Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 16
The Clinical Manifestations and Efficacy of Different Treatments Used for Nephrogenic Systemic Fibrosis: A Systematic Review
Authors Farooqi S , Mumtaz A , Arif A , Butt M, Kanor U , Memoh S , Qamar MA , Yosufi A
Received 17 October 2022
Accepted for publication 10 January 2023
Published 12 January 2023 Volume 2023:16 Pages 17—30
DOI https://doi.org/10.2147/IJNRD.S392231
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Shaheer Farooqi,1 Afshan Mumtaz,2 Aabiya Arif,1 Mehwish Butt,3 Una Kanor,4 Samuel Memoh,5 Mohammad Aadil Qamar,1 Abubakr Yosufi6
1Department of Medicine, Ziauddin University, Karachi, Pakistan; 2Department of Medicine and Allied, Karachi Medical and Dental College, Karachi, Pakistan; 3Department of Medicine and Allied, Jinnah Medical and Dental college, Karachi, Pakistan; 4Department of Medicine, Bogomolets National Medical University, Kyiv, Ukraine; 5Department of Medicine, Windsor University School of Medicine, Cayon, Saint Kitts and Nevis; 6Medical School, Kabul University of Medical Sciences, Kabul, Afghanistan
Correspondence: Abubakr Yosufi, Kabul University of Medical Sciences, Kabul, Afghanistan, Tel +93 747236767, Email [email protected]
Aim: Nephrogenic systemic fibrosis (NSF) is a rare disorder that occurs in association majorly with chronic kidney disease (CKD). The lack of collective quantitative data on its clinical manifestations and the different treatment options’ efficacy, call the need for our investigation.
Methods: A systematic review was conducted covering a timeline from inception up to July 2022 without any restrictions. Article screening and data extraction were performed independently on PubMed, Google Scholar, ScienceDirect, and Cochrane Library. The keywords that we used were CKD, NSF, Gadolinium enduced fibrosis, etc; shortlisted articles were assessed for risk of bias. Data were presented as frequencies and percentages, with a confidence interval of 95%. A chi-square test was also done to find significant relationships, with a p-value < 0.05 considered significant.
Results: We had 83 patients in this review consisting of 44 (55.7%) females with a mean age of 51.4± 14.6 years. Sixty-nine (83.1%) patients had chronic kidney disease predisposition to NSF. Previous exposure to gadolinium-based contrast dyes was seen in 66 (79.5%) patients). The most common symptom in patients was cutaneous lesions in 69 (83.1%) patients. The most used treatments were ultraviolet therapy, renal transplant, and extracorporeal photopheresis; in 13.3% of the patients each. Condition in most patients either improved (67.1%) or remained stable (11.8%). Chi-square testing found that the treatments offered were also seen to be significantly related to outcome (p=0.015).
Conclusion: The findings in this study provide a quantitative measurement of NSF’s presentations and treatment efficacies. This serves to make way for researchers to form comprehensive guidelines on the presentation-based treatment of NSF.
Keywords: chronic kidney disease, fibrosis, scleroderma, renal impairment, CKD
Introduction
Nephrogenic systemic fibrosis (NSF) is a rare scleroderma-like fibrosing disorder which occurs in majorly association with chronic kidney disease (CKD), most likely in patients of stages 4 and 5 chronic kidney disease (CKD), but sometimes also in patients with stage 3 CKD.1–3 Majorly a cutaneous manifestation but also effecting mobility, NSF was first reported in 1997; in that study, all 15 patients were dialysis dependent, with varying levels of renal impairment.1,4 Histological studies show characteristic CD 34 and procollagen-positive dermal spindle cells, as well as randomly arranged collagen fibers along with mucin deposition.1,5 Typically, patients present with pruritus and burning sensation of the skin, as well as swelling of the arms and legs. The induration of skin and development of contractures is accompanied by debilitating disability due to loss of range of motion.1,6,7 The disease shows no inclination towards a certain age group or gender but does show some level of occurrence in patients with renal disease exposed to gadolinium-based contrast agents (GBCA).1,8
Although still a relatively new phenomenon, there are several treatment options utilized in the patients with NSF, depending on the extent of disease and renal impairment. Improvement in renal function mostly leads to simultaneous improvement in the symptoms of NSF, but addressing the dermatological manifestation directly might also aid in achieving positive outcomes. Extracorporeal photopheresis, plasmapheresis, phototherapy, sodium thiosulfate, physical therapy, and kidney transplant are few of the many such treatment options.1,9–11
Previous literature discusses different aspects related to NSF extensively, such as NSF as a risk of GBCA in patients of late-stage CKD, or in patients of liver disease.12,13 Other studies discuss the clinical features and the pathology of the disease, while also discussing few of the treatment options available.14,15 Nonetheless, these studies lack data on the efficacy of the different treatment options available for NSF. Hence, to the best of our knowledge, this systematic review of the published literature is the first of its kind, and aims to provide an extensive insight of NSF by answering the following questions: (i) What are the different clinical presentations in patients with NSF? (ii) What is the extent of these clinical manifestations? (iii) What are the different treatment options and their efficacy in patients with NSF?
Materials and Methods
Our study follows the most recent Preferred Reporting Items for Systematic Reviews and Meta-Analyses standards (PRISMA) statement.16 The protocol of our study was registered to PROSPERO.
