Back to Journals » Journal of Inflammation Research » Volume 17
Tat-CIRP Peptide Facilitates Frozen Wound Healing by Ameliorating Inflammation and Promoting Angiogenesis
Authors Li J, Ding J, Wu H, Lu C , Wu J , Luo Q
Received 7 December 2023
Accepted for publication 4 April 2024
Published 11 April 2024 Volume 2024:17 Pages 2205—2215
DOI https://doi.org/10.2147/JIR.S450288
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Jiayan Li,1,* Jie Ding,1,* Haoyang Wu,1 Chenyan Lu,1 Jian Wu,2 Qianqian Luo1
1Department of Hypoxic Biomedicine, Institute of Special Environmental Medicine and Coinnovation Center of Neuroregeneration, Nantong University, Nantong, 226019, People’s Republic of China; 2Department of Pharmacy, Huashan Hospital, Fudan University, Shanghai, 200040, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jian Wu, Department of Pharmacy, Huashan Hospital, Fudan University, 12 Middle Urumqi Road, Shanghai, 200040, People’s Republic of China, Tel +86-21-54601295, Email [email protected] Qianqian Luo, Department of Hypoxic Biomedicine, Institute of Special Environmental Medicine and Coinnovation Center of Neuroregeneration, Nantong University, 9 Seyuan Road, Chongchuan District, Nantong, Jiangsu, 226019, People’s Republic of China, Tel +86-513-85503378, Email [email protected]
Background: Frostbite is a chemia resulting from cold-induced skin damage. The process of frostbite is often accompanied by inflammation, and the therapeutic strategies focusing on anti-inflammation are the main direction to data. Tat-CIRP is a 15 amino acid peptide containing HIV protein and cold-inducible RNA-binding protein (CIRP), which is believed to compete with endogenous CIRP for myeloid differentiation 2 (MD2) binding. This study aims to investigate the efficacy of Tat-CIRP in the treatment of frostbite.
Methods: A mouse model of frostbite was established, and on the first day after frostbite occurrence, Tat-CIRP peptide was administered intravenously via the tail with a dosage interval of one day for a total of three doses. Frozen mouse skin sections were subjected to histological analysis, including hematoxylin–eosin (HE) staining, Masson staining, and immunohistochemical examination. Western blotting was performed to detect the expression level of Ki-67 in mouse skin tissue.
Results: One day after frostbite, mice exhibited skin swelling and a solid appearance. From day 1 to 5 after frostbite, MD2 expression was significantly upregulated, while CIRP expression was downregulated. Compared to the frostbite group, mice treated with Tat-CIRP showed accelerated frostbite recovery, reduced levels of inflammatory factors and MD2. Furthermore, the expression of cell proliferation-associated protein Ki-67 and angiogenesis-related protein CD31 was upregulated.
Conclusion: Tat-CIRP promotes frozen wound healing via inhibiting inflammation and promoting angiogenesis in frostbitten mice.
Keywords: frostbite, Tat-CIRP, inflammation, angiogenesis
Introduction
Frostbite is a prevalent disease in cold regions, occurring at temperatures below freezing points. It is a common affliction in coastal areas.1 Notably, in recent years, many regions have been experiencing unprecedented harsh winters due to global climate change, and if this trend continues, frostbite cases are expected to increase significantly.2 Researchers now widely believe that if frostbite is not treated promptly, it can lead to severe tissue damage and eventual cell death. Despite considerable advances in the field of medicine, research on frostbite treatment has lagged behind.3 Thus, it is crucial and necessary to explore effective therapeutic strategies or medications to mitigate the serious damage caused by frostbite.
