Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Serum Folate and Vitamin B12 Modify the Associations of N6AMT1 Genetic Variants with Gestational Diabetes Mellitus: A Cross-Sectional Study in Chinese Pregnant Women
Authors Guo G, Chen X, Zhang J, Meng X, Jia A, Xing X, Huang F, Zhang X, Liu J, Li S, Zhang Q
Received 5 January 2024
Accepted for publication 1 April 2024
Published 17 April 2024 Volume 2024:17 Pages 1781—1791
DOI https://doi.org/10.2147/DMSO.S451045
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Guanshuai Guo,1,2,* Xi Chen,1,2,* Jingran Zhang,1,2 Xiangmin Meng,3 Aifeng Jia,4 Xinli Xing,5 Fenglei Huang,6 Xumei Zhang,2,7 Juan Liu,8 Shuying Li,3,9 Qiang Zhang1,2
1Department of Occupational and Environmental Health, School of Public Health, Tianjin Medical University, Tianjin, People’s Republic of China; 2Tianjin Key Laboratory of Environment, Nutrition and Public Health, School of Public Health, Tianjin Medical University, Tianjin, People’s Republic of China; 3Department of Endocrinology, Tianjin Xiqing Hospital, Tianjin, People’s Republic of China; 4Department of Obstetrics and Gynecology, Tianjin Xiqing Hospital, Tianjin, People’s Republic of China; 5Department of Obstetrics and Gynecology, Women’s and Children’s Health Center of Dongchangfu District, Liaocheng, People’s Republic of China; 6Department of Reproductive Health, Women’s and Children’s Health Center of Dongchangfu District, Liaocheng, People’s Republic of China; 7Department of Nutrition and Food Science, School of Public Health, Tianjin Medical University, Tianjin, People’s Republic of China; 8Department of Biomedical Information and Library, Tianjin Medical University, Tianjin, People’s Republic of China; 9NHC Key Laboratory of Hormones and Development, Tianjin Key Laboratory of Metabolic Diseases, Chu Hsien-I Memorial Hospital & Tianjin Institute of Endocrinology, Tianjin Medical University, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shuying Li, Department of Endocrinology, Tianjin Xiqing Hospital, Tianjin, 300380, People’s Republic of China, Email [email protected] Qiang Zhang, Department of Occupational and Environmental Health, School of Public Health, Tianjin Medical University, Tianjin, 300070, People’s Republic of China, Tel +86 022 83336633, Email [email protected]
Purpose: This study aimed to explore the association between N-6 adenine-specific DNA methyltransferase 1 (N6AMT1) single nucleotide polymorphisms (SNPs) and gestational diabetes mellitus (GDM) and the modification of the relationship by folate and vitamin B12.
Methods: A cross-sectional study involving 1303 pregnant women (262 GDM and 1041 non-GDM) was performed in Tianjin, China. Nine SNPs in N6AMT1 were genotyped, and serum folate, vitamin B12, and homocysteine (Hcy) levels were measured. The logistic regression models determined the odds ratios (ORs) for SNPs in N6AMT1 and the gene-nutrition interactions on GDM.
Results: N6AMT1 rs7282280, rs1048546, and rs1997605 were related to GDM under the dominant model after adjusting for multiple covariates. Individuals carrying the N6AMT1 rs7282280 TC/TT genotypes had a lower risk of developing GDM, regardless of serum folate and vitamin B12 levels. However, rs1048546 TG/GG genotypes were associated with lower GDM risk when serum folate ≥ 6.0 ng/mL. Pregnancies with the risk genotypes in N6AMT1 and higher serum folate or lower vitamin B12 are more prone to GDM. The study also showed a statistically significant additive interaction between N6AMT1 rs1997605 GG genotypes and lower vitamin B12 (RERI: 2.54; 95% CI: 0.17, 4.92).
Conclusion: SNPs in N6AMT1 were found to be associated with GDM, and serum folate and vitamin B12 levels can modify their associations.