Literature Search
We performed an extensive literature search of PubMed, Google Scholar, ScienceDirect, and Cochrane Library, which was conducted from inception to the last updated month of July 2022, using a combination of database-specific subject headings and keywords as search terms that included “nephrogenic systemic fibrosis”, “renal impairment”, and “treatment”, with the help of Boolean operators “OR” and “AND”. Complete search string will be provided in Supplementary File 1. Preprint articles on medRxiv and bioRxiv were also screened, as well as the bibliographies of the retrieved articles.
Study Selection
Any human observational studies including case reports, case series, and retrospective studies relevant to the research question, presenting original data, and fulfilling the criteria: (i) patients with histologically proven NSF, (ii) reported data either between clinical presentation and outcome or both, and (iii) reports the use of any treatment were included. All review articles, opinion articles, commentaries or any study that reported (i) data on a patient that may have a condition that may produce similar symptoms and (ii) were not written in English were excluded. In addition to this, for retrospective studies and case series, only cases fitting the criteria were included if their data were distinct.
Following the criteria set, both the title and abstract screening, and then the full-text screening were performed by two of the authors SF and AA, any conflicts were settled by the third author AM.
Data Extraction
Two of the authors MB and UK separately and in duplicate extracted the shortlisted papers into a structured extraction sheet.
Extracted data will include study details (author names, year of publication, study design, number of patients, country of origin), patient demographics (age, gender, comorbidities), CKD, time since onset and stage of CKD, acute kidney injury (AKI), pain, mobility, edema, anemia, extent of cutaneous manifestation, past surgery, use of GBCA, dialysis, duration of dialysis, treatment used, duration of treatment, duration of follow-up, improvement in symptoms (skin induration, stiffness, edema, pigmentation, pain, mobility, skin (softening), and thickness) and outcomes of treatment (overall improvement/stable/deterioration, relapse, death, cause of death).
Data Analysis
Means, ranges, and standard deviations were calculated for continuous variables, such as age, while frequencies and percentages were tabulated for descriptive data using SPSS Version 25 (Chicago, IL: IBM® SPSS® Statistics). Significance analysis done via chi-square test made use of the same software. A confidence-interval (CI) of 95% was used and a p-value less than 0.05 was considered statistically significant.
Risk of Bias Assessment
The quality assessment tool developed by the National Heart, Lung, and Blood Institute (NHLBI) was used.17 Two of the authors SF and AM assessed the quality of the included articles separately, while a third author resolved any disagreements. Case reports were scored out of 6 and categorized as good quality (score 5–6), fair quality (score 2–4), and poor quality (score <2). Case series were scored out of 7 and categorized as good quality (score 5–7), fair quality (score 2–4), and poor quality (score <2). Retrospective studies were scored out of 12 and categorized as good quality (score 9–12), fair quality (score 5–8), and poor quality (score <5).
Results
Study Selection and Bias Assessment
All 27,890 studies yielded from our initial search were exported to Mendeley, with the help of which duplicates were removed. This resulted in 379 studies eligible for title and abstract screening. Eventually, 69 studies were assessed for full-text eligibility, out of which 39 were eventually included in our review.10,11,18–54 From these 39 studies, 21 were case reports, 16 were case series, and 2 were retrospective studies. Figure 1summarizes each stage.
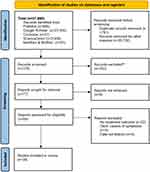 |
Figure 1 PRISMA 2020 flow diagram of included studies (n=39). Notes: Adapted from Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ [Internet]. 2021 Mar 29 [cited 2022 Sep 3];372.16 |
The bias assessment rating for case reports was good for 18, and fair for 3 articles, that for case series was good for 14 and fair for 2 articles, while both retrospective studies were rated good. Results of bias assessment are present in the Supplementary File 2.
Patient Demographics
We had a total of 83 patients in our study with a mean age of 51.4±14.6 years, the youngest patient was 17 years old, and the oldest was 87. Data on patient ages was available for 79 cases and showed that our study included 44 (55.7%) females.
In our study, 73 (88.0%) of the patients had renal impairment predisposing to the development of NSF, including 69 with chronic kidney disease (CKD) and 4 with acute kidney injury (AKI). The stage of CKD was given for 39 patients, with only 1 (2.6%) patient each having stages 3a and 4 and the rest (94.9%) all having stage 5 CKD. Time elapsed since onset of CKD was available for 18 patients and the mean time was 131.1±88.5 (8–336) months. The major causes of CKD included diabetes (26.5%), hypertension (32.5%), and autoimmune diseases (21.7%), of which systemic lupus erythematosus (SLE) (14.5%) was the most common. Other causes included focal segmental glomerulosclerosis (FSGS), IgA nephropathy, hemolytic uremic syndrome (HUS), interstitial nephritis, chronic pyelonephritis, and hypertrophic kidney.
Patients also had other comorbidities including neoplasia (12.0%), hepatitis C (2.4%), deep vein thrombosis (DVT) (4.8%), thyroid disease (3.6%) and heart disease (7.2%).
Gadolinium use was common in our study, as 66 (79.5%, 95% CI: 70.3–88.4%) patients were exposed to gadolinium-based contrast agents in the past.
In this study, 64 (77.1%, 95% CI: 67.5–86.4%) patients were undergoing dialysis at the time they presented with NSF. Duration of dialysis was available for 54 (65.1%) of these cases, having an average duration of 78.6±90.8 (0.8–336) months. Previous history of kidney transplant was seen in 19 (22.9%) patients.
