Back to Journals » Research and Reports in Urology » Volume 15
Surgical Management of Male Stress Incontinence: Techniques, Indications, and Pearls for Success
Authors Smith WJ, VanDyke ME, Venishetty N, Langford BT, Franzen BP, Morey AF
Received 1 February 2023
Accepted for publication 3 June 2023
Published 21 June 2023 Volume 2023:15 Pages 217—232
DOI https://doi.org/10.2147/RRU.S395359
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Panagiotis J Vlachostergios
Wesley J Smith, Maia E VanDyke, Nikit Venishetty, Brian T Langford, Bryce P Franzen, Allen F Morey
Department of Urology, University of Texas Southwestern Medical Center, Dallas, TX, USA
Correspondence: Allen F Morey, Department of Urology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX, 75390-9110, USA, Tel +214-648-0202, Fax +214-648-8786, Email [email protected]
Purpose: Male stress urinary incontinence (SUI) has detrimental and long-lasting effects on patients. Management of this condition is an evolving field with multiple options for surgical treatment. We sought to review the pre-operative evaluation, intra-operative considerations, post-operative care, and future directions for treatment of male SUI.
Methods: A literature review was performed using the PubMed platform to identify peer-reviewed, English-language articles published within the last 5 years pertaining to management of male stress urinary incontinence with an emphasis on devices currently on the market in the United States including the artificial urinary sphincter (AUS), male urethral slings, and the ProACTTM system. Patient selection criteria, success rates, and complications were compared between the studies.
Results: Twenty articles were included in the final contemporary review. Pre-operative workup most commonly included demonstration of incontinence, PPD, and cystoscopy. Definition of success varied by study; the most common definition used was social continence (0– 1 pads per day). Reported rates of success were higher for the AUS than for male urethral slings (73– 93% vs 70– 90%, respectively). Complications for these procedures include urinary retention, erosions, infections, and device malfunction. Newer treatment options including adjustable balloon systems and adjustable slings show promise but lack long-term follow-up.
Conclusion: Patient selection remains the primary consideration for surgical decision-making for management of male SUI. The AUS continues to be the gold standard for moderate-to-severe male SUI but comes with inherent risk of need for revision. Male slings may be a superior option for appropriately selected men with mild incontinence but are inferior to the AUS for moderate and severe incontinence. Ongoing research will shed light on long-term results for newer options such as the ProACT and REMEEX systems.
Keywords: AdVance sling, AdVance XP sling, artificial urinary sphincter, male stress incontinence, male urethral sling, ProACT, REMEEX sling, Virtue sling
Introduction
Urinary incontinence affects 19.3% of US males, with 4.6% of men experiencing stress urinary incontinence (SUI). The majority of SUI is iatrogenic, due to radical prostatectomy (RP), pelvic radiation, and surgical management of benign prostatic hyperplasia (BPH).1 Radical prostatectomy has the highest risk of SUI, with rates of 5–72% being recorded.2 This wide variability has been attributed to surgical technique, surgeon experience, differing definitions of incontinence, and length of follow-up, amongst other reasons.3 Primary radiation for prostate cancer and outlet procedures causes SUI much less commonly with rates reported as 6–7% and 1–5%, respectively.4,5
Stress urinary incontinence has a significant emotional and financial impact, affecting daily living and quality of life (QOL).6 Incontinence has been associated with weight gain, decreased sexual activity, depression, anxiety, and lack of interest in leaving one’s home.7 From a financial perspective, SUI yields both direct and indirect costs. The direct costs – including cost of pads, diapers, clamps, and doctors’ visits – are estimated at 3.8 billion annually in the US.8 Additional indirect costs include time off work, disability leave, and early retirement.9 While the exact magnitude of indirect cost is not known, it is estimated that about 40% of patients with SUI will request time off work directly related to their incontinence.10
Conservative options for management of SUI include behavioral therapy, pads, condom catheters, and various clamps.11 When these methods fail, patients turn to surgical options, with urethral slings and the artificial urinary sphincter (AUS) accounting for the vast majority of procedures performed in the United States. Newer FDA-approved options include the ProACT (Uromedica Inc., Plymouth, MN, USA) adjustable balloon system and the REMEEX (Neomedic, Terrassa, Barcelona, Spain) adjustable sling. Given the heterogeneity of this population with regard to degree of stress incontinence, radiation history, and comorbidities, there is no “one size fits all” approach. Rather, the decision of which device to utilize is a nuanced one that relies on open discussion between the patient and surgeon.12,13 In this narrative review, we sought to review the initial workup, surgical decision-making, and outcomes of the most popular devices on the market in the US today. Additionally, we have provided commentary based on our extensive, 16-year experience with the AUS and AdVance urethral sling at a tertiary referral center, including pearls from pre-operative workup to intra-operative tips and tricks for success.
Methods
A comprehensive literature review was conducted using the PubMed platform to identify peer-reviewed articles published in the last 5 years on the management of male SUI with an emphasis on devices currently FDA-approved and available in the US. IRB approval was exempt due to the nature of the study. Keywords included “male stress incontinence”, “artificial urinary sphincter”, “male urethral sling”, “AdVance sling”, “AdVance XP sling”, “Virtue sling”, “REMEEX sling” and “ProACT”. A summary of search methodology is summarized in Figure 1. Non-English language articles and those pertaining to devices not currently on the market in the US were excluded. Also excluded were any articles including female patients and review articles.
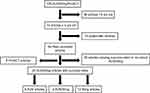 |
Figure 1 Literature selection criteria. |
Each article was reviewed by three separate authors (WS, MV, BF) between August 2022 and April 2023. Articles were primarily evaluated for definition of success, reported success rates, and reported complete continence (“cure”) rates. Secondary outcomes included complications, reintervention rate, and follow-up period.
Evaluation of Stress Incontinence
Initial evaluation of incontinence is centered on determination of etiology (stress, urge, or mixed). Patient reports of leakage while coughing/sneezing indicate stress incontinence, while urgency and frequency raise the question of an urge component. Voiding diaries may also be helpful to identify frequency patterns. Overflow incontinence can be ruled out using in-office post-void bladder ultrasound. Stress incontinence can be elicited by standing cough test (SCT) which is easily performed in the office setting. In our practice, we do not obtain urodynamics to assess bladder dynamics on all patients; however, others do.14 If patient history suggests a significant urge component, then we recommend an empiric trial of anticholinergic or beta-3 agonist to address these symptoms prior to addressing the stress component.
