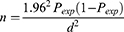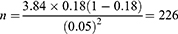Back to Journals » Veterinary Medicine: Research and Reports » Volume 14
Seropositivity, Comparison Between the Efficiency of Serological Tests and Risk Factors of Brucella Infection in Small Ruminants with History of Abortion in the Afar Region of North-Eastern Ethiopia
Authors Tekle M, Legesse M, Mamo G
Received 29 October 2023
Accepted for publication 19 December 2023
Published 1 January 2024 Volume 2023:14 Pages 245—252
DOI https://doi.org/10.2147/VMRR.S446714
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Muluken Tekle,1 Mengistu Legesse,2 Gezahegne Mamo1
1College of Veterinary Medicine and Agriculture, Addis Ababa University, Bishoftu, Ethiopia; 2Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Muluken Tekle, Addis Ababa University, College of Veterinary Medicine and Agriculture, Tel +251910117279, Email [email protected]
Purpose: Brucellosis is one of the most important reproductive diseases that cause abortion and breeding failure in small ruminants in Ethiopia. Therefore, our objective was to detect the seropositivity and risk factors of Brucella infection in small ruminants with history of abortion using modified RBPT, cELISA, and CFT in the Amibara district of the Afar Region, Ethiopia.
Methods: Sera were collected from 226 animals (195 goats and 31ewes) and assessed for seropositivity of Brucella infection using modified RBPT, CFT, and competitive ELISA.
Results: The overall seroprevalence was 12.0% (27 out of 226), 7.5% (17 out of 226), and 26.5% (60 out of 226) by mRBPT, CFT, and cELISA, respectively. Out of 27 sera that were reactive by mRBPT, 17 (63.0%) were also reactive by (CFT). Out of the 17 sera that were reactive by CFT and mRBPT, 14 (82.4%) were reactive by cELISA. Out of the 29 sera that were non-reactive by both mRBPT and CFT, 10 (34.5%) were found reactive by cELISA. Out of the 226 sera that were tested by both mRBPT and cELISA, 20 (8.9%) were reactive by both tests, while 159 (70.4%) were non-reactive by both tests. The percentage of test agreement (79.2%) between mRBPT and cELISA was poor (k=0.353). High seropositivity for Brucella infection was significantly associated with the presence of retained placenta in the studied animals (adjusted OR=2.2, 95% CI, 1.1– 4.4, P=0.030) as detected by cELISA.
Conclusion: The current study revealed that a cELISA-based seroepidemiological survey increases the likelihood of detecting individuals with brucellosis and provides reliable evidence for mRBPT. Furthermore, there was a significant association between seropositivity for Brucella infection and retained placenta. These findings emphasize the necessity for proactive measures to reduce the economic impact of brucellosis and mitigate the risk of zoonotic transmission.
Keywords: abortion, brucellosis, seropositivity, small ruminants
Introduction
Brucellosis is a common disease in tropical and subtropical countries and causes enormous economic losses due to it causes abortion and breeding failure in small ruminants and also it affects the health of livestock and diminishes their products.1,2 It has also been considered the most common re-emerging zoonotic disease in many areas of the world.3–6 However, the magnitude and distribution of brucellosis both in animals and humans vary in different geographical localities.7 Studies on small ruminant brucellosis in pastoral areas of Ethiopia determined seroprevalences ranging from 1.5% to 16% in Afar, Oromia, and Somali Regional States using the rose Bengal plate (RBPT) and complement fixation (CFT) tests.8,9 A higher risk of Brucella infection in goats has been associated with agro-pastoral and pastoral production systems. In pastoral areas, the contribution of brucellosis to abortion in goats has been highlighted and an upsurge of infection has been associated with an increased abortion rate.10,11 Hence, an extensive epidemiological survey of brucellosis in humans and animals in different settings using rel iable diagnostic methods would be useful to provide reliable epidemiological data.