Patient Symptoms
All patients in our study were affected with either isolated manifestations, or combinations of cutaneous lesions, impaired mobility, pain, edema, or anemia. Cutaneous manifestations affecting single or multiple body parts were present in 69 patients (83.1%, 95% CI: 73.3–90.5%). Restriction in mobility, ranging from mild hand or leg stiffness to complete disability was seen in 62 (74.7%, 95% CI: 64.0–83.6%) patients. Pain in movement was reported in 33 (40.0%, 95% CI: 34.0–58.0%) patients, 18 (21.7%, 95% CI: 13.4–32.1%) presented with edema, and 5 (6.0%, 95% CI: 2.0–13.5%) were anemic.
The areas affected and the extent of cutaneous lesions, as well as the severity of mobility impairment are summarized in Table 1. In summary, the lower limb (91.3%, 95% CI: 80.5–95.9%) was the most affected body part. The term “lower limb” in our study represents any combination of feet, ankles, calves, and thighs, while “upper limb” represents the fingers, hands, wrists, forearms, elbows, and arms. The upper and lower limbs presented in conjunction in 41 (59.3%) cases. Most studies included in our review mentioned a restriction of movement in patients but did not specify the degree to which the mobility was impaired. This restriction of movement was seen in 26 (36.6%, 95% CI: 25.5–48.9%) patients.
 |
Table 1 Clinical Manifestations |
Treatments
A variety of treatment modalities were used in our included studies. In 13 (15.9%) patients, many of these treatment options were used as part of a combination therapy regimen, the most compared to any other treatment.
In our study, 11 (13.4%) patients each received renal transplant, extracorporeal photopheresis and Ultraviolet (UV)-therapy, 9 (11.0%) received sodium thiosulfate, 8 (9.8%) patients each received corticosteroids and imatinib mesylate therapies, 5 (6.1%) patients underwent dialysis, 4 (4.9%) used immunosuppressants, and 2 (2.4%) patients received other treatments.
Physical therapy is a common NSF treatment, although it was always used in conjunction with other therapies in our study. A total of 15 (18.1%) patients in our study received physical therapy.
We analyzed patient outcomes as improved, stable, or deterioration of disease. An improved outcome was judged using the progress of various symptoms. These symptoms included skin induration, thickness, and pigmentation, edema, pain, and mobility.
Ultraviolet Therapy Group
UV therapy included UV-A1, UV-B, and Psoralen plus UV-A (PUVA) therapies. The characteristics of the UV treatment group, which had a mean age of 52.0±16.8 years, are summarized in (Table 2). Patients were treated with UV therapy for an average of 2.7±1.9 months and were followed up for an average of 15.2 ±8.6 months. Both corticosteroids and physical therapy were used alongside the UV therapy in 1 (9.1%) patient each, although 1 (9.1%) patient received both these additional treatments simultaneously. An overall improvement was reported on 9 (81.8%) occasions, while stable disease and deterioration were seen in 1 (9.1%) patient each. Cutaneous manifestations were the most common symptom seen in this group of patients with extent ranging from 1 to 3 areas, skin induration improved in 90.0% of these patients.
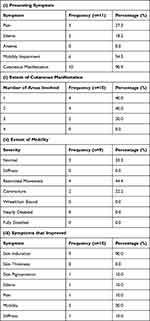 |
Table 2 Characteristics of Ultraviolet Therapy Group |
Renal Transplant Group
Renal transplant was used to achieve a favorable outcome for patients with renal impairment with NSF in 11 cases, with a mean age of 43.1±12.1 years. 2 (18.2%) of these patients had already received a kidney transplant in the past. Cutaneous manifestations were the most common symptom (9 patients) seen in this group of patients with extent ranging from 1 to 3 areas. After transplant, the patients were followed up for 23.2±35.5 months, and data on overall improvement was seen for 10 patients of which 8 (80.0%) patients improved and 2 (20.0%) remained stable Furthermore, 2 of the patients who received a transplant used corticosteroids after transplant. The various characteristics of patients in the renal transplant group are comprehensively described in (Table 3).
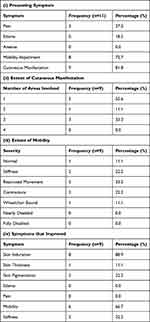 |
Table 3 Characteristics of Renal Transplant Group |
Extracorporeal Photopheresis Group
Extracorporeal photopheresis was given to 11 patients, with mean age 57.1±9.9 years for an average of 10.8±6.5 months. Out of them, 9 (81.8%) patients improved, 1 (9.1%) remained stable, and 1 (9.1%) deteriorated over a mean follow-up duration of 1.3±0.6 months. The patients treated with extracorporeal photopheresis were more severely affected by NSF compared to the UV therapy and transplant groups, as these patients had cutaneous manifestations involving 2 to 4 body areas. Also, this treatment was used for 3 patients who were nearly disabled, and none of the patients treated had normal mobility. Also, 1 (9.1%) patient was given physical therapy in addition to extracorporeal photopheresis. Other important characteristics of these patients are provided in (Table 4).
