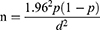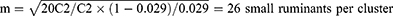Back to Journals » Veterinary Medicine: Research and Reports » Volume 14
Sero-Prevalence and Associated Risk Factors of Peste Des Petits Ruminants in Dera and Gerar Jarso Districts of Oromia Region, Ethiopia
Authors Ejigu E , Tolosa T , Begna F , Tegegne H
Received 31 March 2023
Accepted for publication 1 July 2023
Published 7 July 2023 Volume 2023:14 Pages 111—123
DOI https://doi.org/10.2147/VMRR.S410904
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Eyoel Ejigu,1 Tadele Tolosa,1 Feyissa Begna,1 Hailehizeb Tegegne2
1Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine Jimma University, Jimma, Oromia, Ethiopia; 2Department of Veterinary Science, College of Agriculture and Environmental Science, Debre Tabor University, Debre Tabor, Amhara, Ethiopia
Correspondence: Eyoel Ejigu, Email [email protected]
Introduction: Peste des petits ruminants is a transboundary disease of major economic importance and imposes significant constraints on small ruminant production.
Methods: A cross-sectional study was employed in Dera and Gerar Jarso districts of the North Shewa zone, Oromia Region from February 2021 to March 2022, to estimate the antibody of PPRV and assess the associated risk factors. Blood samples (n = 662) were collected from sheep and goats. Cluster sampling strategy was employed to collect the data. Villages/Kebeles and individual small ruminants were randomly selected, while households were designated using a systematic random sampling method.
Results: An overall individual animal and flock level sero-prevalence was 10.3% (95% CI = 8.2– 12.8) and 100% (95% CI = 96.3– 100), respectively, from the c-ELISA test result. A sero-prevalence of 11.2% (95% CI = 8.7– 14.4) in Dera and 8% (95% CI = 5– 12.7) in Gerar Jarso districts was recorded.
Discussion: Flock size, age, sex, communal grazing, and watering system, new small ruminant introduction into a flock, and mixed rearing were significantly associated with PPR sero-positivity in sheep and goats. The chance of PPR occurrence in goats was 4 times (OR = 4; P = 0.000) more than sheep. Female sheep and goats were more likely to be sero-positive to PPR by 3 times (OR = 3.2; P = 0.003) than males. The newly introduced small ruminants had 4 times more odds (OR = 4.4; P = 0.000) of sero-positivity than animals being born at home. Small ruminants kept under communal grazing and watering system were nearly 12 times (OR = 11.5; P = 0.024) more likely sero-positive than privately managed small ruminants. Likewise, sheep and goats reared together were almost 9 times (OR = 9.4; P = 0.000) a higher chance of being sero-positive compared with separately reared small ruminants.
Conclusion: The finding of PPR virus antibodies in small ruminants from all study areas indicates endemic circulation of the virus. The implementation of regular vaccination could minimize the occurrence of PPR.
Keywords: PPR, prevalence, risk factor, small ruminant, North Shewa
Introduction
Ethiopia possesses the largest population of small ruminants, with an estimated 42.9 million sheep and 52.5 million goats1 in Africa. Livestock ownership currently contributes to the livelihoods of an estimated 80% of the rural population.2 There are 39,854 sheep, 123,675 goats in Dera, 50,564 sheep, and 19,750 goats in Girar Jarso. Despite production and the disease challenges in Ethiopia, farmers prefer to rear sheep and goats for their low cost of production, prolificacy, adaptive capacity to the environment through dynamic feeding behavior, and fast reproduction cycle and growth rate.3 The degree to which sheep and goats survive to marketable age is one of the key indicators of the efficiency of their production. They also play an important role in food security and livelihood resilience in many parts of the country, but several constraints are reducing productivity in this sector. Peste des petits ruminants are considered a major restriction factor causing direct losses, such as death and decreased production, and indirect losses, such as, animal movement restriction and trade banns.4–6
Peste des petits ruminants (PPR) is one of the transboundary diseases of major economic importance and imposes significant constraints on small ruminant production due to its high mortality rate. It is an acute, highly contagious, and frequently fatal disease of sheep and goats caused by PPR virus, a member of the genus morbillivirus of the family Paramyxoviridae.7,8 Therefore, it is considered one of the most damaging animal diseases in Africa, the Middle East, and Asia. It is also one of the priority diseases indicated in the FAO-OIE Global Framework for the Progressive Control of Trans-boundary Animal Diseases.3
PPR is more pronounced in goats than in sheep, and mortality approaches 100% when associated with other disease complications. Following infection via the respiratory tract, PPRV replicates in the oropharynx and mandibular lymph node. The incubation period of PPRV is about three to four days before the onset of clinical disease.8,9 Pyrexia (40–41°C), nasal and ocular discharge, respiratory tract infection, and inflammation of the gastrointestinal tract that resulted in severe diarrhea are the disease clinical manifestation.10 Viremia may develop within 2–3 days and via blood, it spreads to other organs and tissues like the spleen, lungs, bone marrow, and mucosa of the gastrointestinal tract.11
