Back to Journals » Hepatic Medicine: Evidence and Research » Volume 16
Synchronous Double Primary Tumors of Liver (Small Cell Neuroendocrine Carcinoma and Hepatocellular carcinoma): A Case Report
Authors Bu Y , Wen J, Wang F, Dong S, He L , Li Y, Liang J, Zhang H
Received 10 November 2023
Accepted for publication 12 March 2024
Published 20 April 2024 Volume 2024:16 Pages 31—36
DOI https://doi.org/10.2147/HMER.S449206
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Yuqing Bu,1,* Junye Wen,2,* Fayan Wang,1,3 Shibo Dong,4 Liya He,1 Yang Li,1,5 Jinlong Liang,1,3 Hongzhen Zhang1,3,5
1Department of Oncology, Hebei General Hospital, Shijiazhuang, Hebei Province, 050051, People’s Republic of China; 2Department of Hepatobiliary Surgery, Hebei General Hospital, Shijiazhuang, Hebei Province, 050051, People’s Republic of China; 3Department of Medicine, Hebei North University, Zhangjiakou, Hebei Province, 075000, People’s Republic of China; 4Department of Imaging, Hebei General Hospital, Shijiazhuang, Hebei Province, 050051, People’s Republic of China; 5Department of Medicine, North China University of Science and Technology, Tangshan, Hebei Province, 063210, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongzhen Zhang, Email [email protected]
Abstract: This study presents a case of dual primary liver cancer involving small cell neuroendocrine carcinoma and hepatocellular carcinoma. The 58-year-old Chinese male patient, who has a medical history of viral hepatitis B, presented with right upper abdominal pain persisting for one month. Imaging studies indicated the presence of multiple liver masses in segments V and VII–VIII, as well as a mass in the left lung. Subsequent hepatic biopsy performed on both segments confirmed the presence of hepatocellular carcinoma in segment V and small cell neuroendocrine carcinoma in segment VII–VIII. After undergoing one cycle of chemotherapy, the lung mass exhibited a reduction in size, while the liver masses showed an inadequate response. Subsequently, the patient underwent Transcatheter Arterial Chemoembolization (TACE) and Hepatic Artery Infusion Chemotherapy (HIAC), resulting in partial remission (PR). However, the patient was diagnosed with brain metastasis and subsequently treated with Sorafenib and Tirelizumab, a Programmed Death 1 (PD-1) immune checkpoint inhibitor. The efficacy evaluation indicated stability, and no severe adverse effects were observed at the time of writing. The patient’s survival time was 16 months.
Keywords: synchronous tumor, liver tumor, primary tumor, small cell neuroendocrine carcinoma, hepatocellular carcinoma
Introduction
The detection rate of multiple primary malignant tumors (MPMTs) is increasing according to the development of screening and diagnostic technologies, but the liver MPMTs are extremely rare, hepatocellular carcinoma (HCC) is the most common hepatic malignancy, but the incidence of neuroendocrine carcinoma (NEC) in hepatic malignancies was only 0.46%.1 According to previous study, surgery maybe the most effective treatment, but there was no established treatment for MPMTs, especially for the recurrence patients, 1 year survival rate of mixed HCC and NEC was about 58%.2 Here, we reported a patient with synchronous small cell neuroendocrine carcinoma and HCC, combined therapy was given to patient at different periods with stable disease.