Frostbite is a result of excessive heat loss and hypothermia in cold, humid, or windy environments. Recent advances in molecular mechanism research of frostbite have shed light on the complex processes that occur during tissue freezing and subsequent thawing. One key finding is that ice formation within cells and tissues can cause damage through a variety of mechanisms, including disruption of cell membranes, alteration in protein structure, and activation of inflammatory pathways.4 To date, three pathways triggering pathological changes in frostbite have been identified: tissue freezing, hypoxia, and the release of inflammatory mediators. These pathways occur almost simultaneously and exacerbate the damage caused by each other.5–9
It was reported that frostbite leads to damage and death of endothelial cells, resulting in the formation of blood clots and the release of inflammatory mediators that cause inflammation. Myeloid differentiation factor 2 (MD2) is an essential co-receptor for Toll-like receptor 4 (TLR4) activation, forming the TLR4-MD2 complex.10,11 It plays a crucial bridging role between inflammation, apoptosis, and necrosis, and recognizes common patterns in structurally diverse endotoxins.12,13 Cold-inducible RNA-binding protein (CIRP) is a newly identified damage-associated molecular pattern (DAMP) molecule that activates macrophages by binding to the TLR4-MD2 complex, leading to NF-κB activation and nuclear translocation. This, in turn, causes the release of cytokines/chemokines, further stimulating the inflammatory response and resulting in tissue damage.14–16
Tat-CIRP, as a peptide, penetrates into cells via the HIV Tat sequence and interferes with MD2 via the 15 aa sequence in CIRP. To date, several studies have proven the potential therapeutic applications of Tat-CIRP in various diseases, including stroke17 and sepsis,18 through inhibiting proinflammatory cytokines production and improving survival rates. However, whether Tat-CIRP peptide exerts its effects in frostbite treatment through the suppression of inflammation remains unclear.
In this study, we established a frostbitten mice model to observe and investigate the potential therapeutic effects and molecular mechanisms of Tat-CIRP in frostbite. We found that Tat-CIRP through intravenous administration improves skin healing in frostbitten mice, the underlying mechanism of which was through reducing inflammatory cells infiltration and promoting angiogenesis. Our research explored and provided Tat-CIRP as a new effective and thorough treatment medication for frostbite.
Materials and Methods
Animals and Reagents
All the ICR mice used in this study were provided by the Animal Experimental Centre of Nantong University. Mice were fed in individual ventilated caging system at 21 ± 2°C with a relative humidity of 55–60% and 12-h rotation periods of light and dark. All animal treatment procedures were conducted according to the Chinese Animal Management Rules of the Ministry of Health and were authorized by the Animal Ethics Committees of Nantong University research program protocol (#NTU-19-023). The study was carried out in accordance with ARRIVE guidelines. Unless otherwise described, all reagents used in this study were supplied by Sigma (St. Louis, MO, United States). The Tat-CIRP peptide was synthesized by Hefei KS-V Peptide Biological Technology Co., Ltd (Hefei, Anhui, China).
Tat-CIRP Peptide Synthesis
Based on a previous study on the high affinity between CIRP and MD2,14,17,18 we synthesized a peptide that mimics the CIRP 106–125 domain and linked it to the human immunodeficiency virus type 1 transcriptional activator TAT peptide (YGRKKRRQRRR) to form Tat-CIRP (YGRKKRRQRRR-GRGFSRGGGDRGYGG) for the next study.
Frostbitten Mice Model Establishment
Twenty-four male mice weighing 20–25 g were randomly divided into four groups, including control group, Tat-CIRP treatment group, frostbite group, and frostbite with Tat-CIRP treatment group. Following the method described by Auerbach et al,19 the mice were anesthetized with afodin (2.5%) and then subjected to continuous freezing treatment. In brief, standardized 3 cm diameter circles were prepared on the dorsal skin of the mice before frostbite treatment. Two 2.8 cm diameter cooled magnets (previously placed in dry ice for 15 minutes) were applied to perform a frostbite injury from opposite sides of the intervening skin. The two cooled magnets were left on the frozen skin for 1 minute, and then immediately replaced with new magnets at the same position on the frozen skin. The time for each magnet exchange was less than 5 seconds. This process was repeated 5 times, resulting in a total freezing time of 5 minutes.
Tat-CIRP Peptide Treatment
The Tat-CIRP peptide treatment involved dissolving Tat-CIRP peptide in 0.9% saline solution and administering it through tail vein injection on the 1st, 3rd, and 5th days after frostbite (12.5 mg/kg/day)
Morphology and Wound Area Analysis
Morphological changes, such as frostbite skin color, swelling, and scab formation, were assessed by visual observation of the wound. Changes in the wound were recorded using a camera at 0, 1, 3, 5, 7, 14, 21, 28, and 35 days after frostbite treatment. On the day 0, 1, 3, 5, 7, and 29, the mice were euthanized according to the approved euthanasia protocol, and the dorsal skin of the mice was collected for subsequent experiments.