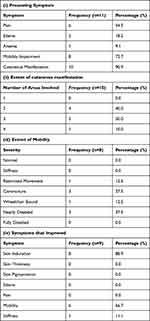 |
Table 4 Characteristics of Extracorporeal Photopheresis Group |
Combined Therapy Group
The combined therapy regimen included multiple different combinations of renal transplant (2 patients), Ultraviolet (UV)-therapy (7 patients), extracorporeal photopheresis (10 patients), sodium thiosulfate (1 patient), acitretin (4 patients), corticosteroids (4 patients), immunosuppressants (1 patient), plasmapheresis (1 patient), rituximab (2 patients), and cyclophosphamide (1 patient). In this group, 7 (53.8%) of patients had a positive response to combined therapy (Table 5) over a mean treatment duration of 11±10.1 months and follow-up of 16±18.9 months, that is, almost an equal proportion of patients improved compared to those that did not improve. Relapse was seen in 1 (7.7%) patient.
 |
Table 5 Treatment Modality vs Outcome |
Dialysis Group
All the patients in the dialysis group were already undergoing dialysis at time of presentation with NSF. The mean duration of dialysis was 21.2±17.7 months, ranging from 1 to 53 months. Outcome detail was available for 4 patients, and the condition of 1 (25.0%) patient improved, while 3 (75.0%) deteriorated (Table 5). All 4 of these patients used immunosuppressants alongside their dialysis.
Imatinib Mesylate Group
Patients were treated with imatinib mesylate for an average of 6.0±4.5 months. Additionally, corticosteroids were provided to 1 (20.0%) patient, and 3 (60.0%) patients received physical therapy. 2 (25.0%) patients reported a relapse of their condition over 13 months and 12.5 months of follow-up, respectively. All imatinib patients reported an improvement in symptoms (Table 5) over a mean follow-up of 10.4±4.1 months.
Corticosteroid Group
Patients using solely corticosteroids were followed up for an average of 57.8±65.0 months. This group had 3 (37.5%) patients who improved, 3 (37.5%) who remained stable, and 2 (25.0%) who deteriorated (Table 5). As can be seen, less patients improved compared to those that did not improve.
Sodium Thiosulfate Group
Patients were treated for an average of 3.4±1.4 months. In this group, 3 (50.0%) patients received physical therapy. Data on outcome were available for 6 patients in this group, and none of the patients reported a deterioration in their condition, as 3 (50.0%) improved and 3 (50.0%) remained stable (Table 5) over an average of 7.2±2.9 months.
Immunosuppressant Group
Physical therapy was provided to 2 (50.0%) patients using immunosuppressants. Data on duration of treatment and follow-up were available for only 1 patient, and both these times were 8 months. Patients either improved (50.0%) or remained stable (50.0%) (Table 5).
Other Treatment Groups
Other treatments included intravenous immunoglobulin, plasma exchange and plasma therapy, for an average of 0.75±0.25 months. Fifty percent of the patients improved, and 50.0% of the patients remained stable (Table 5) over an average follow-up duration of 9±3 months.
Outcomes
We evaluated the data of 76 patients for whom details on outcome were provided and found 51 (67.1%, 95% CI: 55.4–77.5%) to have an overall improvement, 16 (21.1%, 95% CI: 12.5–31.9%) to have remained stable, and 9 (11.8%, 95% CI: 5.6–21.3%) patients to have deteriorated. Skin induration improved in 45 patients, pigmentation, and thickness in 4 patients each. Edema and pain became better for 5 patients each, while 32 patients reported better mobility and 7 reported an improvement in their stiffness. Although these measures provided a good estimate of the outcome in most cases, there was an eventual deterioration in 2 cases despite individual improvement of the symptoms.
Chi-square test was done to find relationships between clinical symptoms and the outcome. We found that mobility, pain, anemia, and edema were all non-significantly related. Another chi-square test determined that the modality of treatment used is significantly related to the outcome (p=0.015), these details are summarized in Table 5.
Relapse was rarely seen among patients, as only 5 (6.0%) patients experienced a return of their disease after improvement.
In this review, 9 patients died, but the cause of death in all these patients was either unknown, or due to reasons unrelated to NSF such as pneumonia, carcinoma, and sepsis. Only 1 patient died due to kidney-related complications.
Discussion
Nephrogenic systemic fibrosis is a rare disease discovered in 1997, defined as a multiorgan failure that is progressive in nature. It is mainly reported as an iatrogenic disease of the skin as most of the thickening of the skin and subcutaneous tissue arises secondary to exposure to gadolinium-based contrast agents (GBCAs).8,55
GBCAs are used intravenously to improve MRI images. A strong link between GBCAs and NSF was reported in the early 2000s, with around 500 cases reported from 1997 to 2007.8,56,57 In our study, 79.5% of the patients were exposed to GBCAs. Literature reports that patients with renal insufficiency, especially stage 4 or 5 CKD, are more prone to developing NSF.58 Similarly, in our study, 88% of the patients had renal impairment. In addition, in patients where CKD stage was reported, 94.9% of the patients had stage 5 CKD.