Bacteriological isolation using the culture method, which is a reliable method for the diagnosis of Brucella infection in animals and humans. However, as the Brucella organism grows very slowly in vitro, it requires advanced laboratory, trained manpower, and high biosafety situations. In addition, the culture method is not feasible to carry out an epidemiological study of brucellosis.12 Hence, serological tests such as modified Rose Bengal Plate Test (mRBPT), Complement Fixation Test (CFT), and Enzyme Linked Immuno Sorbent Assay (ELISA) in a single or combination are commonly used for the screening of Brucella infection.12 Among others, RBPT with or without CFT is the most commonly used for preliminary diagnosis of brucellosis in many resource-limited countries including Ethiopia.9–12 However, RBPT has been criticized for its drawbacks such as false-positive results due to cross-reactivity with other bacteria.13,14
Several studies have also suggested that ELISA is more effective for a seroepidemiological survey of brucellosis as compared to RBPT and good diagnostic results have been obtained in sheep and goats with indirect (I-) or competitive (C-) enzyme linked immunosorbent assays (ELISAs) using various antigens, but generally the ELISAs that use antigens with a high content of smooth lipopolysaccharide (sLPS) are the most useful.15–20
In Ethiopia, several seroprevalence studies of brucellosis have shown the occurrence of brucellosis among livestock using mRBPT and CFT.21–24 Nevertheless, few studies have used ELISA to assess the seroprevalence of brucellosis in animals.23,25 Previous studies have shown that cELISA is highly specific compared to mRBPT for the diagnosis of brucellosis in animals.18,26,27
Therefore, the present study was conducted in the Amibara district of the Afar Region, Ethiopia, to detect the seropositivity and risk factors of Brucella infection in small ruminants with a recent history of abortion using modified RBPT, cELISA, and CFT.
Methods
Study Area and Animal Population
The study was conducted in a pastoralist area of Amibara district of Afar National Regional State, Ethiopia. Amibara district is located in the Middle Awash Valley about 260 km to the North East of Addis Ababa. The livestock populations of the Amibara district are 103,959 cattle, 122,526 goats, 48,043 sheep, 3888 donkeys, and 39,995 camels. The production system of the Afar region is dominated by pastoralism (90%) from which agro-pastoralism (10%) is now emerging, following some permanent and temporary rivers on which small-scale irrigation is developed.28,29 In Ethiopia to date, no brucellosis vaccination has been undertaken in animals.25,30,31
Sample Size Estimation
With the assumption of 18% seroprevalence of Brucella infection in small ruminants in the study area25 5% precision and 95% confidence level, about 226 animals were intended to be included in the study.
Where,
n=required sample size,
Pexp=expected prevalence, and
d=desired absolute precision
Hence, based on the above formula, taking the expected prevalence of brucellosis in clinically aborted small ruminants as 18%25 a desired absolute precision of 5% and 95% confidence level, 226 animals are required.
Therefore, a total of 226 clinically aborted small ruminants were considered for this study from Pastoral kebeles.
Study Animals and Procedure of Data Collection
A community-based cross-sectional study was conducted from October 2015 to April 2016. A house-to-house survey was conducted to include goats and/or sheep that had a history of recent abortion (abortion occurred in the last 30 days at the time of data collection) and were included in the study. After the aim of the survey had been explained, the owners were interviewed using a structured questionnaire regarding the history of abortion, the duration of abortion, age of the animal, frequency of abortion, and retained placenta. Then, about 3 mL of blood samples were collected from each animal, serum was separated and stored at −20°C until processed for serological analysis.
Serological Tests
All sera were screened using mRBPT and cELISA as per the manufacturers’ instruction (Svanova, Brucella-ab c-ELISA Uppsala Business Park. Rapsgatan 7,75174 Uppsala, Sweden). All sera found positive by mRBPT are further tested by CFT. In addition, 29 randomly selected sera that were negative by mRBPT were also tested by CFT.
Data Analysis
Data were entered into EpiData Software v.3.1 and analyzed using Stata version 11. Frequencies and percentages were used to summarise baseline characteristics of the study animals and the seroprevalence of brucellosis as diagnosed using mRBPT, CFT, and cELISA. Univariable and multivariable logistic regression analyses were used to assess the effect of each of the independent variables (such as age, history of abortion, and retained placenta). A p-value less than 0.05 was considered statistically significant. Agreement between the tests was assessed using Cohen’s Kappa (k) coefficient. K values greater than 0.75 between 0.4 and 0.75 and less than 0.4 were considered excellent, fair, and poor agreement, respectively.
Results
Baseline Characteristics of the Study Animals and Seroprevalence of Brucellosis
According to a report by the owners, 98/226 (43.4%) animals had abortion history for two or more times. More than half 119/226 (52.7%) of the studied animals had retained their placenta during the data collection. Out of the total 226 animals, 27 (12.0%) were positive for Brucella infection by the mRBPT. Out of those 27, 17 (63.0%) were positive for Brucella infection by the CFT. None of the 29 animals that were negative by mRBPT were found to be positive by CFT.