Histologic Evaluation
Hematoxylin and Eosin (HE) staining was used to evaluate the structural changes in the wound skin. The skin tissues were fixed in 4% paraformaldehyde for 24 hours, embedded in paraffin, and then cut into 10-μm-thick sections for the subsequent staining process. For HE staining, the HE staining kit (C0105S, Beyotime Biotechnology, China) was used. For Masson staining, the Modified Masson’s Trichrome Stain Kit (G1346, Solarbio, China) was employed. In summary, the sections were deparaffinized in xylene and then rehydrated through a series of graded alcohols (30%, 50%, 75%, 80%, 95%, 95%, 100%, and 100%) for 2 minutes at each step. Subsequently, the sections were immersed in a mixture of equal volumes of absolute ethanol and xylene, as well as xylene I and xylene II, for 15 minutes. The sections were then stained with hematoxylin, differentiated in an acidic solution, and then counterstained with eosin. Following that, the sections were dehydrated in a series of graded alcohols (30%, 50%, 75%, 80%, 95%, 95%, 100%, and 100%) for 2 minutes at each step and finally sealed with neutral resin. Images were captured using a Leica fluorescent microscope (DM4000B, Leica, Germany).
Western Blot
The proteins of skin tissue were collected and homogenized in RIPA lysis buffer (BL504A, Biosharp, China) and then sonicated with a sonifier (Scientz-48L, Xinzhi, China) for ultrasonic treatment. The protein concentration was determined by BCA assay kit (U10007A, Keyoubo, China). Skin tissue samples containing 20 μg of protein were loaded onto SDS-PAGE gels for separation and then transferred to PVDF membranes. The PVDF membranes containing the proteins were blocked with 5% non-fat dry milk and then incubated with different primary antibodies, including anti-MD2 (NBP1-75513, Novus Biologicals, USA), anti-Ki67 (A2094, ABclonal, China), or anti-CIRP (10209-2-AP, Proteintech, China), at a dilution of 1:1000 at 4°C overnight. Subsequently, the membranes were incubated with secondary antibodies (anti-rabbit or anti-mouse IgG, 1:10000 dilution, 115–035-003, Jackson, USA) at room temperature for 1 hour. Anti-β-actin monoclonal antibody (66009, Proteintech, China) at a 1:10000 dilution was used as a loading control.20 The signal was developed by an enhanced chemiluminescence substrate (U10010A, Keyoubo, China) and captured by a chemiluminescent imaging system (4100, Tanon, China). Scanning and analysis were performed using Imaging J software and GraphPad software. The results were shown as the ratio of the optical density normalized to β-actin.
Immunohistochemistry
The skin tissue paraffin sections (10 μm) were permeabilized with 0.3% Triton X-100, followed by blocking with 10% donkey serum and incubation with specific primary antibodies against MD2 (1:200, NBP1-75513, Novus Biologicals, USA), IL-6 (1:200, ab11575, Abcam, UK), TNFα (1:200, 11948, Cell Signal Technology, USA), Ki67 (1:100, A2094, ABclonal, China), CIRP (1:100, 10209-2-AP, Proteintech, China), or CD31 (1:200, AF3628, R&D, USA). Then, the skin tissue sections were sequentially incubated with secondary antibodies, reaction enhancer, and 3,3’-diaminobenzidine (MXB, Fuzhou, China). Tissue images were captured using a fluorescent microscope (DM4000B, Leica, Germany) and analyzed using Imaging J software.
Statistical Analysis
The data were presented as mean ± standard deviation (SD), and GraphPad Prism 8.0 was utilized for statistical analysis. Unpaired t-test was used to determine the statistical significance of a single comparison between two groups. One-way analysis of variance (ANOVA) was performed for multiple comparisons, followed by the Tukey’s test for pairwise comparisons between two groups. A P-value of less than 0.05 (P<0.05) was considered statistically significant for differences.
Results
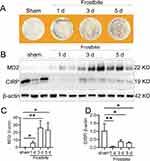
Frostbite Induced MD2 Expression
We established the frostbitten mice model according to the study of Auerbach et al.19 After frostbite 0, 1, 3, and 5 days we collected images and detected MD2 and CIRP expression in the damaged skin tissues. Figure 1A shows that while there was no discernible variation in color and apparent wounds, slight surface wrinkles were observed on the frozen skin. Moreover, the skin exhibited swelling and solid appearance at 1 day after frostbite (Figure 1A). Besides, from 1 to 5 days after frostbite, there was a significant increase in MD2 expression (Figure 1B and C), and a noticeable decrease in CIRP expression (Figure 1B and D). Consequently, in our subsequent study, we opted to administer Tat-CIRP 1 day after frostbite to inhibit MD2-mediated inflammation.