The exact mechanism by which NSF develops post GBCAs exposure is unknown; however, it is hypothesized that chelation of gadolinium to form free gadolinium from its compound, which then reacts with macrophages and monocytes, eliciting a cascading response from these cells to produce cytokines, chemokines, profibrotic cytokines and collagen which in turn leads to fibrosis of organs, is one of the possible mechanisms.8,59 The other suggested pathway is gadolinium ion-induced continuous formation of transforming growth factor-beta 1 and dendritic cell maturation leading to unrestrained soft tissue collagen deposition.52 Moreover, the strong association of NFS with renal impairment is thought to be due to the slow excretion of the contrast material from the body, increasing the chances of gadolinium chelation and tissue deposition through the process of transmetalation with calcium, copper, iron, and zinc ions in the body, which are present in greater concentrations in individuals with impaired renal function, which may possibly lead to greater tissue deposition of gadolinium, hence increasing the possibility of developing toxicities.8,60 However, GBCAs exposure is not necessary for developing NSF; in our study, 17 patients that developed NSF had no history of GBCAs’ exposure. Any event, such as surgery, infection, or malignancy that can induce an inflammatory state in the body can be the reasoning behind NSF in these patients; however, the exact mechanism by which this happens is not known.61
The clinical signs and symptoms range depending on the severity of the disease. Initially, it presents with pain, pruritis, and edema and then progresses to develop skin tightening and hyperpigmentation. In the latter stages, skin induration begins, also known as “peau d’orange” appearance, which leads to the development of contractures and restricts mobility.8,62 In our study, 83% of the patients had cutaneous manifestations, while 74.7% had some form of mobility issues, ranging from mild stiffness to complete disability. In 36.6% of the patients, the disease progressed to the point of mobility restriction, which was the most reported mobility issue. Progression to complete disability was reported only 2.8% of the time. The literature suggests that the disease commonly involves the limbs and always spares the face; moreover, it is not uncommon for both limbs to be involved.61 Our study’s findings mostly support this, as, in our study, 91.3% of the patients had manifestations in the lower limb, making the lower limb the most affected area. Both limbs were most frequently involved (30%), with just a slightly less percentage of patients presenting with manifestation only in their upper limb (24.6%) and all three, upper limb, lower limb, and one more body part (21.7%). Even though it is an unusual finding, two of the patients reported ocular involvement, which goes against the idea that NFS does not appear on the facial skin.
Until now, no treatment has proven to be effective for NSF.59 Various treatment regimens have been employed, which result in variable success, as prognosis greatly depends on the severity of the disease. Hence, treating impaired renal function is crucial as it halts the progression to its severe forms and may simultaneously improve the symptoms of NFS. In addition, multiple studies have reported either stability or improvement in the NFS manifestations after renal transplant.11,59,63,64 However, renal transplant itself is not the treatment of NSF rather it indirectly effects in the improvement of symptoms by directly improving renal function. Our study finds that 80% of the patients who went through renal transplants improved, while the rest remained stable UV-A therapy has proven successful in multiple cases; it blocks the procollagen and TGF-β1 production, improving skin thickness.52 Similarly, in our study, UV therapy was found to be beneficial, with around 82% of the patients who underwent UV therapy reporting an overall improvement in their condition, 90% of these patients experienced an improvement in cutaneous manifestations, and 54.5% improved in terms of mobility. Extracorporeal Photopheresis was also a successful treatment, with 81.1% of the patients improving overall; however, 9.1% deteriorated. This can perhaps be explained by the more severe manifestations in this group. Imatinib mesylate was another treatment that led to improved conditions in all the patients who were administered the drug. The use of corticosteroids resulted in a variable response, with a more significant percentage of the patients deteriorating compared to patients with another treatment regimen. In the case of NSF, some of the symptoms improving does not translate to the overall improvement in disease. Hence, different frequencies are seen between the two improvements.
The prognosis of this disease and effectivity of the treatment almost depends on the extent of the disease. If the condition progresses from the skin to visceral fibrosis, chances of symptoms regression on the improvement of kidney function decrease. A higher mortality rate is reported in patients with visceral involvement, particularly in the cardiac and respiratory systems. Mortality due to NSF can be due to worsening renal function due to fibrosis of the tubules, cardiomyopathy, or even falls.65,66 In our study, the mortality rate was 10.8%. However, only one patient died due to NFS. One important observation from literature was that majority of cases of NSF that developed in patients were because of linear gadolinium-based contrast and no other medium was found to cause severed complications like NSF with this frequency and incidence.67
A major limitation of this study was the small sample size for each treatment option that was analyzed, which is why a more extensive analysis could not be done. There were not many studies that provided data on either clinical presentation or outcome along with the treatment used, which was needed so that relationships can be developed between treatment, and either or both variables. Moreover, since information regarding whether all cases of NSF were diagnosed through skin biopsy or not was not possible to be extracted due to the limitation of literature, this was another limitation of the literature and in turn the study itself. Furthermore, the small sample sizes also do not allow us to generalize our findings to the entire population. There was a lack of standardization between included studies and those used for comparison. However, our study can be used to produce better results as more data is available in the future, hence determining a better estimate of the efficacies of treatment options used.
Conclusion
Our research aimed to collect studies that reported data on the different clinical manifestations and their extent, as well as the different treatment options and their outcomes in patients of NSF. Hence, we report the common findings and highlight the efficacies of the most used treatment modalities. The commonly seen symptoms were cutaneous lesions (83.1%) and impaired mobility (74.7%). We have found that the most used treatments for the disease are UV-therapy, renal transplant, and extracorporeal photopheresis, these treatments were used in 13.4% of the patients each. UV-therapy yielded improvement in 81.8% of the patients, renal transplant in 80.0% of the patients, and extracorporeal photopheresis in 81.8% of the patients. Modality of treatment used was significantly related to outcome (p=0.015). Using the results of our study, future researchers may be able to develop comprehensive treatment guidelines for the condition based on the various presenting symptoms.
Summary at a Glance
A comprehensive systematic review was conducted, to identify the association of Nephrogenic Systemic Fibrosis (NSF) with kidney disease. We had 83 patients in this review and found that most patients improved with no long-term consequences.
Data Sharing Statement
All data are available within the main manuscript and Supplementary File.