In addition, out of 226 animals, 60 (26.6%) animals were found to be positive for Brucella infection by cELISA. Out of the 27 animals that were positive for Brucella infection by mRBPT, 7(25.9%) were negative by cELISA. Out of the 17 animals that were positive for Brucella infection by CFT and mRBPT, 14 (82.4%) were positive by cELISA.
In general, cELISA revealed high seropositivity for Brucella infection in the studied animals with retained placenta compared to animals without retained placenta (34.5% versus 17.8%, x2=8.05, P=0.005). In bivariate logistic regression analysis, age over 6 years (Crude OR=2.3, 95% CI, 1.1–5.1, P=0.030) and retained placenta (crude OR=2.4, 95% CI, 1.3–4.5, P=0.005) were significantly associated with high seropositivity for Brucella infection as detected by cELISA. In multivariable logistic regression analysis, only retained placenta (adjusted OR=2.2, 95% CI, 1.1–4.4, P=0.030) was significantly associated with high seropositivity for Brucella infection (Table 1).
 |
Table 1 Association Between Baseline Characteristics of the Studied Animals and Seropositivity for Brucella Infection by mRBPT, CFT, and cELISA |
Agreement of the Tests for the Screening of Brucella Infection in Small Ruminants
Table 2 shows the test agreement between mRBPT and cELISA for the screening of Brucella infection in the studied animals. Out of the 226 sera that were tested by both mRBPT and cELISA, 20 (8.9%) were positive by both tests, while 159 (70.4%) were negative by both tests. Hence, the percentage of agreement (79.2%) between mRBPT and cELISA was poor (k=0.353). Out of the total 56 sera that were tested both by CFT and cELISA, 14 (25%) were positive by both tests, while 29 (51.8%) were negative by both tests and the percentage of agreement (76.8%) between cELISA and CFT was also poor (k=0.193).
 |
Table 2 Tests Agreement for the Serodiagnosis of Brucella Infection in the Studied Animals |
Discussion
The present study determined the overall seroprevalence of brucellosis in sheep and goats with a history of recent abortion is between 12.0% and 7.5% with mRBPT alone and using combined mRBPT CFT tests, respectively. The observed seroprevalence is higher than seroprevalence reported in small ruminants in other areas of Ethiopia using CFT22,32 but relatively lower than previously reported overall seroprevalence of brucellosis in small ruminants using CFT in the Afar Region.30,31
In this study, we assessed a seroprevalence of brucellosis in small ruminants with a history of recent abortion by mRBPT and cELISA. The seroprevalence of brucellosis was 12.0% in the studied animals as detected by mRBPT, which is similar to a recently reported seroprevalence of brucellosis in small ruminants in other districts of the Afar Region.25,26 The similarities could be due to resemblance to animal husbandry, communal grazing of rangelands and watering areas, and possibly similar climatic conditions.25 Another study in Afar Region showed a low seroprevalence (3.1%) of brucellosis in small ruminants using mRBPT.21 Studies in other pastoral areas of Ethiopia also revealed relatively a low seroprevalence (8.5%) of the diseases using mRBPT.22 In addition, Tadeg et al33 reported a high seroprevalence of brucellosis (17.4%) using mRBPT in small ruminants in another area of the Afar Region. This difference might have originated mainly from variation in the sample type and method of detection.23
In this study, cELISA revealed a high seroprevalence for Brucella infection in the studied animals as compared to that of mRBPT (26.6% VS 12.0%, x2=35.5, p<0.001). Previous studies in Ethiopia also showed a significantly higher seroprevalence of brucellosis in small ruminants using iELISA as compared to mRBPT.25 Similar to the findings of the present study, previous studies on small ruminants suggested that ELISA-based test is more effective for serological-based survey of brucellosis as compared to mRBPT.16–19
In this study, the agreement between mRBPT and cELISA for the serodiagnosis of Brucella infection in small ruminants was poor. However, a previous study in Ethiopia revealed a fair agreement between mRBPT and indirect ELISA for the serodiagnosis of Brucella infection in small ruminants.25 On the other hand, a study in Rwanda has shown an excellent agreement between mRBPT and cELISA (K=0.92) for the serodiagnosis of Brucella infection in cattle.34 The low agreement between mRBPT and cELISA in the present study was attributed to the high seroprevalence of brucellosis detected by cELISA, which might be due to the ability of this ELISA-based technique to detect low level of antibody even at the early stage of infection.17,35
In this study, a high seroprevalence for Brucella infection was detected in those animals with the retained placenta, which is similar to the results of previous studies elsewhere.36–39 This indicates that abortion and retained placenta are typical outcomes of brucellosis.40 A review on clinical features of brucellosis also showed that placenta retention is one of the main clinical signs of B. melitensis infection in aborted small ruminants.1
To evaluate the reliability of the mRBPT and/or cELISA for the serodiagnosis of brucellosis, the gold standard (bacteria culture) needs to be used. However, in this study, the seroprevalence of brucellosis in animals with a history of recent abortion was assessed and compared using mRBPT and cELISA without using a gold standard like several previous studies and this could be one of the limitations of this study.