Case Presentation
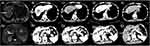
A 58-year-old Chinese man was admitted to our hospital due to a one month history of right upper abdominal pain, The pain significantly disrupted his sleep and was accompanied by symptoms of loss of appetite and fatigue. The patient’s previous medical examination at a local hospital revealed elevated tumor markers, specifically an alpha-fetoprotein (AFP) level of 172.9ng/mL, a Carcinoembryonic antigen (CEA) level of 9.16 ng/mL, a Carbohydrate antigen 125 (CA 125) level of 42.64 U/mL, and a Carbohydrate antigen 199 (CA 199) level of 53.08 U/mL. Hepatitis B virus DNA quantitative was 5.35E+05IU/mL. Hepatobiliary ultrasound findings indicated the presence of liver cirrhosis and fibrosis, with visible liver masses. The patient’s past medical history included hepatitis B-associated liver cirrhosis for 10 years, and antiviral therapy was not regularly applied. His personal history included smoking for more than 20 years, approximately 6–7 cigarettes per day, without a history of alcoholism. In terms of family history, his mother had Esophagogastric Junction Cancer, his sister had breast cancer, his brother had liver cirrhosis. During the physical examination, liver palm and spider nevus were observed. There was tenderness in the right upper abdomen, and the liver could be palpated 1cm below the costal area. After being admitted to our hospital, chest computed tomography (CT) and abdominal magnetic resonance imaging (MRI) was performed to assess the tumor, revealing the presence of mass in the left lung and multiple masses in the liver (Figure 1), brain MRI and bone scintigraphy showed no brain or bone metastases. Biopsy pathology of the liver masses in segment VII–VIII confirmed the presence of a malignant tumor (Figure 2), Immunohistochemical staining results were as follows: CKpan (weak +), Syn (+), CgA (+), CD56 (+), CD34 (-), CK19 (-), Arginase-1 (-), HepPar-1 (-), AFP (-), Vimentin (partly weak +), CK7 (-), CK20 (-), Villin (-), TTF-1 (-), CDX-2 (-), Ki-67 (active zone about 70%+), MLH1 (+), MSH2 (+), MSH6 (+), PMS2 (+), PD-L1 (22C3)(CPS 1), Subsequently, the patient underwent one cycle of chemotherapy with etoposide and cisplatin. However, two weeks after the chemotherapy, the patient developed febrile neutropenia. Following treatment with recombinant human granulocyte colony-stimulating factor (GSF), the patient’s white blood cell count returned to the normal range.
 |
Figure 1 Chest CT and abdominal MRI showed left lung and multiple liver masses (A–D). (white arrow indicate lung and liver masses). |
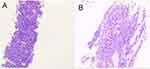 |
Figure 2 Pathological findings of liver mass in segment VII-VIII. Hematoxylin and eosin (H&E) staining. Original magnification: (A)×40. (B) ×100. |
In light of the patient’s prior history of hepatitis B-associated liver cirrhosis, the possibility of primary liver cancer cannot be disregarded. Consequently, a puncture biopsy of the mass in liver segment V was conducted, yielding findings consistent with hepatocellular carcinoma (Figure 3), Immunohistochemistry results were as follows: GS(+), GPC3 (focal +), HSP70 (focal +), CD34 (+), CK19 (-), Arginase-1 (+), HepPar-1 (+), AFP (-), Ki-67 (active zone about 15%+). The patient received a combination treatment of sorafenib (0.4g Bid) with Transcatheter arterial chemoembolization (TACE) and hepatic artery infusion chemotherapy (HIAC), One month after treatment, a partial response (PR) was achieved. TACE was administered monthly, totaling two treatments, during the treatment, the patient experienced a first-degree gastrointestinal reaction and liver function impairment. Approximately three months later, abdominal CT scans indicated stability in the liver masses, although a head MRI revealed the presence of a new brain metastasis in the left parietal lobes without accompanying symptoms. The patient received one cycle of Zimberelimab (240mg) as treatment, which was unfortunately discontinued due to the onset of the COVID-19 pandemic.
 |
Figure 3 Pathological findings of liver mass in segment V. Hematoxylin and eosin (H&E) staining. Original magnification: (A)×40. (B) ×100. |
Three months after the treatment, imaging studies revealed stable tumors in chest, head and liver was stable (Figures 4–6), but an enlargement of retroperitoneal lymph nodes and enhanced margins of a liver mass in the V segment. But an enlargement of retroperitoneal lymph nodes and enhanced margins of a liver mass in the V segment. To control hepatocellular carcinoma, a third TACE procedure was performed, along with the addition of sorafenib and six cycles of tirelizumab. Efficacy evaluation was stable, and the brain tumor exhibited a reduction in size without any accompanying symptoms, no sever adverse was discovered by the time of writing, and the patient was in a good physical condition.