Effects of Tat-CIRP on Wound Healing
To monitor the morphological changes of the skin after frostbite, we continuously captured images of the mice skin from day 1 to day 29 post frostbite. As shown in Figure 2A, on the 7th day, depressed scars were observed on the surface of the frostbitten skin. By the 15th day, these depressed scars had been replaced by a crusty layer. However, the aforementioned phenomena were not observed in the sham group or the Tat-CIRP treatment group. Additionally, compared to the frostbite group, the incrustation disappeared and proliferated skin increased after Tat-CIRP treatment and the skin surface exhibited a significant acceleration in fur growth rate from the 15th day. Moreover, the frozen wounds in the Tat-CIRP group exhibited smaller than those in the frostbite group from the 5th day until the last day of observation (Figure 2B).
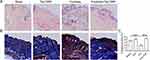
Effects of Tat-CIRP on Histopathology and Collagen Deposition
To further investigate the structural changes of the skin after frostbite, histological staining techniques including HE and Masson staining were employed. The HE staining results (Figure 3A) revealed that both the sham group and Tat-CIRP administration group exhibited intact skin morphology. The epidermis, dermis, and adipose layers were clearly visible, with an intact epidermis and well-preserved dermal striated muscle structure. In the frostbite group, epidermal hyperplasia and degeneration were accompanied by dermal necrosis of the striated muscles. Some striated muscles exhibited loss of transverse striations, along with edema and prominent inflammatory cell infiltration. In the skin tissue sections of the mice treated with Tat-CIRP after frostbite, the epidermis appeared intact and significantly thicker compared to the frostbite group, indicating the presence of new skin tissue with intact dermal striated muscle structure and no noticeable inflammatory cell infiltration. Masson staining revealed pronounced collagen deposition in the skin tissue of the sham and Tat-CIRP groups, while only a small amount of collagen existed in the skin tissue of the frostbite group (Figure 3B). However, compared to the frostbite group, the skin of mice treated with Tat-CIRP after frostbite was new and exhibited evident and complete collagen presence in the dermis. Immediate Tat-CIRP treatment after frostbite significantly enhanced collagen deposition (Figure 3B and C).
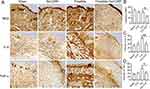
Effects of Tat-CIRP on Inflammation
We next examined the impact of Tat-CIRP treatment on the inflammatory responses, including pro-inflammatory cytokines IL-6 and TNFα, as well as MD2 expression, under both normal and frostbite conditions. The immunohistochemical results demonstrated that Tat-CIRP treatment significantly reduced the levels of MD2 (Figure 4A and B), IL-6 (Figure 4A and C), and TNFα (Figure 4A and D) in comparison to the frostbite group. However, these indicators did not show significant changes in normal conditions following Tat-CIRP treatment when compared to the sham group. The findings demonstrated that Tat-CIRP had an inhibitory effect on the release of DAMPs and inflammatory cytokines in frostbitten mice.
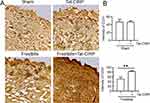
Tat-CIRP Promoted Cell Proliferation and Angiogenesis
Our subsequent investigation aimed to elucidate the potential role of Tat-CIRP in promoting cell proliferation by assessing Ki-67 and CD31 expression in skin tissues during frostbite treatment. The immunohistochemical results showed no change in Ki-67 expression in the Tat-CIRP group compared to the sham group, indicating that Tat-CIRP did not exert any discernible impact on mice under normal physiological conditions (Figure 5A and B). However, compared to the frostbite group, immediate Tat-CIRP administration after frostbite induction induced an increased expression of Ki-67, indicating enhanced cell proliferation in the skin (Figure 5A and C). Western blot analysis also revealed a significant increase in Ki-67 protein expression in the skin with immediate Tat-CIRP treatment after frostbite, whereas no effects were observed under normal conditions (Figure 5D and E). Meanwhile, CD31 expression was also increased in the skin of mice treated with Tat-CIRP (Figure 6A and B). The findings suggested that Tat-CIRP enhances cell proliferation and angiogenesis in frostbitten mice.