Conclusion
This study demonstrated high seropositivity of Brucella infection in small ruminants with a history of recent abortion could suggest that brucellosis is the main responsible cause of abortion and breeding failure in small ruminants of pastoral communities. The results associated with the presence of abortion are consistent with serological investigations and indicate a high level of exposure to Brucella infection. Besides this, high seropositivity of Brucella infection in small ruminants was detected by cELISA as compared to mRBPT. Moreover, seropositivity for Brucella infection was significantly associated with retained placenta.
Hence, this finding suggests that cELISA-based test is more effective for serological-based surveys of brucellosis in small ruminants as compared to mRBPT in the present study area though additional conformational studies are important. This finding also warrants appropriate control strategies to reduce its economic impact and risk of zoonotic transmission of the disease in the study area.
Abbreviations
AAU, Addis Ababa University; ALIPB, Aklilu Lemma Institute of Pathobiology; CVMA, College of Veterinary Medicine Agriculture; ITM, Institute of Tropical Medicine.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request and with permission from IRB and/or other responsible bodies in Ethiopia.
Ethics Approval and Consent to Participate
We confirmed that the study protocol was approved by the Institutional Review Board (IRB) of the College of Veterinary Medicine and Agriculture, Addis Ababa University. The purpose of the study was clearly explained to all goat and sheep owners who participated in the study, and their verbal consent was obtained before collecting samples from goats. The IRB specifically approved the use of verbal consent for obtaining blood samples from aborted goats, as this procedure was considered a routine clinical practice for confirming Brucella diagnosis.
Acknowledgments
We acknowledge Dr. Mekonin Bayissa (Amibara District Pastoral Office, Afar Region) for his positive cooperation during data collection; Administration of Afar Pastoral Regional State of Amibara district, animal owners, and Melka Werer Agriculture Research Center are highly acknowledged. We acknowledge the Animal Health Institute (AHI) in Sebeta, Ethiopia, for their support in serology laboratory analysis.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This Study was financially supported by a collaborative research project entitled “Integrated Community and Health facility-based study of brucellosis in Pastoralists and their livestock in Afar Regional State of Ethiopia” between ALIPB and CVMA of Addis Ababa University funded by the Institute of Tropical Medicine (ITM-Belgium) No: 1123/2016. The funding organization had no any role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Disclosure
The authors declare that they have no competing interests
References
1. Megid J, Mathias LA, Robles CA. Clinical manifestations of brucellosis in domestic animals and humans. Vet Sci J. 2010;4:119–126.
2. Rossetti CA, Arenas AM, Maurizio E. Caprine brucellosis: a historically neglected disease with significant impact on public health. PLoS Negl Trop Dis. 2017;11:56–92. doi:10.1371/journal.pntd.0005692
3. Pal M, Gizaw F, Fekadu F, Alemayehu G, Kandi V. Public health and economic importance of bovine brucellosis: an overview. Amer J Epidemiol Infect Dis. 2017;5:27–34.
4. Donev D, Karadzovski Z, Kasapinov B, Lazarevik V. Epidemiological and public health aspects of brucellosis in the Republic of Mecedonia. Biology Med Scie. 2010;1:33–54.