Keywords: N6AMT1, folate, vitamin B12, gene-nutrition interaction, gestational diabetes mellitus
Introduction
Gestational diabetes mellitus (GDM) is the most prevalent metabolic complication that occurs during pregnancy. It is characterized by abnormal glucose tolerance that arises or is first detected during pregnancy. The global prevalence of GDM was 14.0%, and China’s was 14.8%.1,2 Studies indicate that GDM can have long-term adverse effects on the mother and their offspring, in addition to affecting the course of pregnancy.3 As the prevalence of GDM continues to rise, there is an urgent need for more cost-effective strategies to alleviate the burden on both the mother and child. The etiology of GDM remains to be fully understood. It is a multifactorial metabolic disorder resulting from genetic, epigenetic, and environmental factors.4,5
The most common nongenetic risk factors for GDM are older age, higher body mass index (BMI), parity, and family history of diabetes.3 Recent studies have shown that environmental pollutants (such as arsenic and air pollution) and nutrients involved in the one-carbon metabolism (OCM) pathway may be essential in GDM.6–10 Our previous study indicated that genetic variants in N-6-adenine-specific DNA methyltransferase 1 (N6AMT1) were associated with arsenic methylation metabolism. In addition, they can interact to affect GDM occurrence in the Chinese population. Interestingly, we observed a novel and independent association of N6AMT1 rs1997605 and rs1003671 with GDM.11 However, the relationship between other single nucleotide polymorphisms (SNPs) of the N6AMT1 gene and GDM remains unclear.
The N6AMT1 methyltransferase plays an essential role in arsenic methylation metabolism and has recently been identified as responsible for DNA N-6-methyladenine (6mA) modification.12–14 Abnormal epigenetic modifications, such as methylation of DNA or RNA, have been linked to GDM.15,16 Fortunately, these epigenetic modifications are reversible, making them promising targets for GDM prevention and therapy. DNA 6mA modification is a newly discovered epigenetic mark widespread among several species.12,17 This modification is produced by adding a methyl group from S-adenosy-L-methionine (SAM) to the sixth position of the adenine ring through specific methyltransferases, such as N6AMT1.18 DNA 6mA is emerging as crucial in regulating gene expression and cell defense. Dysregulation of DNA 6mA modification has been linked to embryogenesis, neuropsychiatric disorders, hypertension, chronic kidney disease, and tumorigenesis.19–23 A recent study reported that 6mA is enriched in mammalian mitochondrial DNA (mtDNA) and regulates the mitochondrial stress response.24 While the biological function of 6mA was initially studied, its role in metabolic disease, mainly the function of N6AMT1, has yet to be explored.
OCM is a biochemical pathway that provides SAM for the methylation reaction and is influenced by folate and other nutrients, such as vitamin B12, choline, and betaine.25 Homocysteine (Hcy) is the most commonly used biomarker of folate or vitamin B12 deficiency in the OCM pathway. The effects of micronutrient supplements, especially folate and vitamin B12, on GDM risk have received increasing attention worldwide. Some prospective studies have confirmed that excessive folic acid intake or higher blood folate increases the risk of GDM.26–28 Other studies found that lower blood vitamin B12 correlates with a higher GDM risk.29,30 Due to the interaction effect of folate and vitamin B12 on the OCM pathway, some studies have shown that the imbalance of folate and vitamin B12 (higher folate and lower vitamin B12) is associated with a higher GDM risk.31,32 Furthermore, folate and vitamin B12, involved in SAM generation, play essential roles in DNA methylation.
In light of previous research that has shown a correlation between N6AMT1 SNPs and GDM, it is of great interest to investigate whether micronutrients related to SAM generation may modify the association between N6AMT1 and the risk of GDM. In the present study, we further investigated the association between N6AMT1 genetic variants and GDM by expanding the study sample size and increasing the number of potential SNP loci. At the same time, we examined the modification effects of serum folate and vitamin B12 on the association between N6AMT1 SNPs and GDM and the gene-nutrition interactions on GDM occurrence.
Subjects, Materials and Methods
Study Population
The study design has been described in our previous studies.6,10 In brief, a total of 1505 pregnant women who underwent GDM screening between 24–28 weeks of gestation were recruited according to the following criteria: (1) older than 18 years; (2) have lived in Tianjin for one year and plan to live in Tianjin for the next six years (3) have no history of pre-pregnancy diabetes or previous GDM. Of the 1505 pregnancies, 202 were excluded for various reasons, such as being from ethnic minorities (n = 105), not having GDM (n = 38) and OCM results (n = 33), and missing covariate results (n = 26). This left 1303 subjects available for the subsequent analysis (Figure S1). All procedures involving human participants followed the ethical standards of the Helsinki Declaration of Ethical Principles, and the research proposal was approved by the Ethics Committee of Metabolic Diseases Hospital and Institute of Endocrinology, Tianjin Medical University (DXBYYhMEC2018-14) and Tianjin Xiqing Hospital (xqyyll-2020-07). Written informed consent was obtained from all pregnant women before participation in this study.