The next step is to stratify the severity of SUI. While this is commonly assessed by patient-reported pads per day (PPD), there are distinct limitations to this method including patient recall bias, differing size and absorbency of pads and degree of saturation at time of exchange.13,15,16 Obtaining 24-hour pad weights provides a more objective measurement; however, this can be cumbersome to both patients and office staff.17 As an alternative, we have utilized the standing cough test in our own practice in order to provide an objective display of SUI and to assist with severity stratification. Degree of leakage is then categorized according to the Male Stress Incontinence Grading Scale (MSIGS) as seen in Figure 2, which ranges from 0 (no incontinence) to 4 (early, persistent stream with cough).16 This rapid, objective test has been shown to correlate well with 24-hour pad weight measurements and is easy to perform in a clinical setting.18
 |
Figure 2 Standing cough test (SCT) scoring by the Male Stress Incontinence Grading Scale. Note: Reproduced with permission from Shakir NA, Fuchs JS, McKibben MJ, et al. Refined nomogram incorporating standing cough test improves prediction of male transobturator sling success. Neurourol Urodyn. 2018;37(8):2632–2637. © 2018 Wiley Periodicals, Inc.16 |
In patients with history of radical prostatectomy, cystoscopy should be performed to rule out bladder neck contracture. If present, bladder neck contracture should be managed first, with SUI being addressed only after ensuring stability of the bladder neck.19–21 There is currently no consensus on duration of stability that must be ensured prior to addressing SUI, with some authors advocating AUS placement as early as 12 weeks after bladder neck contracture intervention.22,23 While we find that most patients with concurrent bladder neck contracture and stress incontinence are most appropriate for artificial sphincter placement, occasionally a patient is a candidate for sling surgery. In this instance, we would wait a similar period of time to ensure bladder neck stability.
Management Options for Stress Incontinence
Patients should always be counseled regarding non-operative interventions available. These range from the use of various pads, to condom catheters, to incontinence clamps. The latter have been shown to be quite effective at stopping urine leakage but vary widely in design and can be uncomfortable to some patients.24 For men who desire surgical intervention, options currently available in the US include male slings, adjustable balloon devices, and the artificial urinary sphincter. Bulking agents have been studied for men with SUI but are currently not recommended by the AUA guidelines due to poor response. Decision of which method to pursue is a nuanced one, and depends on the severity of SUI, patient history and comorbidities, and patient preference.
Devices Currently Available in the US
The Artificial Urinary Sphincter
The first modern multi-component artificial urinary sphincter was developed by F. Brantley Scott in 1973. In 1980, the AMS 800™ (AMS, Minnetonka, MN, USA) was introduced, and remains the gold standard for management of refractory SUI to this day.25–28 This device has undergone a number of modifications over the intervening decades to increase device reliability, from the introduction of kink-resistant tubing in 1987 to the addition of antibiotic coatings in 2008.29 The modern-day AMS 800 consists of a urethral cuff, pressure regulating balloon (PRB), and pump.30 Various cuff sizes and PRBs are available, with each component being chosen intra-operatively based off patient measurements and joined using the proprietary Quick Connect™ system.30
Pressure regulating balloon placement and design have evolved over time. The PRB was traditionally placed in the Space of Retzius (SOR). While this remains a common practice, SOR placement has rarely been associated with catastrophic complications given proximity to vasculature, bowel, and the bladder – especially at the time of revision surgery.31 Ectopic PRB placement, first described by Wilson in 2001, offered an alternative, virgin space for PRB placement in patients with a potentially hostile abdomen.30,31 However, early experiences with this approach were marred by reports of balloon herniation, bothersome palpability, and pain.32 Today, however, surgeons may select from a variety of PRB placement locations including high submuscular, low submuscular, lateral retroperitoneal, and of course traditional SOR placement.33–35 At our institution, we prefer high submuscular placement, which we now perform in all patients via a counter-incision, allowing precise balloon placement under direct vision and minimizing variability in reservoir location. Adjunctive maneuvers may also be performed such as trans-fascial fixation which virtually eliminates the possibility of PRB herniation.34
While the modern-day AUS has been shown to be effective for all levels of SUI, its use tends to be reserved for those with moderate-to-severe incontinence, where it has been shown to outperform slings.12 It also remains the procedure of choice for patients with history of pelvic radiation, despite worse continence outcomes and higher revision rates when compared to patients without such history.36 Patient selection and counseling remain key to success. In the office, patients must demonstrate the cognitive ability and dexterity to operate the scrotal control pump.37 Patients must also understand that the goal of the device is to improve but not necessarily cure the incontinence. In fact, although 73–93% of patients achieve “social continence”, defined as 0–1 PPD, only around 15–20% of patients achieve “cure” or the ability to live completely pad-free (Table 1).38,39 Even so, satisfaction rates remain high at 95%, with 90% choosing to undergo the procedure again and 96% recommending the device to a friend or family member.40
 |
Table 1 Outcomes after AUS placement |
Perioperative reasons for AUS failure predominately include urinary retention and infection. Retention may occur in up to 31% of patients, most commonly resolving with temporary catheter placement.29 Patients with persistent retention are best served with suprapubic tube placement to avoid prolonged catheterization. Since the advent of antibiotic-impregnated devices in 2006, rates of AUS infection have decreased drastically, with contemporary rates of infection ranging from 0.5% to 10.6%.47,48 Interestingly, however, the only two studies to directly evaluate the impact of InhibiZone™ on infection found no significant effect.47,49 Infection may occur early (within 6 weeks of surgery) or in delayed fashion (after 6 weeks) and is more common in patients with diabetes mellitus, smoking history, and those on anticoagulants.40,48 Regardless of timing, infection mandates urgent device removal. Infection may occur concurrently with cuff erosion, which also requires device explant. Risk factors for erosion include history of pelvic radiation, hypogonadism, hypertension, diabetes, and prolonged indwelling catheter duration, among others.50
Overall device survival of the AUS is around 90% at one year, 74–80% at five years, 57% at ten years, and 41% at 15 years.40 Reasons for revision include mechanical failure (leak, tubing fracture, pump malfunction) and non-mechanical failure (infection, component erosion, urethral atrophy). Mechanical failure may be responsible for 39–48% of revisions; while malfunction can occur at any component, it is most common at the urethral cuff and PRB.51–53 Rates of long-term infection and erosion are 1–10% and 4–19%, respectively, both of which require device explant and a return to a state of florid incontinence.29,30,32,54 Some patients also experience recurrent incontinence due to urethral atrophy; various techniques have been described to manage this entity including cuff downsizing or repositioning, capsulotomy, and use of advanced techniques such as tandem cuffs and transcorporal cuff placement to restore continence.55–58 At our institution, we begin by measuring the urethra after cuff removal; if this measurement is appropriate for a 4.0cm cuff or larger, we will simply install a new cuff at the same site. If, however, the urethra measures 3.5cm or below, we preferentially relocate the cuff to a new, more proximal location. If this is not possible, then we prefer a transcorporal approach.