5. Liu Q, Cao L, Zhu XQ. Major emerging and re-emerging zoonoses in China: a matter of global health and socioeconomic development for 1.3 billion. Inter J Infect Dis. 2014;25:65–72. doi:10.1016/j.ijid.2014.04.003
6. Seleem MN, Boyle SM, Sriranganathan N. Brucellosis: a re-emerging zoonosis. Vet Microbiol. 2010;140:392–398. doi:10.1016/j.vetmic.2009.06.021
7. Dean AS, Crump L, Greter H, Hattendorf J, Schelling E, Zinsstag J. Clinical manifestations of human brucellosis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6(12):e1929. doi:10.1371/journal.pntd.0001929
8. Asmare K, Megersa B, Denbarga Y, et al. A study on seroprevalence of caprine brucellosis under three livestock production systems in southern and central Ethiopia. Trop Anim Health Prod. 2013;45:555–560. doi:10.1007/s11250-012-0258-2
9. Bekele WA, Tessema TS, Melaku SK. Camelus dromedarius brucellosis and its public health associated risks in the Afar National Regional State in northeastern Ethiopia. Acta Vet Scandi. 2013;5:1–8.
10. Megersa B, Biffa D, Abunna F, Regassa A, Godfroid J, Skjerve E. Seroprevalence of brucellosis and its contribution to abortion in cattle, camel, and goat kept under pastoral management in Borana, Ethiopia. Trop Anim Health Prod. 2011;43(3):651–656. doi:10.1007/s11250-010-9748-2
11. Ashagrie T, Deneke Y, Tolosa T. Seroprevalence of caprine brucellosis and associated risk factors in South Omo Zone of Southern Ethiopia. Afr J Microbiol. 2011;5(13):1682.
12. Nielsen K, Yu WL. Serological diagnosis of brucellosis. Prilozi. 2010;31(1):65–89.
13. Nielsen K, Smith P, Yu W, et al. Serological discrimination by indirect enzyme immunoassay between the antibody response to Brucella sp. and Yersinia enterocolitica O:9 in cattle and pigs. Vet Immunol Immunopathol. 2006;109(1–2):69–78. doi:10.1016/j.vetimm.2005.07.025
14. Chart H, Okubadejo OA, Rowe B. The serological relationship between Escherichia coli O157 and Yersinia enterocolitica O9 using sera from patients with brucellosis. Epidemiol Infect. 1992;108(1):77–85. doi:10.1017/S0950268800049529
15. Gall D, Nielsen K. Serological diagnosis of bovine brucellosis: a review of test performance and cost comparison. Rev Sci Tech. 2004;23(3):989–1002. doi:10.20506/rst.23.3.1545
16. Nielsen K, Smith P, Yu WL, et al. Validation of a second generation competitive enzyme immunoassay (CELISA) for the diagnosis of brucellosis in various species of domestic animals. Vet Immunol Immunopathol. 2008;125(3–4):246–250. doi:10.1016/j.vetimm.2008.02.015
17. Perrett LL, McGiven JA, Brew SD, Stack JA. Evaluation of competitive ELISA for detection of antibodies to Brucella infection in domestic animals. Croat Med J. 2010;51(4):314–319. doi:10.3325/cmj.2010.51.314
18. Ahmed M, Islam MDA, Khatun MM, Baek BK. Evaluation of four serological tests for the detection of brucellosis in goats and cattle under the field condition of Bangladesh. Asian J Biolog Scie. 2011;4:477–482. doi:10.3923/ajbs.2011.477.482
19. Saxena N, Singh BB, Gill JPS, Aulakh RS. Frequency of occurrence of brucellosis in goats in ludhiana District of Punjab state of India. Microbiol Research J Int. 2017;21(6):1–7. doi:10.9734/MRJI/2017/35974
20. World Organisation for Animal Health (WOAH). Bovine Brucellosis; caprine and ovine brucellosis and porcine brucellosis. In: World Assembly of Delegates of the OIE Chapter 2.4.3. Paris: WOAH Terrestrial Manual; 2009:1–35.
21. Ashenafi F, Teshale S, Ejeta G, Fikru R, Laikemariam Y. Distribution of brucellosis among small ruminants in the pastoral region of Afar, eastern Ethiopia. Rev Sci Technol. 2007;26:731–739. doi:10.20506/rst.26.3.1781
22. Tsehay H, Getachew G, Morka A, Tadesse B, Eyob H. Seroprevalence of brucellosis in small ruminants in pastoral areas of Oromia and Somali regional states, Ethiopia. J Vet Med. 2014;6(11):289–294.
23. Tschopp R, Bekele S, Moti T, Young D, Aseffa A. Brucellosis and bovine tuberculosis prevalence in livestock from pastoralist communities adjacent to Awash National Park, Ethiopia. Prev Vet Med. 2015;120(2):187–194. doi:10.1016/j.prevetmed.2015.03.004
24. Sintayehu G, Melesse B, Abayneh D, et al. Epidemiological survey of brucellosis in sheep and goats in selected pastoral and agro-pastoral lowlands of Ethiopia. Rev Sci Tech Int Epiz. 2015;34(3):1.