Sample Collection and Covariate Assessment
For GDM screening, a fasting venous blood sample was obtained from each pregnant woman. The samples and serum aliquots were sent to Tianjin Medical University for storage at −80 °C until further analysis. Well-trained interviewers used a structured questionnaire to gather baseline characteristics such as age, ethnicity, education, smoking and drinking habits, height, current and pre-pregnancy weight, parity, and family history of diabetes. Pre-pregnancy BMI (kg/m2) was estimated by dividing the weight (kg) by the square of height (m). Serum folate, vitamin B12, and Hcy were analyzed using a previously described method.10 Folate and vitamin B12 levels were determined through a chemiluminescence immunoassay system. Hcy levels were determined using an enzymatic cycling method with an automatic biochemical analyzer.
Diagnosis of GDM
Between 24 and 28 weeks of pregnancy, a 75-g oral glucose tolerance test (OGTT) screening test was conducted to check for GDM. The diagnosis of GDM is made if any one of the following values meets or exceeds the criteria set by the International Association of Diabetes and Pregnancy Study Groups (IADPSG): fasting plasma glucose (FPG) of 5.1 mmol/L or higher, 1-hour plasma glucose (1-h PG) of 10.0 mmol/L or higher, and 2-hour plasma glucose (2-h PG) of 8.5 mmol/L or higher.10
Genotyping of N6AMT1 SNPs
According to literature reports, seven additional SNPs were detected in N6AMT1 (rs1006903, rs1003671, rs4816333, rs7282280, rs7282257, rs1048546, rs1997605, rs2738966, rs2248501), in addition to the two SNPs (rs1997605 and rs1003671) previously studied.13,14,33 The RelaxGene Blood DNA System was used to extract genomic DNA from the blood sample, following the manufacturer’s instructions. Biowing Biotechnology genotyped all nine SNPs using a Hi-SNP method based on multiplex PCR coupled with next-generation sequencing as previously described. The success rate of this method was greater than 97%. The concordance rate was above 99% when 10% of duplicates were reanalyzed.
Statistical Analysis
We used descriptive statistical methods to summarize the study participants’ baseline characteristics [n (%) and median and interquartile range (IQR) were present for categorical variables and continuous variables, respectively]. We used the Wilcoxon Mann–Whitney U-test to examine the difference in continuous variables with skewed distributions between the GDM and non-GDM groups. We employed the Chi-square or Fisher’s exact test to determine each SNP genotype’s Hardy-Weinberg equilibrium (HWE) and compare categorical data between groups. Using Haploview, we evaluated linkage disequilibrium (LD) and selected tag SNPs (see Figure S1).
We performed logistic regression to obtain odds ratios (ORs) and 95% confidence intervals (CIs) for genetic variants in N6AMT1 SNPs on GDM risk. All models were adjusted for potential maternal confounding factors, including age, education levels, smoking and drinking habits, family history of diabetes, parity, pre-pregnancy BMI, and serum OCM nutrients (folate, vitamin B12, and Hcy). The dominant model was found to be the best genetic model for the N6AMT1 SNPs on GDM risk based on the Akaike information criterion (AIC) and Bayesian information criterion (BIC) minima (see results section).
Under the dominant model, we reanalyzed the association of three tag SNPs in N6AMT1 with GDM at different serum folate and vitamin B12 levels. Subnormal folate and vitamin B12 were defined as serum folate less than six ng/mL and vitamin B12 less than 200 pg/mL, respectively.34,35 Additionally, additive and multiplicative interactions were used to determine whether N6AMT1 SNPs had a varying effect on GDM across different folate and vitamin B12 subgroups. For a more detailed explanation of these models, please refer to our previous study.36 The relative excess risk due to interaction (RERI) assessed additive interaction using a multiple logistic regression model, and a significant additive interaction was indicated by RERI > 0. We used a multiple logistic regression model to evaluate multiplicative interactions using the P value of a cross-product interaction term of the N6AMT1 SNPs, folate, and vitamin B12. In addition to the maternal confounding factors, Hcy, serum folate, and vitamin B12 were mutually adjusted in the stratification and interaction analysis.
We conducted all statistical analyses in R (version 4.1.3; R Project for Statistical Computing), and a P value < 0.05 was considered statistically significant.
Results
Baseline Characteristics of the Study Population
Table 1 shows the study participants’ baseline characteristics and serum folate, vitamin B12, and Hcy levels. Among 1303 pregnant women, 262 (20.1%) cases of GDM were identified. Women with GDM tended to be older, overweight or obese, multiparous, and have a family history of diabetes compared with non-GDM women. Education, smoking, and drinking habits showed no significant differences between the two groups. Pregnancies with GDM had a higher median serum folate level (10.4 ng/mL) than non-GDM participants (9.1 ng/mL). On the other hand, the average serum vitamin B12 level in GDM women (260 pg/mL) was lower than that in non-GDM women (273 pg/mL). No significant difference in serum Hcy levels was observed between the two groups.