Over the past few years, multiple competing devices have been introduced, including the Zephyr ZSI 375 (Zephyr Surgical Implants, Geneva, Switzerland), and the VICTO (Promedon, Cordoba, Argentina). The former consists of a pre-connected device with an inflatable cuff which can be adjusted for continence after the initial post-operative period.59 Success rates as high as 91.8% have been reported, but with 22.6% of patients requiring explant during the 24-month follow-up.60 The VICTO is another adjustable sphincter device which features a subcutaneous adjustment port; this allows percutaneous adjustment of fluid levels as needed for better continence results. Literature has suggested that the VICTO has a post-surgery continence rate of 80%. Neither device is currently approved for use in the US.
Fixed Male Slings
Fixed male slings are an alternative option for patients with mild-to-moderate SUI. There is significant variability in sling design, with two main types currently on the market in the US today: the AdVance XP sling (Boston Scientific, Minnetonka, MN, USA) and the Virtue quadratic sling (Coloplast, Humlebaek, Denmark).
The AdVance sling is a self-anchoring polypropylene mesh sling which is positioned around the proximal bulbar urethra and placed via a transobturator approach. Continence is achieved not by compression but by a 3–4cm proximal elevation of the bulbar urethra, which lengthens the functional membranous urethra.61 The second iteration – the AdVance XP – was introduced in 2010 and features polypropylene barbs to minimize slippage. The Virtue sling, by contrast, was designed to provide both urethral elevation and compression and features a broader sling that suspends the entire bulbar urethra. This is achieved via four mesh arms, two of which are transobturator and provide urethral elevation and two of which are prepubic and provide urethral compression.
Regardless of sling design, overall success rates are similar and have been reported from 70 to 90% (Table 2). The published definitions of success are variable, with definitions ranging from improvement with no more than 1 pad per day leakage to complete cure.62,63 Success is inversely correlated with SUI severity, with 80–90% success rates for mild incontinence, even outperforming the AUS.64 Conversely, for those with moderate SUI, the AUS has been shown to outperform the sling, with sling success rates only reported at 32–83%.12,16,65 One question has been the durability of these results; one study found that although 82.7% of patients were dry at 12 months after Virtue sling placement, this declined to only 58.6% at 36 months.61,66 Despite this, patient satisfaction has been shown to be maintained over time, which may be a testament to pre-operative counseling.66,67
 |
Table 2 Outcomes of urethral sling placement |
Overarchingly, the key to success with sling placement is appropriate patient selection. Slings should be reserved for men with “mild incontinence”, defined variably as 1 PPD leakage, 24-hour pad weight of less than 400cc, or at our institution, standing cough test of MSIGS 1.12,78 Others reserve slings for those with elevated Valsalva leak point pressure as determined by urodynamics. Increasing SUI severity has been associated with worse outcomes, and therefore this evaluation is paramount.79,80 Multiple studies have shown a higher failure rate in patients with history of radiation, with some reporting failure rates as high as 50%.62,69 Some authors also recommend performing in-office cystoscopy on any patient considering a sling.81 This evaluation includes confirmation of sphincter competence, as well as appropriate urethral coaptation with perineal pressure.78 In our own practice, we utilize the following selection criteria: a patient with one pad per day SUI, standing cough test of MSIGS 1, and no history of radiation. If there is any question, cystoscopy to confirm intact sphincter mechanism and good urethral mobility with perineal pressure can also be performed.
We primarily utilize the AdVance XP at our institution and have found that by following a few tenets we can achieve durable results. Intra-operatively, care must be taken to position the sling appropriately, which requires taking down the central tendon to ensure appropriate urethral mobility upon sling tensioning. Extra time is taken to ensure that the trocar passes as anteriorly as possible via the obturator foramen; we have found that too-proximal placement of the sling can paradoxically worsen incontinence due to distortion of the membranous urethra. Intra-operative complications – such as damage to the bladder or urethra with trocar passage – are uncommon, and the risk decreased by emptying the bladder prior to trocar passage and controlled guidance of the needle over the tip of the surgeon’s finger. Bleeding from the trocar sites tends to be self-limited and controlled with pressure alone; while we have not required these adjunctive maneuvers, others have described the instillation of hemostatic agents via the sling sheath. We leave a catheter overnight and have the patient remove this the following morning.81,82
The reported post-operative complication rates for slings of any type in the literature vary widely from 1 to 45%.65 Pelvic pain is the most common complaint, with rates as high as 45% reported; however, this tends to be self-limited and mild.82 Rates of urinary retention vary from 3 to 23% and depend in part on sling selection. For example, retention rates have been reported to be slightly higher for the AdVanceXP model compared to its AdVance predecessor.82 Regardless, retention is typically transient, with most patients passing a void trial after 5–7 days of urethral catheterization.83 Other complications, such as urethral erosion and infection, are quite rare. The risk of infection is lower when compared to AUS, ranging from 1 to 3%. Revision rates are reported at approximately 1%, another benefit compared to the AUS.61,83
Overall, male urethral slings provide an excellent tool in the urologist’s armamentarium for management of mild stress incontinence. Given the similar success rates of the various models, we counsel surgeons to use the technique with which they are most comfortable. The key, however, is proper patient selection and counseling; in the appropriate setting, success and satisfaction rates are high.