Discussion
Frostbite is defined as the acute freezing of tissues resulting from exposure to temperatures below the freezing point of intact skin. It occurs due to the induction of crystallization into ice by locally cooled tissue water, leading to free radicals formation and subsequent tissue damage. Its pathogenesis is linked to tissue freezing, hypoxia, and the release of inflammatory mediators. The frostbite model used in this study was constructed according to the research of Auerbach et al,19 which can be replicated and effectively utilized for studying the mechanisms associated with frostbite in mice. In terms of frostbite therapy, the main aim of most methods is to facilitate wound surface healing or enhance blood circulation.
Preventing exposure to cold from the beginning is one of the best preventive measures against frostbite. Another approach addresses the severe thrombosis that occurs after frostbite, which is one of the major factors contributing to late-stage ischemic damage of frostbite.21 Currently, most therapeutic approaches following the occurrence of frostbite involve the use of antibiotics like penicillin and methicillin to prevent infection. Additionally, some anti-thrombotic medications like aspirin are utilized due to their anti-inflammatory and platelet-inhibitory properties, making them widely applicable in frostbite treatment.22 However, the pathogenesis of frostbite is a prolonged process, and long-term medication carries certain risks including potential drug resistance, which further aggravates the challenge of clinical frostbite therapy. For example, it has been reported that aspirin exerts irreversible inhibition on cyclooxygenase activity, which is essential for the biosynthesis of PGE2 and PGF2α. However, prostaglandins play a crucial role in maintaining gastric mucosal integrity, and aspirin’s inhibition of their synthesis has been shown to increase epithelial shedding, decrease the rate of gastric mucosal renewal, exacerbate ulcer severity, and reduce gastric mucous generation. Thus, when using aspirin to treat frostbite, it might lead to gastric mucosal erosions, bleeding, and ulcers.23 Therefore, further exploration of the pathophysiological mechanisms of frostbite, development of appropriate treatment strategies, and identification of potential therapeutic targets remain of significant importance.
In this study, we aimed to identify a novel therapeutic approach that can effectively impede the progression of frostbite and expedite recovery with minimal adverse effects. MD2 has been considered as a therapeutic target for various mouse disease models, including acute lung injury,24 stroke,17 perioperative neurocognitive disorder,25 and sepsis18,26 mice models. Besides, CIRP-related peptide has demonstrated effective anti-DAMP damage effects in stroke and sepsis mouse models with low confirmed side effects. We hypothesized whether it might also possess potential therapeutic functions in frostbite. We utilized a mouse frostbite model to preliminarily investigate the expression of MD2 and CIRP proteins in frozen skin tissue. The results demonstrated a significant upregulation in MD2 expression from day 1 to day 5 after frostbite. Therefore, we administered Tat-CIRP on day 1, 3, and 5 post-frostbite to target endogenous enhanced MD2 function, aiming to attenuate the endogenous proinflammatory activity of MD2 during the subsequent stages of frostbite progression. Based on morphological observations, Tat-CIRP was found to significantly enhance the frozen wound recovery on day 5 after frostbite. While CIRP has been reported to be induced under cold conditions and identified as a DAMP molecule in several diseases, we observed an obvious decrease in CIRP protein expression from day 3 to day 5 after frostbite. However, the absence of extracellular CIRP content in the serum leaves its role as a DAMP molecule in frostbite unclear.
To eliminate the potential side effects of Tat-CIRP, we evaluated the effects of Tat-CIRP peptide under normal conditions in mice. The results suggested that Tat-CIRP did not elicit any alterations in mouse tissue structure, inflammatory factors expression, cell proliferation, and angiogenesis. Instead, Tat-CIRP peptide exerted its effects by suppressing the expression of inflammatory factors, inducing cell proliferation, and promoting hair growth, thereby facilitating skin tissue recovery in the frostbitten mice. Our data suggested that the progression of frostbite tissue damage could be inhibited through the reduction of inflammation, which was consistent with previous reports.27,28 Immediate administration of Tat-CIRP after frostbite reduced the elevated inflammation induced by increased MD2 level. The findings suggested that targeting MD2 function could effectively inhibit inflammation, which aligned with the results of Fang et al’s study. However, whether Tat-CIRP intervened MD2/TLR4 signal pathway and subsequently influenced inflammation required further exploration.