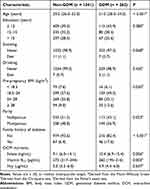 |
Table 1 Clinical Characteristics of the Study Population (n = 1303) |
Associations Between N6AMT1 Polymorphisms and the Risk of GDM
The genotypic distribution of the nine N6AMT1 SNPs is shown in Table S1, and eight SNPs were in Hardy-Weinberg equilibrium (P > 0.05). Three tag SNPs (rs7282280, rs1048546, rs1997605) were selected using Haploview (Figure S2). Table 2 displays the associations of the three N6AMT1 SNPs with GDM under five genetic models. After adjustment for maternal characteristics (age, education, smoking, drinking habits, family history of diabetes, parity, and pre-pregnancy BMI) and serum OCM nutrients (folate, vitamin B12, and Hcy), N6AMT1 rs7282280 and rs1048546 were found to be associated with GDM in the codominant, dominant, overdominant, and log-additive models. However, rs1997605 was associated with GDM only in the dominant model after adjustment for multiple covariates. For instance, pregnancies with rs7282280 T alleles (TC/TT), rs1048546 G alleles (TG/GG), or rs1997605 A alleles (AG/AA) had a lower risk of developing GDM than those with C alleles in rs7282280 (OR: 0.63; 95% CI: 0.47, 0.85), T alleles in rs1048546 (OR: 0.65; 95% CI: 0.48, 0.87), or G alleles in rs1997605 (OR: 0.72; 95% CI: 0.53, 0.98) under the dominant model, respectively (Table 2). The dominant model was determined to be the best inheritance model for the three tag SNPs based on the AIC and BIC values (Table 2). Therefore, the following analysis is based on the dominant model.
 |
Table 2 Association Between N6AMT1 SNPs and GDM Risk |
Associations Between N6AMT1 SNPs and GDM Stratified by OCM Nutrients
The stratified analysis revealed that N6AMT1 rs7282280 TC/TT genotypes were negatively associated with GDM regardless of folate levels after adjustment for maternal covariates and serum vitamin B12 and Hcy levels. However, pregnancies with rs1048546 G alleles (OR: 0.63; 95% CI: 0.46, 0.88) had a lower GDM risk than those with T alleles in rs1048546 when the serum folate level was ≥ 6.0 ng/mL. Although a negative correlation between rs1997605 AG/AA genotypes and GDM was observed in the higher folate group, the results were insignificant (Table 3). Similar results were found between N6AMT1 rs7282280 and rs1048546 and GDM when stratified by vitamin B12 levels. However, pregnancies with rs1997605 AG/AA genotypes had a decreased GDM risk than pregnancies with the GG genotype in the lower vitamin B12 group (OR: 0.41; 95% CI: 0.21, 0.80) after adjustment for maternal covariates and serum folate and Hcy levels (Table 4).
 |
Table 3 Association Between N6AMT1 SNPs and GDM Risk in the Dominant Model Stratified by Folate Levels |
 |
Table 4 Association Between N6AMT1 SNPs and GDM Risk in the Dominant Model Stratified by Vitamin B12 Levels |
Joint Effects of N6AMT1 SNPs and OCM Nutrients on GDM Risk
The following table (Table 5) outlines the combined effects of N6AMT1 SNPs with serum folate and vitamin B12 on GDM risk. According to the table, the odds ratios of GDM were significantly higher among pregnancies with the rs7282280 CC genotype and higher serum folate (OR: 3.37; 95% CI: 1.87, 6.43) compared to the reference group (pregnancies with rs7282280 TC/TT genotypes and lower serum folate). Similar joint effects were found between the N6AMT1 rs1048546 TT and rs1997605 GG genotypes with higher serum folate on GDM risk. However, no significant interactions existed between N6AMT1 SNPs and serum folate on GDM (Table 5). In addition, the odds ratios of GDM were significantly higher among pregnancies who had homozygous for rs7282280 CC, rs1048546 TT, or rs1997605 GG genotype and lower serum vitamin B12 [(OR: 3.13; 95% CI: 1.91, 5.09), (OR: 3.15; 95% CI: 1.81, 5.41), and (OR: 4.09; 95% CI: 2.22, 7.45), respectively] compared to the reference group (pregnancies with rs7282280 TC/TT, rs1048546 TG/GG, or rs1997605 AG/AA genotypes and higher serum vitamin B12). Notably, there was significant additive (RERI: 2.54, 95% CI: 0.17, 4.92) and multiplicative interactions (Pinteraction < 0.05) between the N6AMT1 rs1997605 GG genotype and lower serum vitamin B12 on GDM (Table 5).