Adjustable Male Slings
Multiple adjustable male slings have been proposed, including – but not limited to – the Adjustable Trans-Obturator Male Sling (ATOMS), Argus, and Readjustment Mechanical External (REMEEX) System.84 The theoretical advantage of such systems is the ability to adjust the level of urethral compression provided without requiring additional surgery. Currently, the REMEEX sling is the only model available in the US; this device consists of a modified suburethral sling which is situated at the proximal bulbar urethra via a retropubic approach. The design features adjustable sutures which are tensioned at the rectus fascia with a varitensor device. Tension may be adjusted in the office setting.
At this time, studies regarding the REMEEX system are small and lacking in long-term follow-up. One meta-analysis found a dry rate of 53% for the system, with improvement rates of 80%.85 However, complication rate was relatively high at 36% with pooled explant rates of 14%. Further multi-center studies with long-term follow-up are clearly needed in this space.
Adjustable Balloon Devices
In 2015, the ProACT Adjustable Continence Therapy System received pre-market approval by the FDA for management of men with stress incontinence after radical prostatectomy or transurethral resection of the prostate.86 The system consists of two separate devices which are placed percutaneously under fluoroscopic guidance via the perineum to the level of the bladder neck. Each device consists of a self-sealing volume adjustment port, a balloon, and connective tubing.86,87 During implantation, each balloon is filled with about 1.0mL of isotonic solution (sterile water and contrast) in order to provide compression at the bladder neck. The volume in each balloon may then be adjusted at post-operative visits (typically starting at around 6 weeks) until the desired level of continence is obtained.
Eighteen-month follow-up on the initial United States trial demonstrated significant improvement in 24-hour pad weight from 399g at baseline to 160g at the 18-month follow-up visit. Results were found to be similar across all levels of SUI severity, with 61% of patients experiencing a pad weight reduction of at least 50%. Mean reported pads per day were reduced from 4.1 pre-op to 2.2 at 18-month follow-up. Notably, the study showed a high device explant rate of 24.2% during follow-up, most commonly due to device migration. Even so, 22/30 (73%) of patients elected to undergo re-implantation of the device. With regard to safety, one in five patients in this study experienced a procedure-related adverse event (AE), including bladder or urethral perforation during implant in 16/124, 13%.
A more recent meta-analysis suggests potentially even better outcomes, with daily pad count reduced from 4.0 to 1.1 over a mean follow-up for 3.6 years.88 A full 60% of patients were considered “dry”, with 82% of patients showing at least 50% improvement. Similar to the male slings, studies on the ProACT system suggest that patients with history of pelvic radiation may see lower efficacy when compared to their non-irradiated counterparts.89–91 Likewise, they appear to be at higher risk of urethral erosion, which requires explant of the affected device.89,90,92 However, re-implantation is possible after healing of the affected tissues.93 Alternatively, patients may elect for placement of an AUS instead.
Given these promising results, it seems likely that the ProACT system will continue to gain popularity in the United States as it has done in Europe. Despite relatively high complication and explantation rates, the system offers a minimally invasive alternative to the AUS for patients with severe incontinence that is not limited by patient dexterity or cognition. However, studies with longer-term follow-up will be required to assess the durability of these outcomes and the devices themselves.
Innovations on the Horizon
Ongoing device innovation will keep the field of male stress urinary incontinence an exciting one for years to come. Hardly a week goes by in our clinic when a patient does not inquire about remote technology; animal studies are currently being conducted by Boston Scientific on a Bluetooth-activated version of the AMS 800 device.94 In addition to the convenience of a phone-controlled sphincter, this would also expand the potential patient population to those with limited dexterity or poor pinch strength, for whom the AMS 800 is not an option. Furthermore, decreased manual manipulation of the pump might theoretically lead to decreased inflammation and mechanical strain on the system. In addition, competitors such as Rigicon (Innovative Urological Solutions, Ronkonkoma, NY, USA) are currently pursuing trials of alternative devices including the ContiClassic® and ContiReflex® artificial urinary sphincters to challenge the AMS 800™.95
Refinement and innovation of urethral slings continues to evolve. For example, the Andrianne Mini-Jupette sling has gained popularity in patients with ED undergoing IPP who also suffer from climacturia or mild SUI. This technique involves securing a graft of the surgeon’s choice to the medial aspect of the corporotomies prior to corporotomy closure. Yafi et al recently evaluated this approach specifically in men with ED and mild-to-moderate SUI; they found improvement of SUI in 80% and resolution in 60%, with only 3/35 ultimately going on to subsequent AUS placement.96,97 Valenzuela et al explored a modification to this technique (the so-called “male urethral mini-sling” or MUMS) using a tailored Virtue mesh with the sling arms removed. Subjective SUI improved in 85% of patients with a 59% cure rate.75 One patient in this study experienced mesh erosion and required explant and primary urethral repair.
Other agents currently being studied include urethral bulking agents and stem cell therapy. Urethral bulking agents have been utilized in treatment of SUI for decades; however, poor efficacy and durability in the setting of male SUI have led to the AUA recommending against their use.19,98 Research continues to be ongoing in this area, however, and perhaps improvement in technique or injection material will lead to a resurgence of their use.99 Both muscle and adipose stem cell research show promise, with a goal of regenerating sphincter myoblasts and providing bulk to the periurethral space, respectively.100,101 However, studies remain in their infancy, and stem cell utility remains to be seen.
Conclusion
The artificial sphincter and urethral sling continue to be the mainstay of surgical management in the United States for men suffering from stress urinary incontinence. The question is not one of “which treatment is better” but rather “which treatment is better when”. Upfront evaluation of incontinence severity and discussion of patient goals is crucial to appropriate counseling. In those with mild SUI and no history of radiation, one of the three commercially available urethral slings may be an excellent option. For those with more severe incontinence – or other risk factors – the AUS remains the gold standard. Patients who have an unclear or inconsistent history should undergo further objective workup including in-office cystoscopy and/or urodynamics where appropriate. Alternatives such as adjustable male slings and adjustable balloon devices show promise, but more data is needed on these newer modalities.
Abbreviations
SUI, stress urinary incontinence; BPH, benign prostatic hyperplasia; QOL, quality of life; AUS, artificial urinary sphincter; PPD, pads per day; SCT, standing cough test; MSIGS, Male Stress Incontinence Grading Scale; UUI, urge incontinence; PRB, pressure regulating balloon; SOR, Space of Retzius; HSM, high submuscular; REMEEX, Readjustment Mechanical External; MUMS, male urethral mini-sling.