Acknowledgments
We would like to acknowledge and thank Syed Mesum Hussain from DOW University of Health Sciences towards his kind contributions during literature search and extraction.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Taraphder A, Waikhom R. Nephrogenic systemic fibrosis: a brief review. Indian J Dermatol. 2011;56(1):54. doi:10.4103/0019-5154.77554
2. Rota E, Nallino MG, Bainotti S, Formica M. Nephrogenic systemic fibrosis: an unusual scleroderma-like fibrosing disorder. Rheumatol Int. 2010;30(10):1389–1391. doi:10.1007/s00296-009-1083-4
3. Shah AH, Olivero JJ. Gadolinium-induced nephrogenic systemic fibrosis. Methodist Debakey Cardiovasc J. 2017;13(3):172–173. doi:10.14797/mdcvj.751
4. Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356(9234):1000–1001. doi:10.1016/S0140-6736(00)02694-5
5. Deng A, Martin DB, Spillane A, et al. Nephrogenic systemic fibrosis with a spectrum of clinical and histopathological presentation: a disorder of aberrant dermal remodeling. J Cutan Pathol. 2010;37(2):204–210. doi:10.1111/j.1600-0560.2009.01301.x
6. Bhawan J, Swick BL, Koff AB, Stone MS. Sclerotic bodies in nephrogenic systemic fibrosis: a new histopathologic finding. J Cutan Pathol. 2009;36(5):548–552. doi:10.1111/j.1600-0560.2008.01111.x
7. Jiménez SA, Artlett CM, Sandorfi N, et al. Dialysis-associated systemic fibrosis (nephrogenic fibrosing dermopathy): study of inflammatory cells and transforming growth factor beta1 expression in affected skin. Arthritis Rheum. 2004;50(8):220–222. doi:10.1002/art.20362
8. Todd DJ, Kay J. Gadolinium-induced fibrosis. Annu Rev Med. 2016;67:273–291. doi:10.1146/annurev-med-063014-124936
9. Knopp EA, Cowper SE. Nephrogenic systemic fibrosis: early recognition and treatment. Semin Dial. 2008;21(2):123–128.
10. Ishikawa M, Motegi S, Toki S, Endo Y, Yasuda M, Ishikawa O. Calciphylaxis and nephrogenic fibrosing dermopathy with pseudoxanthoma elasticum-like changes: successful treatment with sodium thiosulfate. J Dermatol. 2019;46(7):e240–e242. doi:10.1111/1346-8138.14780
11. Panesar M, Banerjee S, Barone GW. Clinical improvement of nephrogenic systemic fibrosis after kidney transplantation. Clin Transplant. 2008;22(6):803–808. doi:10.1111/j.1399-0012.2008.00886.x
12. Woolen SA, Shankar PR, Gagnier JJ, MacEachern MP, Singer L, Davenport MS. Risk of nephrogenic systemic fibrosis in patients with stage 4 or 5 chronic kidney disease receiving a group II gadolinium-based contrast agent: a systematic review and meta-analysis. JAMA Intern Med. 2020;180(2):223–230. doi:10.1001/jamainternmed.2019.5284
13. Mazhar SM, Shiehmorteza M, Kohl CA, Middleton MS, Sirlin CB. Nephrogenic systemic fibrosis in liver disease: a systematic review. J Magn Reson Imaging. 2009;30(6):1313–1322. doi:10.1002/jmri.21983
14. Bhargava V, Singh K, Meena P, Sanyal R. Nephrogenic systemic fibrosis: a frivolous entity. World J Nephrol. 2021;10(3):29–36. doi:10.5527/wjn.v10.i3.29
15. Nephrogenic systemic fibrosis: a case report and review of the literature; 2022. Available from: https://pubmed.ncbi.nlm.nih.gov/21637902/.
16. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n160. doi:10.1136/bmj.n160
17. Study quality assessment tools. NHLBI, NIH; 2022. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
18. Swaminathan S, Arbiser JL, Hiatt KM, et al. Rapid improvement of nephrogenic systemic fibrosis with rapamycin therapy: possible role of phospho-70-ribosomal-S6 kinase. J Am Acad Dermatol. 2010;62(2):343–345. doi:10.1016/j.jaad.2009.04.022
19. Wilson J, Gleghorn K, Seigel Q, Kelly B. Nephrogenic systemic fibrosis: a 15-year retrospective study at a single tertiary care center. J Am Acad Dermatol. 2017;77(2):235–240. doi:10.1016/j.jaad.2017.02.003
20. Gilliet M, Cozzio A, Burg G, Nestle FO. Successful treatment of three cases of nephrogenic fibrosing dermopathy with extracorporeal photopheresis. Br J Dermatol. 2005;152(3):531–536. doi:10.1111/j.1365-2133.2005.06434.x
21. Kadiyala D, Roer DA, Perazella MA. Nephrogenic systemic fibrosis associated with gadoversetamide exposure: treatment with sodium thiosulfate. Am J Kidney Dis. 2009;53(1):133–137. doi:10.1053/j.ajkd.2008.09.016
22. Yerram P, Saab G, Karuparthi PR, Hayden MR, Khanna R. Nephrogenic systemic fibrosis: a mysterious disease in patients with renal failure--role of gadolinium-based contrast media in causation and the beneficial effect of intravenous sodium thiosulfate. Clin J Am Soc Nephrol. 2007;2(2):258–263. doi:10.2215/CJN.03250906