Morbidity and case fatality rates vary depending on factors such as immune status, age, species, and the presence of other co-infections. The disease can cause mortality rates as high as 90–100% in naive sheep and goat populations. In clean flocks, sheep and goats of all ages can be affected during an outbreak.10,11 However, in endemic areas, the most susceptible ages are between 4 and 24 months. The disease has been associated with an increased animal movement for commercial and trade purposes, transhumance and nomadic customs, climatic changes, and extensive farming practices.12
The control of PPR requires an effective mass vaccination of small ruminants, where the virus is endemic and farmers are unable to afford and implement the strict sanitary control measures, including the stamping out policy, required to contain the virus. Mass vaccination campaigns must achieve high levels of herd immunity (70% to 80%) to block the epidemic cycle of the virus.10 With the tools currently available, disease control and subsequent eradication programs for PPR may be feasible options. An understanding of the cultural and socio-economic circumstances of goat and sheep owners and a keen watch on the endemic nature of PPR in neighboring countries will enhance the success of this approach. Coordinated efforts from all stakeholders, combined with proper funding and execution of control programs, will be needed to achieve the goal of PPR-free zones.13 As a result, Ethiopia has developed a strategy for the progressive control of PPR that builds upon the lessons learned from rinderpest eradication.14,15
However, the control campaign based on repeated vaccination of all susceptible small ruminants is not an easy way to implement. Hence, an epidemiologically based targeting of endemic populations and high-risk zones is essential. Among the highly risky zones of Oromia’s Regional State of Ethiopia is North Shewa, where there is no report on epidemiological and socio-economic studies, even though a large number of small ruminants are raised in the smallholder production system. Therefore, there is a need to assess the prevalence of the disease under village conditions to recommend possible prevention and control strategies that enhance poverty alleviation programs in the area.
Materials and Methods
Study Area Descriptions
The study was conducted in two districts (Dera and Girar Jarso) of the North Shewa zone of the Oromia region. The North Shewa has an area of 8990 km2 and accounts for about 2.5% of the total area of Oromia Region. It is located between 9.05°–10.23° N and 37.57°–39.28 E. It is bordered on the south by Oromia special zone surrounding Addis Ababa, on the southwest by West Shewa, on the north by Amhara Region, and the southeast by East Shewa. The total area of the zone is covered by 20.7% Kola (tropical), 42.6% Woyna Dega (Sub-tropical), and 36.7% Dega (temperate) agro-climatic zones, respectively (Figure 1). Temperate climate prevails in areas having elevation ranging from 1750 to 2700 meter above sea level. Crop-livestock production is the major farming practice in the zone.16
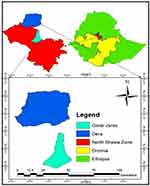 |
Figure 1 Map showing the location of the study sites. |
Dera is the largest district in the North Shewa zone (Oromia region) with 42 administrative PAs. It is situated north of Addis Ababa at 220 km. It is bordered on the south by the Jemma River, which separates it from Hidabu Abote and Wara Jarso districts, the Abay River defines the Western boundary with the East Gojjam zone, on the north and east by South Wollo zone as well as on the southeast by North Shewa of Amhara region. The district falls between 38.66° and 39.28° E and between 8.31° and 10.23° N. The agroecology of the district is mostly characterized by Kola with an altitude ranging from 1500 m to 1860 m. It receives an annual rainfall of 894 mm.
Gerar Jarso is located 114 km north of Addis Ababa at 8.54°–10.23° N and 37°.56–39.24° E. The district has 18 PAs including the zonal administrative town Fiche. The total area of the district is 42,763 hectares. Yaya Gulale and Debrelibanos districts border it on the south, on the west by Degem district, on the east by Amhara region (Ensaro district), and share a border with Hidabu Abote district on the north. The maximum altitude of the district is 2542 m, and the minimum is 1080m. The average minimum and maximum rainfall are 793 mm and 1443 mm, respectively.
Study Population
The study was done on some small ruminants found in Dera and Girar Jarso districts of North Shewa zone, Oromia region. Small ruminants considered for sampling in this study were those animals that were not vaccinated against PPR before. This was to eliminate the chance of being sero-positive due to vaccination. Small ruminants of both sex and greater than six months (0.5 years) old were included in the study.
Study Design
It was a cross-sectional study design that was conducted. Individual animal bio-data was collected using the appropriate format, and flock-level information was collected using a semi-structured questionnaire.
Sample Size Determination
The sample size for the study was determined based on the formula described by Abraham et al,17 using a 95% confidence interval at 5% desired absolute precision and considering a conservative prevalence of 50% to have a maximum sample size.