 |
Figure 4 Chest CT images before and after treatment. The time of CT was (A) 2022-07-25, (B) 2022-09-14, (C) 2023-02-08, (D) 2023–06-28. (white arrow indicate lung mass). |
 |
Figure 6 Brain MRI images before and after treatment. The time of MRI was (A) 2022-06-24, (B) 2022-10-24, (C) 2023-02-09, (D) 2023-05-11. (white arrow indicate brain mass in the left parietal lobe). |
Discussion
The definition of MPMTs is that two or more primary malignant tumors developed in the same patient. MPMTs were divided into two groups, Synchronous MPMTs means that the second primary cancer is diagnosed in 6 months after the first primary cancer, metachronous MPMTs are the opposite of synchronous cancer.3 Metachronous MPMTs are more common than synchronous MPMTs. Due to the development of screening technologies and diagnostic tools, the detection rate of MPMTs is increasing. Zhai et al found that MPMTs harbored tumors whether in the same organ or in the different organs, the incidence of digestive system malignancies was the highest,4 and the clinical reports on MPMTS in the digestive system is increasing,5 but the incidence of synchronous double primary liver cancers is extremely low, about 0.1% to 1%, the most common pathological type was hepatic, neuroendocrine, and biliary components, when synchronous MPMTs occurred in liver independently, they also defined as collision type.6 The mechanism of tumorigenesis remains unclear.
The treatment methods of malignant tumors were developed rapidly, such as radiotherapy, chemotherapy, endocrine therapy, targeted therapy and immunotherapy and so on, the survival rate of MPMTs patient is increasing, but the median survival of neuroendocrine combined with hepatic cancer was only about 17.8 months, and the quality of life is not well, hepatitis viral infection and tumor recurrence time maybe the most influencing factor of survival time.7,8 By now, there are no standard treatment guidelines for MPMTs.
In this patient, when the liver masses were discovered, biopsy confirmed the diagnosis of small cell neuroendocrine carcinoma, according to the imaging examination, it was divided into stage IV, post one cycle of chemotherapy, the mass of lung was smaller, but the liver masses were stable. After conducting a comprehensive analysis of the patient’s previous liver enhanced MRI scans with contrast enhancement and consulting imaging experts, it was advised that the liver masses in segments VII–VIII demonstrated mild irregular edge enhancement in both the arterial and portal phases. In contrast, the liver mass in segment V exhibited noticeable enhancement during the arterial phase, followed by a rapid decline in the venous phase. Both sets of lesions were identified as primary tumors, with the liver masses in segments VII–VIII being diagnosed as NSE. However, the mass observed in segment V of liver are more likely to exhibit imaging characteristics indicative of liver cancer, combined with the previous history of hepatitis, biopsy of another liver segment was performed, the pathology showed hepatocellular carcinoma. Hence, it is imperative to pay attention towards imaging findings and the patient’s medical history when assessing multiple liver masses.
The incidence of synchronous double primary liver cancers is very low, there were several reports on synchronous primary liver cancers included intrahepatic cholangiocarcinoma and hepatocellular carcinoma,9–11 but the occurrence of neuroendocrine carcinoma combined with hepatocellular carcinoma was rare, and with poor prognosis, the median time of overall survival and the median time to recurrence were only 17.88 months and 6 months.8 When simultaneous neoplasms are discovered, systematic therapy should be given according to the tumor with the worst prognosis. In this patient, the disease of small cell neuroendocrine carcinoma was under control, targeted hepatocellular carcinoma therapy was performed, HIAC and TACE combined with sorafenib was given to the patient, considered that Immune escapes is one of the most common pathological mechanism of various malignant tumors, and immunotherapy was effective to the both kinds of tumor,12–14 immunotherapy was given to control the disease, although the treatment was interrupted due to the COVID-19, but restart the immunotherapy, the patient was effective. By now the patient was in good condition without obvious symptom, the survival time was 16 months.
Conclusion
In summary, the incidence of double primary liver cancer is very low, imaging findings and past medical history may be helpful to get the correct diagnosis, the gold standard of diagnostic is pathology. Although there was no standard treatment by now, but the treatment should be targeted the tumor with the worst prognosis, and appropriate treatments should be selected according to different stages of disease progression.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This research was approved by the Institutional Review Board at Hebei General Hospital.