25. Teshale S, Muhie Y, Dagne A, Kidanemariam A. Seroprevalence of small ruminant brucellosis in selected districts of Afar and Somali pastoral areas of Eastern Ethiopia: the impact of husbandry practice. Revue Méd Vét. 2006;157: (11:557–563.
26. Nielsen K, Gall D, Smith P, Balsevicius S, Garrido F, Ferrer MD. Comparison of serological tests for the detection of ovine and caprine antibody to Brucella melitensis. Rev Sci Tech. 2004;23(3):979–987. doi:10.20506/rst.23.3.1532
27. Etman RH, Barsoum SA, Ibrahim IGA, El-Ashmawy WR, Abou-Gazia KH. Evaluation of efficacy of some serological tests used for diagnosis of brucellosis in cattle in Egypt using latent class analysis. Sokoto J Vet Sci. 2014;12(2):1–7. doi:10.4314/sokjvs.v12i2.1
28. Mamo G, Abebe F, Worku Y, et al. Bovine tuberculosis and its associated risk factors in pastoral and agro-pastoral cattle herds of Afar Region, Ethiopia. J Vet Med Ani Health. 2013;5(6):171–179.
29. CSA. Cities & Towns; 2015. Available from: https://www.citypopulation.de/Ethiopia.html.
30. Tegegn AH, Feleke A, Adugna W, Melaku SK. Small ruminant brucellosis and public health awareness in two Districts of Afar Region, Ethiopia. J Vet Sci Technol. 2016;7:335. doi:10.4172/2157-7579.1000335
31. Adugna W, Tessema TS, Keskes S. Sero-prevalence of small ruminants’ brucellosis in four districts of Afar National Regional State, Northeast Ethiopia. J Vet Med Anim Health. 2013;5(12):358–364.
32. Dulo F. Seroprevalence of Caprine Brucellosis and Its Associated Risk Factor in Mirab Abaya district, South Eastern Ethiopia. J Natu Sci Res. 2017;7(9):ISSN 2224–3186.
33. Tadeg WM, Gudeta FR, Mekonen TY, Asfaw YT, Birru AL, Reda AA. Seroprevalence of small ruminant brucellosis and its effect on reproduction at Tellalak District of Afar region, Ethiopia. J Vet Med Anim Health. 2015;7(4):111–116. doi:10.5897/JVMAH2014.0287
34. Manishimwe R, Ntaganda J, Habimana R, et al. Comparison between rose Bengal plate test and competitive enzyme linked immunosorbent assay to detect bovine brucellosis in Kigali City, Rwanda. J Veterinar Sci Technol. 2015;6:211. doi:10.4172/2157-7579.1000211
35. Bishaif R, Galli R. Enzyme-linked immunosorbent assay for detection of antibodies to influenza A and B and parainfluenza type 1 in sera of patients. J Clin Microbiol. 1978;8:648–656. doi:10.1128/jcm.8.6.648-656.1978
36. Islam MA, Samad MA, Rahman AK. Risk factors associated with prevalence of brucellosis in Black Bengal goats in Bangladesh. Bangl J vet Med. 2010;8(2):141–147. doi:10.3329/bjvm.v8i2.11198
37. Kelkay MZ, Gugsa G, Hagos Y, Taddelle H. Seroprevalence and associated risk factors for Brucella seropositivity among small ruminants in Tselemti districts, Northern Ethiopia. J Vet Med Animal Health. 2017;9(11):320–326.
38. Sharma V, Sharma HK, Ganguly S, Berian S, Malik MA. Seroprevalence studies of brucellosis among goats using different serological tests. J Entomol Zool Stud. 2017;5(2):1512–1516.
39. Ahasan MS, Rahman MS, Rahman AK, Berkvens D. Bovine and Caprine Brucellosis in Bangladesh: bayesian evaluation of four serological tests, true prevalence, and associated risk factors in household animals. Trop Anim Health Prod. 2017;49(1):1–11. doi:10.1007/s11250-016-1151-1
40. Quinn P, Markey B, Carter M, Donnelly W, Leonard F. Veterinary Microbiology and Microbial Disease. Blackwell Science Ltd. A Blackwell publishing company; 2005.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.