thromb Haemost. 2018;16(3):458–461. doi:10.1111/jth.13948
7. Umemura Y, Yamakawa K. Optimal patient selection for anticoagulant therapy in sepsis: an evidence-based proposal from Japan. J Thromb Haemost. 2018;16(3):462–464. doi:10.1111/jth.13946
8. Wiedermann CJ. Antithrombin concentrate use in disseminated intravascular coagulation of sepsis: meta-analyses revisited. J Thromb Haemost. 2018;16(3):455–457. doi:10.1111/jth.13950
9. Vincent JL, Francois B, Zabolotskikh I, et al. Effect of a recombinant human soluble thrombomodulin on mortality in patients with sepsis-associated coagulopathy: the SCARLET Randomized Clinical Trial. JAMA. 2019;321(20):1993–2002. doi:10.1001/jama.2019.5358
10. Evans L, Rhodes A, Alhazzani W, et al. Sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063–e1143. doi:10.1097/CCM.0000000000005337
11. Kobayashi M, Asakura R, Ehama Y, et al. Impact of anticoagulant therapy on mortality for sepsis-associated disseminated intravascular coagulation depending on the source of infection. J Intensive Crit Care. 2021;7(3):62. doi:10.36648/2471-8505.21.7.37
12. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
13. Gando S, Saitoh D, Ogura H, et al. Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: results of a multicenter, prospective survey. Crit Care Med. 2008;36(1):145–150. doi:10.1097/01.ccm.0000295317.97245.2d
14. Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi:10.1097/00003246-198510000-00009
15. Kawano N, Yoshida S, Ono N, et al. Clinical features and outcomes of 35 disseminated intravascular coagulation cases treated with recombinant human soluble thrombomodulin at a single institution. J Clin Exp Hematop. 2011;51(2):101–107. doi:10.3960/jslrt.51.101
16. Yamakawa K, Fujimi S, Mohri T, et al. Treatment effects of recombinant human soluble thrombomodulin in patients with severe sepsis: a historical control study. Crit Care. 2011;15:R123. doi:10.1186/cc10228
17. Hayakawa M, Yamamoto H, Honma T, et al. Pharmacokinetics and pharmacodynamics of recombinant soluble thrombomodulin in disseminated intravascular coagulation patients with renal impairment. Shock. 2012;37(6):569–573. doi:10.1097/shk.0b013e318252bc82
18. Ozawa Y, Yamakawa K, Ogura H, et al. Recombinant human soluble thrombomodulin improves mortality and respiratory dysfunction in patients with severe sepsis. J Trauma Acute Care Surg. 2012;72(5):1150–1157. doi:10.1097/ta.0b013e3182516ab5
19. Tagami T, Matsui H, Horiguchi K, et al. Recombinant human soluble thrombomodulin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: an observational nationwide study. J Thromb Haemost. 2014;13(1):31–40. doi:10.1111/jth.12786
20. Tagami T, Matsui H, Horiguchi K, et al. Antithrombin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: an observational nationwide study. J Thromb Haemost. 2014;12(9):1470–1479. doi:10.1111/jth.12643
21. Miura F, Okamoto K, Takada T, et al. Tokyo Guidelines 2018: initial management of acute biliary infection and flowchart for acute cholangitis. J Hepatobiliary Pancreat Sci. 2018;25(1):31–40. doi:10.1002/jhbp.509
22. Morita N, Nakahara K, Morita R, et al. Efficacy of combined thrombomodulin and antithrombin in anticoagulant therapy for acute cholangitis-induced disseminated intravascular coagulation. Intern Med. 2019;58(7):907–914. doi:10.2169/internalmedicine.1923-18
23. Ogura T, Eguchi T, Nakahara K, et al. Clinical impact of recombinant thrombomodulin administration on disseminated intravascular coagulation due to severe acute cholangitis (Recover-AC study). J Hepatobiliary Pancreas Sci. 2021;28(1):1–8. doi:10.1002/jhbp.998
24. Kisch B. Electron microscopy of the capillary wall. Exp Med Surg. 1956;14(2–3):113–121.
25. Rhodin J. Electron microscopy of the glomerular capillary wall. Exp Cell Res. 1955;8(3):572–574. doi:10.1016/0014-4827(55)90136-1
26. Wisse E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J Ultrastruct Res. 1970;31(1–2):125–150. doi:10.1016/s0022-5320(70)90150-4
27. VanTeeffelen JW, Brands J, Stroes ES, et al. Endothelial glycocalyx: sweet shield of blood vessels. Trends Cardiovasc Med. 2007;17(3):101–105. doi:10.1016/j.tcm.2007.02.002
28. Reitsma S, Slaaf DW, Vink H, et al. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. doi:10.1007/s00424-007-0212-8
29. Chelazzi C, Villa G, Mancinelli P, et al. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit Care. 2015;19:26. doi:10.1186/s13054-015-0741-z
30. Iba T, Levy JH. Derangement of the endothelial glycocalyx in sepsis. J Thromb Haemost. 2019;17(2):283–294. doi:10.1111/jth.14371
31. Ando Y, Okada H, Takemura G, et al. Brain-specific ultrastructure of capillary endothelial glycocalyx and its possible contribution for blood brain barrier. Sri Rep. 2018;8:17523. doi:10.1038/s41598-018-35976-2
32. Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012;108(3):384–394. doi:10.1093/bja/aer515
33. Singer M. Sepsis: personalization v protocolization? Crit Care. 2019;23:127. doi:10.1186/s13054-019-2398-5
34. Iba T, Saitoh D, Wada H, et al. Efficacy and bleeding risk of antithrombin supplementation in septic disseminated intravascular coagulation: a secondary survey. Crit Care. 2014;18:497. doi:10.1186/s13054-014-0497-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