 |
Table 5 Interactions of N6AMT1 SNPs and OCM Nutrients on GDM Risk |
Discussion
The GENEMaC cohort baseline data were analyzed to investigate the relationship between N6AMT1 SNPs and GDM and the joint effects of N6AMT1 genetic variants and OCM nutrients on GDM risk. We confirmed the positive association between the rs1997605 GG genotype and GDM discovered in our previous study.11 Additionally, two new SNPs (the rs7282280 CC genotype and rs1048546 TT genotype) were identified to be related to a higher risk of GDM. The study revealed that folate and vitamin B12 can modify the associations between N6AMT1 genetic variants and GDM risk. Significant additive and multiplicative interactions were observed between the rs1997605 GG genotype and subnormal serum vitamin B12 (< 200 pg/mL) on GDM risk. This is the first study to identify the N6AMT1 gene interacting with OCM nutrients to affect the occurrence of GDM, providing feasible strategies for preventing GDM in a more personalized manner in the future.
The cause of GDM is still largely unknown. However, recent studies have suggested that it may result from genetic, epigenetic, and environmental factors. There are two approaches currently available for screening GDM-related genetic variations. The first method is genome-wide association studies (GWAS), which involve testing genetic variants across the whole genomes of many individuals to identify genotype-phenotype associations. Over the past decade, GWAS has been used to identify complex genetic mechanisms that have led to the development of GDM. For example, a two-stage GWAS analysis in Korean women confirmed that CDKAL1 and MTNR1B genetic variants are strongly linked with GDM.37 The second approach involves studying candidate genes and detecting the association between variants of selected genes and the risk of GDM. GDM and type 2 diabetes mellitus (T2DM) share the same risk factors and genetic basis. Therefore, some genetic variations (such as TCF7L2, GCK, and KCNJ11) related to T2DM have also been confirmed to be related to GDM.4,5 In this study, N6AMT1 was selected as a candidate gene because we accidentally observed a direct association between this gene and GDM in our previous study.11 N6AMT1 methyltransferase is essential for arsenic biomethylation and the modulation of arsenic-induced toxicity.13,38 Therefore, our previous study aimed to investigate the synergistic effects of N6AMT1 genetic variants with arsenic metabolism on GDM. Due to the small sample size and limited research objectives, we wondered whether the association between N6AMT1 SNPs and GDM is credible. Therefore, in the present study, we expanded the sample size and further increased the number of detected SNPs to explore the relationship between N6AMT1 gene polymorphisms and the risk of GDM. Finally, the positive association between the GG genotype of rs1997605 and GDM was confirmed, but the relationship between rs1003671 and GDM has yet to be confirmed. In addition, we also found that two new SNPs (rs7282280 CC genotype and rs1048546 TT genotype) are associated with GDM risk. These results suggested that N6AMT1 may be involved in the physiological pathology of GDM.
N6AMT1 was identified as the first writer of DNA 6mA modification. Since this modification is associated with various cancers, N6AMT1 is thought to play a critical role in the occurrence and development of cancer. Abnormal expression of N6AMT1 has been shown to influence drug resistance and tumor progression in breast cancer and is associated with hepatocellular and tongue squamous cell carcinoma.19,39–41 However, its role in diabetes, including T2DM and GDM, has not been investigated. Given the limited studies on the role of N6AMT1 in GDM, it is not easy to draw a solid conclusion right now. Therefore, a comprehensive analysis of N6AMT1 and DNA 6mA modification in GDM development is urgently needed.
N6AMT1 is a SAM-dependent methyltransferase.18 SAM is an essential metabolite in the OCM pathway, which consists of two major components (folate and methionine cycles). Methionine is a precursor of SAM. It can be derived from diet and the remethylation of Hcy, in which vitamin B12 is a crucial cofactor for Hcy remethylation.42 Therefore, factors related to SAM generation may also affect the relationship between N6AMT1 and disease susceptibility. Our studies and other studies have confirmed a significant association of folate and vitamin B12 with GDM, while the relationship between Hcy and GDM is insignificant.10 This study found that individuals with the N6AMT1 rs7282280 TC/TT genotypes had a decreased risk of GDM, regardless of their folate and vitamin B12 levels. This implies that the N6AMT1 rs7282280 CC genotype is an independent risk factor for GDM. Additionally, the study observed a significant association between rs1048546 TG/GG genotypes and GDM, but only in individuals with higher serum folate and vitamin B12. The joint effect analysis showed that pregnant women with risk genotypes (rs7282280 CC genotype or rs1048546 TT genotype) have the highest GDM risk when serum folate is higher (≥ 6.00 ng/mL) or vitamin B12 is less than 200 pg/mL (Table 5). It is worth noting that the association between N6AMT1 rs1997605 and GDM only exists in pregnancies with lower serum vitamin B12 (< 200 pg/L), and the GG genotype is associated with a higher risk of GDM. Additionally, pregnant women with the rs1997605 GG genotype and higher serum folate or lower vitamin B12 are more susceptible to GDM than other women (Table 5). The additive and multiplicative interactions between the rs1997605 GG genotypes and lower vitamin B12 were statistically significant. The RERI of the additive interaction was 2.54 (95% CI: 0.17, 4.92). This indicates that the relative risk of having GDM among pregnant women with the rs1997605 GG genotype is 2.54 more with lower serum vitamin B12 than if there was no interaction between rs1997605 and vitamin B12. Our findings indicated that N6AMT1 SNPs are associated with GDM in Chinese women, and folate and vitamin B12 can strongly modify the effects.