Patient Consent for Publication
The patient provided written informed consent for the publication of any associated data.
Acknowledgments
We gratefully acknowledge the patient and his family for allowing us to publish this clinical case.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from Hebei Provincial Department of Finance, China (ZF2023203).
Disclosure
The authors declare that they have no competing interests.
References
1. Shin WY, Lee KY, Kim KD. Mixed primary Hepatocellular Carcinoma and Hepatic neuroendocrine carcinoma: case report and literature review. Medicina. 2023;59(2):418. PMID: 36837619; PMCID: PMC9959776. doi:10.3390/medicina59020418
2. Nakano A, Hirabayashi K, Yamamuro H, et al. Combined primary hepatic neuroendocrine carcinoma and hepatocellular carcinoma: case report and literature review. World J Surg Oncol. 2021;19(1):78. doi:10.1186/s12957-021-02187-5
3. Gokyer A, Kostek O, Hacioglu MB, et al. Clinical features of the patient with multiple primary tumors: single center experience. North Clin Istanb. 2017;4(1):43–51. doi:10.14744/nci.2017.67044
4. Zhai C, Cai Y, Lou F, et al. Multiple primary malignant tumors - a clinical analysis of 15,321 patients with malignancies at a single center in China. J Cancer. 2018;9(16):2795–2801. doi:10.7150/jca.25482
5. Yang XB, Zhang LH, Xue JN, et al. High incidence combination of multiple primary malignant tumors of the digestive system. World J Gastroenterol. 2022;28(41):5982–5992. doi:10.3748/wjg.v28.i41.5982
6. Khanam R, Pachika PS, Arya P, et al. “A tale of 2 demons”-concomitant presence of hepatocellular carcinoma and primary neuroendocrine tumor of liver: a case report and review of literatures. J Investig Med High Impact Case Rep. 2021;9:23247096211043397. doi:10.1177/23247096211043397
7. Zheng Y, Sun Y, Kuai Y, et al. Gene expression profiling for the diagnosis of multiple primary malignant tumors. Cancer Cell Int. 2021;21(1):47. doi:10.1186/s12935-021-01748-8
8. Mao JX, Teng F, Sun KY, et al. Two-in-one: a pooled analysis of primary hepatic neuroendocrine carcinoma combined/collided with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2020;19(4):399–403. doi:10.1016/j.hbpd.2020.03.012
9. Wu C, Bai DS, Jiang GQ, et al. Synchronous double cancers of primary hepatocellular carcinoma and intrahepatic cholangiocarcinoma: a case report and review of the literature. World J Surg Oncol. 2014;12:337. doi:10.1186/1477-7819-12-337
10. Inaba K, Suzuki S, Sakaguchi T, et al. Double primary liver cancer (intrahepatic cholangiocarcinoma and hepatocellular carcinoma) in a patient with hepatitis C virus-related cirrhosis. J Hepatobiliary Pancreat Surg. 2007;14(2):204–209. doi:10.1007/s00534-006-1134-0
11. Ito Y, Fujioka H, Matsuzaki S, et al. Occurrence of hepatocellular and cholangiocellular carcinoma in different hepatic lobes. Hepatogastroenterology. 2003;50(49):65–68.
12. Song L, Zhou F, Xu T, et al. Clinical activity of pembrolizumab with or without chemotherapy in advanced pulmonary large-cell and large-cell neuroendocrine carcinomas: a multicenter retrospective cohort study. BMC Cancer. 2023;23(1):443. doi:10.1186/s12885-023-10952-w
13. Psilopatis I, Damaskos C, Garmpi A, et al. FDA-approved monoclonal antibodies for unresectable Hepatocellular carcinoma: what do we know so far? Int J Mol Sci. 2023;24(3):2685. doi:10.3390/ijms24032685
14. Wan Y, Wang Z, Yang N, et al. Treatment of multiple primary malignancies with PD-1 inhibitor camrelizumab: a case report and brief literature review. Front Oncol. 2022;12:911961. doi:10.3389/fonc.2022.911961
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.