Disclosure
Dr. Allen Morey receives honoraria for being a guest lecturer/meeting participant for Boston Scientific and Coloplast. The other authors report no potential conflicts of interest in this work.
References
1. Cao C, Zhang C, Sriskandarajah C, et al. Trends and racial disparities in the prevalence of urinary incontinence among men in the USA, 2001–2020. Eur Urol Focus. 2022;8(6):1758–1767. doi:10.1016/j.euf.2022.04.015
2. Boorjian SA, Eastham JA, Graefen M, et al. A critical analysis of the long-term impact of radical prostatectomy on cancer control and function outcomes. Eur Urol. 2012;61(4):664–675. doi:10.1016/j.eururo.2011.11.053
3. Trost L, Elliott DS. Male stress urinary incontinence: a review of surgical treatment options and outcomes. Adv Urol. 2012;2012:1–13. doi:10.1155/2012/287489
4. Kim BH, Byun HJ. Robotic-assisted simple prostatectomy versus holmium laser enucleation of the prostate for large benign prostatic hyperplasia: a single-center preliminary study in Korea. Prostate Int. 2022;10(3):123–128. doi:10.1016/j.prnil.2022.05.004
5. Madalinska JB, Essink-Bot M-L, de Koning HJ, Kirkels WJ, van der Maas PJ, Schröder FH. Health-related quality-of-life effects of radical prostatectomy and primary radiotherapy for screen-detected or clinically diagnosed localized prostate cancer. J Clin Oncol. 2001;19(6):1619–1628. doi:10.1200/JCO.2001.19.6.1619
6. Bong J, de Gregorio G, Schuth W. Quality-of-life-Fragebogen bei Patientinnen mit Harninkontinenz. Geburtshilfe Frauenheilkd. 1998;58(11):597–604. doi:10.1055/s-2007-1023008
7. Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50(6):1306–1315. doi:10.1016/j.eururo.2006.09.019
8. Wilson L, Brown JS, Shin GP, Luc K-O, Subak LL. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98(3):398–406.
9. Bhanvadia R, Dropkin B, Wolfe A, et al. Artificial urinary sphincter reverses weight gain associated with post-prostatectomy incontinence. J Sex Med. 2022;19(4):S81–S82. doi:10.1016/j.jsxm.2022.01.172
10. Drossman DA, Li Z, Andruzzi E, et al. US householder survey of functional gastrointestinal disorders. Dig Dis Sci. 1993;38(9):1569–1580. doi:10.1007/BF01303162
11. Koch GE, Kaufman MR. Male stress urinary incontinence. Urol Clin. 2022;49(3):403–418. doi:10.1016/j.ucl.2022.04.005
12. Khouri RK
13. Krhut J, Zachoval R, Smith PP, et al. Pad weight testing in the evaluation of urinary incontinence. Neurourol Urodyn. 2014;33(5):507–510. doi:10.1002/nau.22436
14. Jura YH, Comiter CV. Urodynamics for postprostatectomy incontinence: when are they helpful and how do we use them? Urol Clin. 2014;41(3):419–427. doi:10.1016/j.ucl.2014.04.002
15. Fuchs JS, Shakir N, McKibben MJ, Scott JM, Morey AF. Prolonged duration of incontinence for men before initial anti-incontinence surgery: an opportunity for improvement. Urology. 2018;119:149–154. doi:10.1016/j.urology.2018.05.006
16. Shakir NA, Fuchs JS, McKibben MJ, et al. Refined nomogram incorporating standing cough test improves prediction of male transobturator sling success. Neurourol Urodyn. 2018;37(8):2632–2637. doi:10.1002/nau.23703
17. Sacco E, Bientinesi R, Gandi C, et al. Patient pad count is a poor measure of urinary incontinence compared with 48‐h pad test: results of a large‐scale multicentre study. BJU Int. 2019;123(5A):E69–E78. doi:10.1111/bju.14566
18. Yi YA, Keith CG, Graziano CE, et al. Strong correlation between standing cough test and 24‐hour pad weights in the evaluation of male stress urinary incontinence. Neurourol Urodyn. 2020;39(1):319–323. doi:10.1002/nau.24200
19. Sandhu JS, Breyer B, Comiter C, et al. Incontinence after prostate treatment: AUA/SUFU guideline. J Urol. 2019;202(2):369–378. doi:10.1097/JU.0000000000000314
20. Brede C, Angermeier K, Wood H. Continence outcomes after treatment of recalcitrant postprostatectomy bladder neck contracture and review of the literature. Urology. 2014;83(3):648–652. doi:10.1016/j.urology.2013.10.042
21. Branche B, Crocerossa F, Carbonara U, et al. Management of bladder neck contracture in the age of robotic prostatectomy: an evidence-based guide. Eur Urol Focus. 2021;8(1):297–301. doi:10.1016/j.euf.2021.01.007
22. Bang SL, Yallappa S, Dalal F, Almallah YZ. Post prostatectomy Vesicourethral stenosis or bladder neck contracture with concomitant urinary incontinence: our experience and recommendations. Curr Urol. 2016;10(1):32–39. doi:10.1159/000447148
23. Gousse AE, Tunuguntla HS, Leboeuf L. Two-stage management of severe postprostatectomy bladder neck contracture associated with stress incontinence. Urology. 2005;65(2):316–319. doi:10.1016/j.urology.2004.09.014
24. Lee A, Mmonu NA, Thomas H, Rios N, Enriquez A, Breyer BN. Qualitative analysis of Amazon customer reviews of penile clamps for male urinary incontinence. Neurourol Urodyn. 2021;40(1):384–390. doi:10.1002/nau.24572
25. Hajivassiliou C. The development and evolution of artificial urethral sphincters. J Med Eng Technol. 1998;22(4):154–159. doi:10.3109/03091909809032533
26. Carson CC. Artificial urinary sphincter: current status and future directions. Asian J Androl. 2020;22(2):154. doi:10.4103/aja.aja_5_20
27. Islah M, Cho SY, Son H. The current role of the artificial urinary sphincter in male and female urinary incontinence. World J Men’s Health. 2013;31(1):21–30. doi:10.5534/wjmh.2013.31.1.21
28. Petero VG
29. Linder BJ, Piotrowski JT, Ziegelmann MJ, Rivera ME, Rangel LJ, Elliott DS. Perioperative complications following artificial urinary sphincter placement. J Urol. 2015;194(3):716–720. doi:10.1016/j.juro.2015.02.2945
30. Brant WO, Martins FE. Artificial urinary sphincter. Transl Androl Urol. 2017;6(4):682. doi:10.21037/tau.2017.07.31
31. Wilson S, Delk J
32. Brant WO, Erickson BA, Elliott SP, et al. Risk factors for erosion of artificial urinary sphincters: a multicenter prospective study. Urology. 2014;84(4):934–939. doi:10.1016/j.urology.2014.05.043
33. Morey AF, Cefalu CA, Hudak SJ. High submuscular placement of urologic prosthetic balloons and reservoirs via transscrotal approach. J Sex Med. 2013;10(2):603–610. doi:10.1111/jsm.12000
34. Bansal UK, Lopez JP, Flores-Sandoval FN, Khera M. Ectopic low submuscular pressure regulating balloon placement with transfascial fixation for artificial urinary sphincter. Can J Urol. 2021;28(6):10936–10940.