23. Schäd SG, Heitland P, Kühn-Velten WN, Gross GE, Jonas L. Time-dependent decrement of dermal gadolinium deposits and significant improvement of skin symptoms in a patient with nephrogenic systemic fibrosis after temporary renal failure. J Cutan Pathol. 2013;40(11):935–944. doi:10.1111/cup.12214
24. Tran KT, Prather HB, Cockerell CJ, Jacobe H. UV-A1 therapy for nephrogenic systemic fibrosis. Arch Dermatol. 2009;145(10):1170–1174. doi:10.1001/archdermatol.2009.245
25. Mathur K, Morris S, Deighan C, Green R, Douglas KW. Extracorporeal photopheresis improves nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: three case reports and review of literature. J Clin Apher. 2008;23(4):144–150. doi:10.1002/jca.20170
26. Ross C, de Rosa N, Marshman G, Astill D. Nephrogenic systemic fibrosis in a gadolinium-naïve patient: successful treatment with oral sirolimus. Australas J Dermatol. 2015;56(3):e59–e62. doi:10.1111/ajd.12176
27. Aires NB, Sotto MN, Nico MMS. Nephrogenic fibrosing dermopathy: report of two cases. Acta Derm Venereol. 2007;87(6):521–524. doi:10.2340/00015555-0296
28. Shin K, Granter SR, Coblyn JS, Gupta S. Progressive arm and leg stiffness in a patient with chronic renal impairment. Nat Clin Pract Rheumatol. 2008;4(10):557–562. doi:10.1038/ncprheum0883
29. Nagai Y, Hasegawa M, Shinmi K, et al. Nephrogenic systemic fibrosis with multiple calcification and osseous metaplasia. Acta Derm Venereol. 2008;88(6):597–600. doi:10.2340/00015555-0518
30. Läuchli S, Zortea-Caflisch C, Nestle FO, Burg G, Kempf W. Nephrogenic fibrosing dermopathy treated with extracorporeal photopheresis. Dermatology. 2004;208(3):278–280. doi:10.1159/000077321
31. Chandran S, Petersen J, Jacobs C, Fiorentino D, Doeden K, Lafayette RA. Imatinib in the treatment of nephrogenic systemic fibrosis. Am J Kidney Dis. 2009;53(1):129–132. doi:10.1053/j.ajkd.2008.08.029
32. Duffy KL, Green L, Harris R, Powell D. Treatment of nephrogenic systemic fibrosis with Re-PUVA. J Am Acad Dermatol. 2008;59(2 Suppl 1):S39–S40. doi:10.1016/j.jaad.2007.08.035
33. Marckmann P, Nielsen AH, Sloth JJ. Possibly enhanced Gd excretion in dialysate, but no major clinical benefit of 3–5 months of treatment with sodium thiosulfate in late stages of nephrogenic systemic fibrosis. Nephrol Dial Transplant. 2008;23(10):3280–3282. doi:10.1093/ndt/gfn217
34. Chung HJ, Chung KY. Nephrogenic fibrosing dermopathy: response to high-dose intravenous immunoglobulin. Br J Dermatol. 2004;150(3):596–597. doi:10.1111/j.1365-2133.2003.05795.x
35. DiCarlo JB, Gupta EA, Solomon AR. A pediatric case of nephrogenic fibrosing dermopathy: improvement after combination therapy. J Am Acad Dermatol. 2006;54(5):914–916. doi:10.1016/j.jaad.2006.01.023
36. Schmook T, Budde K, Ulrich C, Neumayer HH, Fritsche L, Stockfleth E. Successful treatment of nephrogenic fibrosing dermopathy in a kidney transplant recipient with photodynamic therapy. Nephrol Dial Transplant. 2005;20(1):220–222. doi:10.1093/ndt/gfh473
37. Goddard DS, Magee CC, Lazar AJF, Miller DM. Nephrogenic fibrosing dermopathy with recurrence after allograft failure. J Am Acad Dermatol. 2007;56(5 Suppl):S109–S111. doi:10.1016/j.jaad.2006.04.061
38. Kay J, High WA. Imatinib mesylate treatment of nephrogenic systemic fibrosis. Arthritis Rheum. 2008;58(8):2543–2548. doi:10.1002/art.23696
39. Richmond H, Zwerner J, Kim Y, Fiorentino D. Nephrogenic systemic fibrosis: relationship to gadolinium and response to photopheresis. Arch Dermatol. 2007;143(8):1025–1030. doi:10.1001/archderm.143.8.1025
40. Poisson JL, Low A, Park YA. The treatment of nephrogenic systemic fibrosis with therapeutic plasma exchange. J Clin Apher. 2013;28(4):317–320. doi:10.1002/jca.21253
41. Elmholdt TR, Pedersen M, Jørgensen B, Ramsing M, Olesen AB. Positive effect of low-dose imatinib mesylate in a patient with nephrogenic systemic fibrosis. Acta Derm Venereol. 2011;91(4):478–479. doi:10.2340/00015555-1085
42. Kreuter A, Gambichler T, Weiner SM, Schieren G. Limited effects of UV-A1 phototherapy in 3 patients with nephrogenic systemic fibrosis. Arch Dermatol. 2008;144(11):1527–1529. doi:10.1001/archderm.144.11.1527
43. Zhang R, Rose WN. Photopheresis provides significant long-lasting benefit in nephrogenic systemic fibrosis. Case Rep Dermatol Med. 2017;2017:1–4. doi:10.1155/2017/6381479
44. Koratala A, Bhatti V. Nephrogenic systemic fibrosis. Clin Case Rep. 2017;5(7):1184. doi:10.1002/ccr3.993