Our study has several strengths that contribute to its reliability and validity. First, we obtained individual measurements of serum folate and vitamin B12 levels, which allowed us to estimate the impact of these nutrients on the association between N6AMT1 SNPs and GDM. Additionally, our larger sample sizes gave us more accurate and trustworthy results. However, there are also some limitations to our study. First, we conducted the study only on Chinese Han pregnant women, and further research is needed on populations from other regions and races. Second, the application of our findings may be limited due to the need for more data on folate and vitamin B12 from dietary intake. Third, the interaction effects of N6AMT1 and OCM nutrients on GDM risk need to be replicated in an independent population, and the potential mechanism should be fully clarified in subsequent studies.
Conclusion
In conclusion, our study indicates that pregnancies with the N6AMT1 rs7282280 CC genotype, rs1048546 TT genotype, and rs1997605 GG genotype are at a higher risk of developing GDM. Additionally, we found that serum folate and vitamin B12 levels can influence the association between N6AMT1 SNPs and GDM. We found significant additive and multiplicative interactions between the rs1997605 GG genotype and lower vitamin B12 on GDM. These findings suggest that by exploring gene-nutrition interactions, we can gain further insight into predicting and intervening in cases of GDM at a more personalized level. However, there is still much to learn in this field, and more in-depth mechanism studies are needed to explain the relationship between N6AMT1 SNPs and GDM in the future.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
All procedures involving human participants followed the ethical standards of the Helsinki Declaration of Ethical Principles, and the research proposal was approved by the Ethics Committee of Metabolic Diseases Hospital and Institute of Endocrinology, Tianjin Medical University (DXBYYhMEC2018-14) and Tianjin Xiqing Hospital (xqyyll-2020-07). Written informed consent was obtained from all pregnant women before participation in this study.
Acknowledgments
We thank all the participants in this study. This work was supported by the National Natural Science Foundation of China (No. 82373543); Tianjin Xiqing Hospital Science Foundation (No. XQYYKLT202101 and XQYYKLT202103); and Science Foundation of Women’s and Children’s Health Center of Dongchangfu District (No. DCFY-KLT-202301 and DCFY-KLT-202304).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas, took part in drafting, revising or critically reviewing the article, gave final approval of the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang H, Li N, Chivese T, et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by international association of diabetes in pregnancy study group’s criteria. Diabetes Res Clin Pract. 2022;183:109050. doi:10.1016/j.diabres.2021.109050
2. Gao C, Sun X, Lu L, Liu F, Yuan J. Prevalence of gestational diabetes mellitus in mainland China: a systematic review and meta‐analysis. J Diabetes Invest. 2018;10(1):154–162. doi:10.1111/jdi.12854
3. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5(1). doi:10.1038/s41572-019-0098-8
4. Dalfra MG, Burlina S, Del Vescovo GG, Lapolla A. Genetics and epigenetics: new insight on gestational diabetes mellitus. Front Endocrinol. 2020;11:602477. doi:10.3389/fendo.2020.602477
5. Rosik J, Szostak B, Machaj F, Pawlik A. The role of genetics and epigenetics in the pathogenesis of gestational diabetes mellitus. Am J Hum Genet. 2019;84(2):114–124. doi:10.1111/ahg.12356
6. Wang Y, Wang Y, Sun Y, et al. Serum folate mediates the associations ofMTHFRrs1801133 polymorphism with blood glucose levels and gestational diabetes mellitus in Chinese Han pregnant women. Br J Nutr. 2023;130(8):1329–1337. doi:10.1017/s0007114523000314