CIRP has been reported to be a direct negative regulator of angiogenesis both in vitro and in vivo.29 Additionally, CIRP deficiency promotes a microenvironment conducive to angiogenesis and vascular regeneration in ischemic muscle tissue.30 The mechanism by which CIRP deficiency promotes angiogenesis and regeneration in ischemic tissue involves the induction of M2-like macrophage polarization.31 In this study, we also observed that Tat-CIRP treatment induced angiogenesis in frostbitten mice, as evidenced by the upregulation of CD31 and Ki-67 expression. The findings were in line with the reduced expression of CIRP observed in frostbitten mouse skin tissue. However, the exact mechanism by which Tat-CIRP induced frostbite-associated angiogenesis warranted further investigation.
Moreover, collagen is a major component of connective tissue, synthesized by fibroblasts during the process of wound healing to provide a structural scaffold for tissue regeneration and enhance tensile strength.32 Tat-CIRP treatment after frostbite led to a significant increase in collagen deposition level in the proliferative tissues compared to the frostbite group, with even distribution throughout the injured area. Additionally, the hair growth around the damaged skin tissues was stimulated following Tat-CIRP treatment. We are uncertain whether Tat-CIRP has the ability to directly stimulate collagen expression in fibroblasts and hair growth in hair follicle cells. Further investigation is needed to address these issues.
Conclusion
In conclusion, in this study, we targeted a novel frostbite treatment approach and validated the inhibitory effects of Tat-CIRP on frostbite progression in a mouse model by suppressing inflammation and promoting angiogenesis.
Funding
This research was funded by the National Natural Science Foundation of China (32271228, 81873924, and 81803516), Cultivate Candidate of the Jiangsu Province “333” Project, and Shanghai Municipal Health Commission (20184Y0102).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Murphy JV, Banwell PE, Roberts AH, McGrouther DA. Frostbite: pathogenesis and treatment. J Trauma. 2000;48(1):171–178. doi:10.1097/00005373-200001000-00036
2. Joshi K, Goyary D, Mazumder B, et al. Frostbite: current status and advancements in therapeutics. J Therm Biol. 2020;93:102716. doi:10.1016/j.jtherbio.2020.102716
3. Yang R, Yang S, Zhao J, et al. Progress in studies of epidermal stem cells and their application in skin tissue engineering. Stem Cell Res Ther. 2020;11(1):303. doi:10.1186/s13287-020-01796-3
4. Tu H, Zhang D, Barksdale AN, Wadman MC, Muelleman RL, Li YL. Dexamethasone Improves Wound Healing by Decreased Inflammation and Increased Vasculogenesis in Mouse Skin Frostbite Model. Wilderness Environ Med. 2020;31(4):407–417. doi:10.1016/j.wem.2020.07.003
5. Bracker MD. Environmental and thermal injury. Clin Sports Med. 1992;11(2):419–436.
6. Britt LD, Dascombe WH, Rodriguez A. New horizons in management of hypothermia and frostbite injury. Surg Clin North Am. 1991;71(2):345–370. doi:10.1016/S0039-6109(16)45384-3
7. Foray J. Mountain frostbite. Current trends in prognosis and treatment (from results concerning 1261 cases). Int J Sports Med. 1992;13(Suppl 1):S193–196. doi:10.1055/s-2007-1024637
8. Heggers JP, Robson MC, Manavalen K, et al. Experimental and clinical observations on frostbite. Ann Emerg Med. 1987;16(9):1056–1062. doi:10.1016/S0196-0644(87)80758-8
9. McCauley RL, Hing DN, Robson MC, Heggers JP. Frostbite injuries: a rational approach based on the pathophysiology. J Trauma. 1983;23(2):143–147. doi:10.1097/00005373-198302000-00013
10. Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458(7242):1191–1195. doi:10.1038/nature07830
11. Nagai Y, Akashi S, Nagafuku M, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3(7):667–672. doi:10.1038/ni809
12. Hyakkoku K, Hamanaka J, Tsuruma K, et al. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience. 2010;171(1):258–267. doi:10.1016/j.neuroscience.2010.08.054
13. Duckett CS. Apoptosis and NF-kappa B: the FADD connection. J Clin Invest. 2002;109(5):579–580. doi:10.1172/JCI0215197
14. Qiang X, Yang WL, Wu R, et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat Med. 2013;19(11):1489–1495. doi:10.1038/nm.3368
15. Denning NL, Aziz M, Gurien SD, Wang P. DAMPs and NETs in Sepsis. Front Immunol. 2019;10. doi:10.3389/fimmu.2019.00010