45. Khurana A, Nickel AE, Greene JF, Narayanan M, High WA, Foulks CJ. Successful pregnancy in a hemodialysis patient and marked resolution of her nephrogenic systemic fibrosis. Am J Kidney Dis. 2008;51(6):e29–e32. doi:10.1053/j.ajkd.2007.12.037
46. Bangsgaard N, Hansen JM, Marckmann P, Skov L. Nephrogenic systemic fibrosis symptoms alleviated by renal transplantation. Dial Transplant. 2011;40(2):86–87. doi:10.1002/dat.20507
47. Shah N, Ramkumar M, Wu C, et al. Is renal transplantation the cure to fatal nephrogenic systemic fibrosis: a case series of a rare disease. Transplantation. 2008;86(2S):696–697. doi:10.1097/01.tp.0000330832.09951.e0
48. Mackay-Wiggan JM, Cohen DJ, Grossman ME, Knobler EH, Grossman ME. Nephrogenic fibrosing dermopathy (scleromyxedema-like illness of renal disease). J Am Acad Dermatol. 2003;48(1):55–60. doi:10.1067/mjd.2003.78
49. Ramaizel L, Sliwa JA. Rehabilitation in nephrogenic systemic fibrosis. PM&R. 2009;1(7):684–686. doi:10.1016/j.pmrj.2009.05.006
50. Schieren G, Wirtz N, Altmeyer P, Rump LC, Weiner SM, Kreuter A. Nephrogenic systemic fibrosis–a rapidly progressive disabling disease with limited therapeutic options. J Am Acad Dermatol. 2009;61(5):868–874. doi:10.1016/j.jaad.2009.03.040
51. Ragunatha S, Palit A, Inamadar A, Madraki R, Yelikar B. Nephrogenic fibrosing dermopathy. Indian J Dermatol Venereol Leprol. 2009;75(1):63–67. doi:10.4103/0378-6323.45224
52. Lim YJ, Bang J, Ko Y, et al. Late onset nephrogenic systemic fibrosis in a patient with stage 3 chronic kidney disease: a case report. J Korean Med Sci. 2020;35(35). doi:10.3346/jkms.2020.35.e293
53. Ustuner P, Kose OK, Gulec AT, Ozen O. A moderate response to plasmapheresis in nephrogenic systemic fibrosis. Clin Pract. 2011;1(4):e124. doi:10.4081/cp.2011.e124
54. Foshee JP, Griffin TD Jr, Cam K, Rivlin M, Keller M. Adjunct treatment of recalcitrant hand plaques in nephrogenic systemic fibrosis after imatinib therapy. SKIN J Cutan Med. 2019;3(6):438–442. doi:10.25251/skin.3.6.14
55. Malikova H. Nephrogenic systemic fibrosis: the end of the story? Quant Imaging Med Surg. 2019;9(8):1470. doi:10.21037/qims.2019.07.11
56. Cowper SE, Su LD, Bhawan J, Robin HS, LeBoit PE. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23(5):383–393. doi:10.1097/00000372-200110000-00001
57. Kitajima K, Maeda T, Watanabe S, Ueno Y, Sugimura K. Recent topics related to nephrogenic systemic fibrosis associated with gadolinium-based contrast agents. Int J Urol. 2012;19(9):806–811. doi:10.1111/j.1442-2042.2012.03042.x
58. Saab G, Cheng S. Nephrogenic systemic fibrosis: a nephrologist’s perspective. Hemodial Int. 2007;11(Suppl 3):S2–S6. doi:10.1111/j.1542-4758.2007.00222.x
59. Daftari Besheli L, Aran S, Shaqdan K, Kay J, Abujudeh H. Current status of nephrogenic systemic fibrosis. Clin Radiol. 2014;69(7):661–668. doi:10.1016/j.crad.2014.01.003
60. Saab G, Abu-Alfa A. Nephrogenic systemic fibrosis--implications for nephrologists. Eur J Radiol. 2008;66(2):208–212. doi:10.1016/j.ejrad.2008.01.028
61. Nephrogenic Systemic Fibrosis – NORD. National organization for rare disorders; 2022. Available from: https://rarediseases.org/rare-diseases/nephrogenic-systemic-fibrosis/.
62. Cowper SE. Nephrogenic fibrosing dermopathy: the first 6 years. Curr Opin Rheumatol. 2003;15(6):785–790. doi:10.1097/00002281-200311000-00017
63. Panesar M, Yacoub R. What is the role of renal transplantation in a patient with nephrogenic systemic fibrosis? Semin Dial. 2011;24(4):373–374. doi:10.1111/j.1525-139X.2011.00913.x
64. Cuffy MC, Singh M, Formica R, et al. Renal transplantation for nephrogenic systemic fibrosis: a case report and review of the literature. Nephrol Dial Transplant. 2011;26(3):1099–1101. doi:10.1093/ndt/gfq693
65. Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis: predictor of early mortality and association with gadolinium exposure. Arthritis Rheum. 2007;56(10):3433–3441. doi:10.1002/art.22925
66. He A, Kwatra SG, Zampella JG, Loss MJ. Nephrogenic systemic fibrosis: fibrotic plaques and contracture following exposure to gadolinium-based contrast media. BMJ Case Rep. 2016;2016:bcr2016214927.
67. Alfano G, Fontana F, Ferrari A, et al. Incidence of nephrogenic systemic fibrosis after administration of gadoteric acid in patients on renal replacement treatment. Magn Reson Imaging. 2020;70:1–4. doi:10.1016/j.mri.2020.02.012
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