7. Zhang Q, Zhang X, Li S, et al. Joint effect of urinary arsenic species and serum one-carbon metabolism nutrients on gestational diabetes mellitus: a cross-sectional study of Chinese pregnant women. Environ Int. 2021;156:106741. doi:10.1016/j.envint.2021.106741
8. Zhang Q, Tian M, Zhang X, et al. Metabolic biomarkers linking urinary arsenic species to gestational diabetes mellitus: a cross-sectional study in Chinese pregnant women. Sci Total Environ. 2023;892. doi:10.1016/j.scitotenv.2023.164761
9. Yang X, Zhang Q, Sun Y, et al. Joint effect of ambient PM2.5 exposure and vitamin B12 during pregnancy on the risk of gestational diabetes mellitus. Sci Total Environ. 2023;876. doi:10.1016/j.scitotenv.2023.162514
10. Li S, Tian X, Wang Y, et al. Associations of maternal rs1801131 genotype in MTHFR and serum folate and vitamin B(12) with gestational diabetes mellitus in Chinese pregnant women. Nutrients. 2022;14(6). doi:10.3390/nu14061169
11. Liang X, Guo G, Wang Y, et al. Arsenic metabolism, N6AMT1 and AS3MT single nucleotide polymorphisms, and their interaction on gestational diabetes mellitus in Chinese pregnant women. Environ Res. 2023;221:115331. doi:10.1016/j.envres.2023.115331
12. Xiao CL, Zhu S, He M, et al. N(6)-Methyladenine DNA modification in the human genome. Mol Cell. 2018;71(2):306–318 e7. doi:10.1016/j.molcel.2018.06.015
13. Harari F, Engstrom K, Concha G, Colque G, Vahter M, Broberg K. N-6-adenine-specific DNA methyltransferase 1 (N6AMT1) polymorphisms and arsenic methylation in Andean women. Environ Health Perspect. 2013;121(7):797–803. doi:10.1289/ehp.1206003
14. Chen X, Guo X, He P, et al. Interactive influence ofN6AMT1andAs3MTGenetic variations on arsenic metabolism in the population of Inner Mongolia, China. Toxicol Sci. 2017;155(1):124–134. doi:10.1093/toxsci/kfw181
15. Wang J, Wang K, Liu W, Cai Y, Jin H. m6A mRNA methylation regulates the development of gestational diabetes mellitus in Han Chinese women. Genomics. 2021;113(3):1048–1056. doi:10.1016/j.ygeno.2021.02.016
16. Tobi EW, Juvinao-Quintero DL, Ronkainen J, et al. Maternal glycemic dysregulation during pregnancy and neonatal blood DNA Methylation: meta-analyses of epigenome-wide association studies. Diabetes Care. 2022;45(3):614–623. doi:10.2337/dc21-1701
17. Luo G-Z, Blanco MA, Greer EL, He C, Shi Y. DNA N6-methyladenine: a new epigenetic mark in eukaryotes? Nat Rev Mol Cell Biol. 2015;16(12):705–710. doi:10.1038/nrm4076
18. Li W, Shi Y, Zhang T, Ye J, Ding J. Structural insight into human N6amt1–Trm112 complex functioning as a protein methyltransferase. Cell Discov. 2019;5(1). doi:10.1038/s41421-019-0121-y
19. Chen J, Zhuang Y, Wang P, et al. Reducing N6AMT1-mediated 6mA DNA modification promotes breast tumor progression via transcriptional repressing cell cycle inhibitors. Cell Death Dis. 2022;13(3). doi:10.1038/s41419-022-04661-8
20. Liu J, Zhu Y, Luo G-Z, et al. Abundant DNA 6mA methylation during early embryogenesis of zebrafish and pig. Nat Commun. 2016;7(1). doi:10.1038/ncomms13052
21. Yao B, Cheng Y, Wang Z, et al. DNA N6-methyladenine is dynamically regulated in the mouse brain following environmental stress. Nat Commun. 2017;8(1). doi:10.1038/s41467-017-01195-y
22. Guo Y, Pei Y, Li K, Cui W, Zhang D. DNA N6-methyladenine modification in hypertension. Aging. 2020;12. doi:10.18632/aging.103023
23. Ouyang L, Su X, Li W, et al. ALKBH1-demethylated DNA N6-methyladenine modification triggers vascular calcification via osteogenic reprogramming in chronic kidney disease. J Clin Investig. 2021;131(14). doi:10.1172/jci146985
24. Hao Z, Wu T, Cui X, et al. N6-Deoxyadenosine methylation in mammalian mitochondrial DNA. Molecular Cell. 2020;78(3):382–395.e8. doi:10.1016/j.molcel.2020.02.018
25. Ducker GS, Rabinowitz JD. One-carbon metabolism in health and disease. Cell Metab. 2017;25(1):27–42. doi:10.1016/j.cmet.2016.08.009