16. Han JR, Zhang YB, Ge P, et al. Exosome-derived CIRP: an amplifier of inflammatory diseases. Front Immunol. 2023;14.
17. Fang ZP, Wu D, Deng J, et al. An MD2-perturbing peptide has therapeutic effects in rodent and rhesus monkey models of stroke. Sci, trans med. 2021;13(597). doi:10.1126/scitranslmed.abb6716
18. Fan Z, Ma H, Li Y, et al. Neuronal MD2 induces long-term mental impairments in septic mice by facilitating necroptosis and apoptosis. Front Pharmacol. 2022;13:884821. doi:10.3389/fphar.2022.884821
19. Auerbach LJ, Galvez MG, De Clerck BK, et al. A Novel Mouse Model for Frostbite Injury. Wilderness Environ Med. 2013;24(2):94–104. doi:10.1016/j.wem.2012.11.020
20. X-m H, Zheng S, Zhang Q, et al. PANoptosis signaling enables broad immune response in psoriasis: from pathogenesis to new therapeutic strategies. Comput. Struct. Biotechnol. J. 2024;23:64–76. doi:10.1016/j.csbj.2023.11.049
21. Bruen KJ, Ballard JR, Morris SE, Cochran A, Edelman LS, Saffle JR. Reduction of the incidence of amputation in frostbite injury with thrombolytic therapy. Arch Surg. 2007;142(6):546–551. doi:10.1001/archsurg.142.6.546
22. McIntosh SE, Hamonko M, Freer L, et al. Wilderness Medical Society practice guidelines for the prevention and treatment of frostbite. Wilderness Environ Med. 2011;22(2):156–166. doi:10.1016/j.wem.2011.03.003
23. Lee M, Cryer B, Feldman M. Dose effects of aspirin on gastric prostaglandins and stomach mucosal injury. Ann Intern Med. 1994;120(3):184–189. doi:10.7326/0003-4819-120-3-199402010-00002
24. Zhang W, Wang Y, Li CW, et al. Extracellular CIRP-Impaired Rab26 Restrains EPOR-Mediated Macrophage Polarization in Acute Lung Injury. Front Immunol. 2021;12.
25. Zuo WQ, Zhao JS, Zhang JM, et al. MD2 contributes to the pathogenesis of perioperative neurocognitive disorder via the regulation of alpha 5GABA(A) receptors in aged mice. J Neuroinflammation. 2021;18(1). doi:10.1186/s12974-021-02246-4
26. McGinn JT, Aziz M, Ode Y, Wang P. Small Molecule C23 Inhibits CIRP-Induced Pro-Inflammatory ICAM1 Expressing Neutrophils in Sepsis. J Am Coll Surgeons. 2018;227(4):S82–S83. doi:10.1016/j.jamcollsurg.2018.07.112
27. Lipatov K, Komarova E, Asatryan A, et al. Frostbite of the upper extremities: hot issues in diagnosis and surgical treatment (review). Burns. 2022;48(6):1279–1286. doi:10.1016/j.burns.2022.03.006
28. Sheridan RL, Goverman JM, Walker TG. Diagnosis and Treatment of Frostbite. N Engl J Med. 2022;386(23):2213–2220. doi:10.1056/NEJMra1800868
29. Goossens EAC, Zhang L, de Vries MR, Jukema JW, Quax PHA, Nossent AY. Cold-Inducible RNA-Binding Protein but Not Its Antisense lncRNA Is a Direct Negative Regulator of Angiogenesis In Vitro and In Vivo via Regulation of the 14q32 angiomiRs-microRNA-329-3p and microRNA-495-3p. Int J Mol Sci. 2021;22(23). doi:10.3390/ijms222312678
30. Kubler M, Beck S, Peffenkover LL, et al. The Absence of Extracellular Cold-Inducible RNA-Binding Protein (eCIRP) Promotes Pro-Angiogenic Microenvironmental Conditions and Angiogenesis in Muscle Tissue Ischemia. Int J Mol Sci. 2021;22(17):9484. doi:10.3390/ijms22179484
31. Kubler M, Beck S, Fischer S, et al. Absence of Cold-Inducible RNA-Binding Protein (CIRP) Promotes Angiogenesis and Regeneration of Ischemic Tissue by Inducing M2-Like Macrophage Polarization. Biomedicines. 2021;9(4):395. doi:10.3390/biomedicines9040395
32. Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3(1):a004978–a004978. doi:10.1101/cshperspect.a004978
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.