35. Mykoniatis I, Osmonov D, van Renterghem K. A modified surgical technique for reservoir placement during inflatable penile prosthesis implantation. Sex Med. 2020;8(3):378–382. doi:10.1016/j.esxm.2020.04.004
36. Guillaumier S, Solomon E, Jenks J, et al. Radiotherapy is associated with reduced continence outcomes following implantation of the artificial urinary sphincter in men with post-radical prostatectomy incontinence. Urol Ann. 2017;9(3):253. doi:10.4103/UA.UA_25_17
37. Lavi A, Boone TB, Cohen M, Gross M. The patient beyond the sphincter–cognitive and functional considerations affecting the natural history of artificial urinary sphincters. Urology. 2020;137:14–18. doi:10.1016/j.urology.2019.11.031
38. Scott FB, Bradley WE, Timm GW. Treatment of urinary incontinence by an implantable prosthetic urinary sphincter. J Urol. 1974;112(1):75–80. doi:10.1016/S0022-5347(17)59647-0
39. Tutolo M, Cornu JN, Bauer RM, et al. Efficacy and safety of artificial urinary sphincter (AUS): results of a large multi‐institutional cohort of patients with mid‐term follow‐up. Neurourol Urodyn. 2019;38(2):710–718. doi:10.1002/nau.23901
40. Linder BJ, Rivera ME, Ziegelmann MJ, Elliott DS. Long-term outcomes following artificial urinary sphincter placement: an analysis of 1082 cases at Mayo Clinic. Urology. 2015;86(3):602–607. doi:10.1016/j.urology.2015.05.029
41. Deruyver Y, Schillebeeckx C, Beels E, Ridder DD, Van der Aa F. Long-term outcomes and patient satisfaction after artificial urinary sphincter implantation. World J Urol. 2022;40(2):497–503. doi:10.1007/s00345-021-03877-1
42. Hebert KJ, Linder BJ, Morrisson GT, Latuche LR, Elliott DS. A comparison of artificial urinary sphincter outcomes after primary implantation and first revision surgery. Asian J Urol. 2021;8(3):298–302. doi:10.1016/j.ajur.2021.03.003
43. Linder BJ, Rangel LJ, Elliott DS. Evaluating success rates after artificial urinary sphincter placement: a comparison of clinical definitions. Urology. 2018;113:220–224. doi:10.1016/j.urology.2017.10.033
44. Sacco E, Gandi C, Marino F, et al. Artificial urinary sphincter significantly better than fixed sling for moderate post-prostatectomy stress urinary incontinence: a propensity score-matched study. BJU Int. 2021;127(2):229–237. doi:10.1111/bju.15197
45. Sacomani CAR, Zequi SC, Costa WH, et al. Long-term results of the implantation of the AMS 800 artificial Sphincter for post-prostatectomy incontinence: a single-center experience. BJU Int. 2018;44(1):114–120.
46. Sayedahmed K, Olianas R, Kaftan B, et al. Impact of previous urethroplasty on the outcome after artificial urinary sphincter implantation: a prospective evaluation. World J Urol. 2019;38(1):183–191. doi:10.1007/s00345-019-02756-0
47. Hüsch T, Kretschmer A, Thomsen F, et al. Antibiotic coating of the artificial urinary sphincter (AMS 800): is it worthwhile? Urology. 2017;103:179–184. doi:10.1016/j.urology.2016.12.056
48. James MH, McCammon KA. Artificial urinary sphincter for post‐prostatectomy incontinence: a review. Int J Urol. 2014;21(6):536–543. doi:10.1111/iju.12392
49. de Cógáin MR, Elliott DS. The impact of an antibiotic coating on the artificial urinary sphincter infection rate. J Urol. 2013;190(1):113–117. doi:10.1016/j.juro.2013.01.015
50. Diao L, Nealon SW, Carpinito GP, et al. Presenting signs and symptoms of artificial urinary sphincter cuff erosion. Int Braz J Urol. 2022;48:679–685. doi:10.1590/s1677-5538.ibju.2022.0089
51. Vandyke ME, Johnson BE, Ali SS, Langford BT, Franzen BP, Morey AF. MP71-11 artificial urinary sphincter cuff is most common site of mechanical failure. J Urol. 2023;209(Supplement 4):e1018. doi:10.1097/JU.0000000000003339.11
52. Elliott DS, Barrett DM. Mayo Clinic long-term analysis of the functional durability of the AMS 800 artificial urinary sphincter: a review of 323 cases. J Urol. 1998;159(4):1206–1208. doi:10.1016/S0022-5347(01)63557-2
53. Srivastava A, Joice GA, Patel HD, Manka MG, Sopko NA, Wright EJ. Causes of artificial urinary sphincter failure and strategies for surgical revision: implications of device component survival. Eur Urol Focus. 2019;5(5):887–893. doi:10.1016/j.euf.2018.02.014
54. Singla N, Siegel JA, Simhan J, et al. Does pressure regulating balloon location make a difference in functional outcomes of artificial urinary sphincter? J Urol. 2015;194(1):202–206. doi:10.1016/j.juro.2015.01.115
55. Pearlman AM, Rasper AM, Terlecki RP. Proof of concept: exposing the myth of urethral atrophy after artificial urinary sphincter via assessment of circumferential recovery after capsulotomy and intraoperative pressure profiling of the pressure regulating balloon. Investig Clin Urol. 2018;59(4):275–279. doi:10.4111/icu.2018.59.4.275