26. Chen X, Zhang Y, Chen H, et al. Association of maternal folate and vitamin B12 in early pregnancy with gestational diabetes mellitus: a prospective cohort study. Diabetes Care. 2021;44(1):217–223. doi:10.2337/dc20-1607
27. Zhu B, Ge X, Huang K, et al. Folic acid supplement intake in early pregnancy increases risk of gestational diabetes mellitus: evidence from a prospective cohort study. Diabetes Care. 2016;39(3):e36–e37. doi:10.2337/dc15-2389
28. Li Q, Zhang Y, Huang L, et al. High-dose folic acid supplement use from prepregnancy through midpregnancy is associated with increased risk of gestational diabetes mellitus: a prospective cohort study. Diabetes Care. 2019;42(7):e113–e115. doi:10.2337/dc18-2572
29. Saravanan P, Sukumar N, Adaikalakoteswari A, et al. Association of maternal vitamin B(12) and folate levels in early pregnancy with gestational diabetes: a prospective UK cohort study (PRiDE study). Diabetologia. 2021;64(10):2170–2182. doi:10.1007/s00125-021-05510-7
30. Krishnaveni GV, Hill JC, Veena SR, et al. Low plasma vitamin B12 in pregnancy is associated with gestational ‘diabesity’ and later diabetes. Diabetologia. 2009;52(11):2350–2358. doi:10.1007/s00125-009-1499-0
31. Li S, Hou Y, Yan X, et al. Joint effects of folate and vitamin B(12) imbalance with maternal characteristics on gestational diabetes mellitus. J Diabetes. 2019;11(9):744–751. doi:10.1111/1753-0407.12899
32. Lai JS, Pang WW, Cai S, et al. High folate and low vitamin B12 status during pregnancy is associated with gestational diabetes mellitus. Clin Nutr. 2018;37(3):940–947. doi:10.1016/j.clnu.2017.03.022
33. Gao S, Mostofa MG, Quamruzzaman Q, et al. Gene-environment interaction and maternal arsenic methylation efficiency during pregnancy. Environ Int. 2019;125:43–50. doi:10.1016/j.envint.2019.01.042
34. World Health Organization. Serum and red blood cell folate concentrations for assessing folate status in populations; 2015. Available from: https://www.who.int/publications/i/item/WHO-NMH-NHD-EPG-15.01.
35. Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR Modern nutrition in health and disease: eleventh edition, Wolters Kluwer Health Adis (ESP); 2012. Available from: https://jhu.pure.elsevier.com/en/publications/modern-nutrition-in-health-and-disease-eleventh-edition.
36. Zhang Q, Hou Y, Wang D, et al. Interactions of arsenic metabolism with arsenic exposure and individual factors on diabetes occurrence: baseline findings from arsenic and non-communicable disease cohort (AsNCD) in China. Environ Pollut. 2020;265. doi:10.1016/j.envpol.2020.114968
37. Kwak SH, Kim S-H, Cho YM, et al. A genome-wide association study of gestational diabetes mellitus in Korean women. Diabetes. 2012;61(2):531–541. doi:10.2337/db11-1034
38. Ren X, Aleshin M, Jo WJ, et al. Involvement of N-6 adenine-specific DNA methyltransferase 1 (N6AMT1) in arsenic biomethylation and its role in arsenic-induced toxicity. Environ Health Perspect. 2011;119(6):771–777. doi:10.1289/ehp.1002733
39. Xi L, Yang Y, Xu Y, et al. The enhanced genomic 6 mA metabolism contributes to the proliferation and migration of TSCC cells. Int J Oral Sci. 2022;14(1). doi:10.1038/s41368-022-00161-9
40. Sheng X, Wang J, Guo Y, Zhang J, Luo J. DNA N6-Methyladenine (6mA) modification regulates drug resistance in triple negative breast cancer. Front Oncol. 2021;10. doi:10.3389/fonc.2020.616098
41. Lin Q, Chen J-W, Yin H, et al. DNA N6-methyladenine involvement and regulation of hepatocellular carcinoma development. Genomics. 2022;114(2). doi:10.1016/j.ygeno.2022.01.002
42. Clare CE, Brassington AH, Kwong WY, Sinclair KD. One-carbon metabolism: linking nutritional biochemistry to epigenetic programming of long-term development. Annu Rev Anim Biosci. 2019;7(1):263–287. doi:10.1146/annurev-animal-020518-115206
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