56. DiMARCO DS, Elliott DS. Tandem cuff artificial urinary sphincter as a salvage procedure following failed primary sphincter placement for the treatment of post-prostatectomy incontinence. J Urol. 2003;170(4 Part 1):1252–1254. doi:10.1097/01.ju.0000085787.21140.db
57. Guralnick ML, Miller E, Toh KL, Webster GD. Transcorporal artificial urinary sphincter cuff placement in cases requiring revision for erosion and urethral atrophy. J Urol. 2002;167(5):2075–2079. doi:10.1016/S0022-5347(05)65088-4
58. Heah NH, Tan RB. Management of urethral atrophy after implantation of artificial urinary sphincter: what are the weaknesses? Asian J Androl. 2020;22(1):60. doi:10.4103/aja.aja_110_19
59. Montague DK, Angermeier KW, Paolone DR. Long-term continence and patient satisfaction after artificial sphincter implantation for urinary incontinence after prostatectomy. J Urol. 2001;166(2):547–549. doi:10.1016/S0022-5347(05)65981-2
60. Ostrowski I, Ciechan J, Sledz E, Dys W, Golabek T, Chłosta PL. Four-year follow-up on a Zephyr Surgical Implants 375 artificial urinary sphincter for male urinary incontinence from one urological centre in Poland. Cent Eur J Urol. 2018;71(3):320. doi:10.5173/ceju.2018.1704
61. Llorens C, Pottek T. Urinary artificial sphincter ZSI 375 for treatment of stress urinary incontinence in men: 5 and 7 years follow-up report. Urologia J. 2017;84(4):263–266. doi:10.5301/uj.5000243
62. Ameli G, Weibl P, Rutkowski M, Hübner WA. PD37-08 A single center study to evaluate the efficacy and safety of a new adjustable artificial urinary sphincter (VICTO): results after follow-up> 12 months. J Urol. 2020;203:e731. doi:10.1097/JU.0000000000000908.08
63. Rizvi IG, Ravindra P, Pipe M, Sohawon R, King T, Belal M. The AdVance™ male sling: does it stand the test of time? Scand J Urol. 2021;55(2):155–160. doi:10.1080/21681805.2021.1877342
64. Grabbert M, Mumm JN, Klehr B, et al. Extended follow‐up of the AdVance XP male sling in the treatment of male urinary stress incontinence after 48 months: results of a prospective and multicenter study. Neurourol Urodyn. 2019;38(7):1973–1978. doi:10.1002/nau.24101
65. Ye H, Haab F, de Ridder D, et al. Effectiveness and complications of the AMS AdVance™ male sling system for the treatment of stress urinary incontinence: a prospective multicenter study. Urology. 2018;120:197–204. doi:10.1016/j.urology.2018.06.035
66. Davies TO, Bepple JL, McCammon KA. Urodynamic changes and initial results of the AdVance male sling. Urology. 2009;74(2):354–357. doi:10.1016/j.urology.2008.12.082
67. Bole R, Hebert KJ, Gottlich HC, Bearrick E, Kohler TS, Viers BR. Narrative review of male urethral sling for post-prostatectomy stress incontinence: sling type, patient selection, and clinical applications. Transl Androl Urol. 2021;10(6):2682. doi:10.21037/tau-20-1459
68. Abdullah A, Machkour F, Bouchet E, Plainard X, Descazeaud A. Efficacy of the VIRTUE male quadratic sling in the treatment of stress urinary incontinence: a retrospective study. Prog Urol. 2019;29(10):490–495. doi:10.1016/j.purol.2019.07.004
69. Ajay D, Kahokehr AA, Lentz AC, Peterson AC. Valsalva leak point pressure (VLPP) greater than 70 cm H2O is an indicator for sling success: a success prediction model for the male transobturator sling. Int Urol Nephrol. 2022;2022:1–5.
70. Chua ME, Zuckerman J, Mason JB, et al. Long-term success durability of transobturator male sling. Urology. 2019;133:222–228. doi:10.1016/j.urology.2019.07.032
71. Giammo A, Ammirati E, Tullio A, et al. Implant of ATOMS® system for treatment of postoperative male stress urinary incontinence: results of a single centre. BJU Int. 2019;45(1):127–136.
72. Marquez-Sanchez GA, Padilla-Fernandez BY, Peran-Teruel M, et al. Remeex® system effectiveness in male patients with stress urinary incontinence. J Clin Med. 2021;10(10):2121. doi:10.3390/jcm10102121
73. Mumm JN, Klehr B, Rodler S, et al. Five-year results of a prospective multicenter trial: AdVance XP for postprostatectomy-incontinence in patients with favorable prognostic factors. Urol Int. 2021;105(5–6):421–427. doi:10.1159/000512881
74. Queissert F, Rourke K, Schonburg S, et al. ATOMS (adjustable transobturator male system) is an effective and safe second-line treatment option for recurrent urinary incontinence after implantation of an AdVance/AdVance XP fixed male sling? A multicenter cohort analysis. J Clin Med. 2021;11(1):81. doi:10.3390/jcm11010081
75. Towe M, El-Khatib F, Osman M, Choi J, Yafi FA. The use of autologous fascia in the Mini-Jupette graft: two cases. Int J Impot Res. 2020;32(1):140–141. doi:10.1038/s41443-019-0178-z
76. Wright HC, McGeagh K, Richter LA, et al. Transobturator sling for post-prostatectomy incontinence: radiation’s effect on efficacy/satisfaction. Can J Urol. 2017;24(5):8998–9002.
77. Bauer RM, Grabbert MT, Klehr B, et al. 36-month data for the AdVance XP® male sling: results of a prospective multicentre study. BJU Int. 2017;119(4):626–630. doi:10.1111/bju.13704
78. Ferro M, Bottero D, D’Elia C, et al. Virtue male sling for post‐prostatectomy stress incontinence: a prospective evaluation and mid‐term outcomes. BJU Int. 2017;119(3):482–488. doi:10.1111/bju.13672
79. Doudt AD, Zuckerman JM. Male slings for post-prostatectomy incontinence. Rev Urol. 2018;20(4):158. doi:10.3909/riu080
80. Fischer MC, Huckabay C, Nitti VW. The male perineal sling: assessment and prediction of outcome. J Urol. 2007;177(4):1414–1418. doi:10.1016/j.juro.2006.11.061
81. Morey AF. Re: a valsalva leak-point pressure of> 100 cmH2O is associated with greater success in AdVance™ sling placement for the treatment of post-prostatectomy urinary incontinence. J Urol. 2015;193(4):1316. doi:10.1016/j.juro.2015.02.010
82. Cornu JN, Sèbe P, Ciofu C, Peyrat L, Cussenot O, Haab F. Mid‐term evaluation of the transobturator male sling for post‐prostatectomy incontinence: focus on prognostic factors. BJU Int. 2011;108(2):236–240. doi:10.1111/j.1464-410X.2010.09765.x
83. Inouye BM, Premo HA, Weil D, Peterson AC. The male sling for stress urinary incontinence: tips and tricks for success. Int Braz J Urol. 2021;47:1131–1135. doi:10.1590/s1677-5538.ibju.2020.1122
84. Prebay ZJ, Foss HE, Wang KR, Chung PH. A narrative review on surgical treatment options for male stress urinary incontinence. Transl Androl Urol. 2023;12(5):874–886. doi:10.21037/tau-22-629
85. Angulo JC, Ruiz S, Lozano M, Arance I, Virseda M, Lora D. Systematic review and meta-analysis comparing Adjustable Transobturator Male System (ATOMS) and male Readjustment Mechanical External (REMEEX) system for post-prostatectomy incontinence. World J Urol. 2021;39(4):1083–1092. doi:10.1007/s00345-020-03300-1
86. Nash S, Aboseif S, Gilling P, et al. Four‐year follow‐up on 68 patients with a new post‐operatively adjustable long‐term implant for post‐prostatectomy stress incontinence: proACT™. Neurourol Urodyn. 2019;38(1):248–253. doi:10.1002/nau.23838
87. Nash S, Aboseif S, Gilling P, et al. Treatment with an adjustable long‐term implant for post‐prostatectomy stress incontinence: the ProACT™ pivotal trial. Neurourol Urodyn. 2018;37(8):2854–2859. doi:10.1002/nau.23802
88. Larson T, Jhaveri H, Yeung LL. Adjustable continence therapy (ProACT) for the treatment of male stress urinary incontinence: a systematic review and meta‐analysis. Neurourol Urodyn. 2019;38(8):2051–2059. doi:10.1002/nau.24135
89. Rouprêt M, Misraï V, Gosseine P-N, Bart S, Cour F, Chartier-Kastler E. Management of stress urinary incontinence following prostate surgery with minimally invasive adjustable continence balloon implants: functional results from a single center prospective study. J Urol. 2011;186(1):198–203. doi:10.1016/j.juro.2011.03.016
90. Gregori A, Romano AL, Scieri F, et al. Transrectal ultrasound–guided implantation of adjustable continence therapy (ProACT): surgical technique and clinical results after a mean follow-up of 2 years. Eur Urol. 2010;57(3):430–436. doi:10.1016/j.eururo.2009.11.031
91. Lebret T, Cour F, Benchetrit J, et al. Treatment of postprostatectomy stress urinary incontinence using a minimally invasive adjustable continence balloon device, ProACT: results of a preliminary, multicenter, pilot study. Urology. 2008;71(2):256–260. doi:10.1016/j.urology.2007.08.062
92. Venturino L, Dalpiaz O, Pummer K, Primus G. Adjustable continence balloons in men: adjustments do not translate into long-term continence. Urology. 2015;85(6):1448–1453. doi:10.1016/j.urology.2015.01.045
93. Kjær L, Fode M, Nørgaard N, Sønksen J, Nordling J. Adjustable continence balloons: clinical results of a new minimally invasive treatment for male urinary incontinence. Scand J Urol Nephrol. 2012;46(3):196–200. doi:10.3109/00365599.2012.660986
94. Hüsch T, Kretschmer A, Thomsen F, et al. The AdVance and AdVanceXP male sling in urinary incontinence: is there a difference? World J Urol. 2018;36(10):1657–1662. doi:10.1007/s00345-018-2316-5
95. Chung E, Liao L, Kim JH, et al. The Asia‐Pacific AMS800 artificial urinary sphincter consensus statement. Int J Urol. 2023;30(2):128–138. doi:10.1111/iju.15083
96. Ko KJ, Kim SJ, Cho ST. Sling surgery for male urinary incontinence including post prostatectomy incontinence: a challenge to the urologist. Int Neurourol J. 2019;23(3):185. doi:10.5213/inj.1938108.054
97. Biardeau X, Hached S, Loutochin O, Campeau L, Sawan M, Corcos J. Montreal electronic artificial urinary sphincters: our futuristic alternatives to the AMS800™. Can Urol Assoc J. 2017;11(10):E396. doi:10.5489/cuaj.4493
98. Yafi FA, Andrianne R, Alzweri L, et al. Andrianne mini-jupette graft at the time of inflatable penile prosthesis placement for the management of post-prostatectomy climacturia and minimal urinary incontinence. J Sex Med. 2018;15(5):789–796. doi:10.1016/j.jsxm.2018.01.015
99. Valenzuela RJ, Ziegelmann MJ, Hillelsohn JH, Farrell MR, Kent MA, Levine LA. Preliminary outcomes of the male urethral “mini-sling”: a modified approach to the Andrianne Mini-Jupette procedure with penile prosthesis placement for climacturia and mild stress urinary incontinence. J Sex Med. 2019;16(8):1310–1317. doi:10.1016/j.jsxm.2019.04.009
100. Herschorn S, Bruschini H, Comiter C, et al. Surgical treatment of stress incontinence in men. Neurourol Urodyn. 2010;29(1):179–190. doi:10.1002/nau.20844
101. Kerr LA. Bulking agents in the treatment of stress urinary incontinence: history, outcomes, patient populations, and reimbursement profile. Rev Urol. 2005;7(Suppl 1):S3.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