Where n = required sample size, P = expected prevalence, and d = desired absolute precision. Substituting each value gives n = 384. Given that cluster sampling was employed, the sample size was recalculated to get a closer accuracy to that of simple random sampling by considering the design effect. The design effect was determined using a formula described by Dohoo et al.18 Thus, the design effect = 1+ ρ (m − 1), where ρ is the intra-cluster correlation coefficient and m is the number of small ruminants sampled per cluster. A flock of sheep/goats in one village was considered a cluster. Rho of 0.029 was used, which was obtained from the national survey of PPR in 1999 in Ethiopia.19 The average number of sheep and goats to be sampled from each cluster (m) was estimated to be 26 according to,20 formula.
Where C1 is the cost of sampling a cluster, C2 is the cost of sampling a sheep or a goat within a cluster and ρ is the intra-cluster correlation coefficient (0.029). C1 was estimated to be 20 times more than C2 by considering the distance between the clusters.
Design effect = 1+0.029(26–1) =1.725
The new sample size (n’) was calculated by multiplying n = 384 by the design effect.21 Hence, n’ = n × Design effect. Therefore, n’ = 384 × 1.725 = 662. The number of clusters/villages to be sampled (25) in the seven kebeles was calculated by dividing the total sample size of small ruminants (662) by the average sample size per cluster (26). Sampling with probabilities proportional to the number of small ruminants in each district was used to determine the number of small ruminants included in the study in each district and Kebele. Similarly, the number of villages and PAs were proportionally allocated per district.20
Sampling Techniques
A cluster sampling strategy with probability sampling was used to meet the objectives of this study. Two districts (Dera and Gerar Jarso) from the North Shewa zone of Oromia were purposively selected from thirteen districts. Information on the general distribution of small ruminants was taken from the respective district livestock and fishery development offices. Further sampling was done according to the local administrative partition (Kebele, village, household) of the district. A total of seven Kebeles were selected randomly from Dera (4 Kebeles) and Gerar Jarso (3 Kebeles). Villages (25); 19 villages from Dera and 6 villages from Gerar Jarso were randomly selected. Averagely, nine households per village were designated using a systematic random sampling method. In this sampling technique, the total number of households found in a village (N = 27) was divided by the desired sample size (n = 9) to get the sampling interval (k=27/9). Thus, the households were sampled at every 3rd after randomly selecting the starting point of selection. Individual small ruminants were randomly selected (in average three animals per household).
Villages were considered as a single-level cluster of small ruminants (epidemiological unit) concerning disease risk, because the residents of the village are living very close to each other and had common watering and browsing/grazing lands. The number of small ruminants sampled from each district and Kebeles was proportional to the sheep and goats population in the district and Kebele. Accordingly, 463/163,529 and 199/70,314 small ruminants per district were sampled from Dera and Gerar Jarso, respectively. A total of 25 clusters/villages (considered as flocks of sheep and/or goats) were sampled from both districts. Thus, districts, Kebeles, species, flock size (small; 26–45 and large; >45), sex, age group, grazing and watering management, housing system, new animal introduction (mixing of the newly bought or brought animal to the flock) and mixed species rearing (rearing of sheep and goats together) were recorded being hypothesized as risk factors. Three local agroecological classifications (highland, midland, and lowland with an altitude range of >2300–3700, >1500–2300, and 500–1500 meters, respectively) were also included. Age was classified as young (>0.5–2 years) and adult (>2 years) and determined using dentition.
Laboratory Protocols
Sample Collection, Transportation, and Storage
Before collection of a sample, the small ruminant from which the sample was going to be taken was identified for vaccination history, and animals were handled in humane manner during sample collection to ensure the ethical treatment of animals and obtain accurate results, and to promote animal welfare, scientific integrity, and ethical conduct in research. We have used appropriate restraining techniques, providing a calm and quiet environment, and minimizing the duration of the procedure to minimize the stress and discomfort experienced by animals during the sample collection process. We confirm that the animals were treated with best practice of veterinary care. Then, blood samples for serological analysis were aseptically collected. Accordingly, the jugular vein area was disinfected using alcohol and about 2mL blood was collected from the vein using a venoject needle and plain vacutainer tube. Then, the collected blood samples were labeled with the date of collection, specific identification number, age, sex, agroecology, and species of the animals. In Selale University’s laboratory, the blood samples were allowed to stand in a slant position for 24 hours at room temperature. After 24 hours, the serum was harvested using clean sterile test tubes and centrifuged at 1500 rpm for 10 minutes to remove the remaining red blood cells. The serum was transferred into a sterile cryovials tube bearing the identification number, transported to the National Animal Health Diagnostic and Investigation Center (NAHDIC), Sebeta, Ethiopia, for laboratory analysis, and then stored at −20◦C until analyzed.
Serological Examination
All the 662 serum samples were analyzed in NAHDIC using the nucleoprotein-based competitive ELISA platform, ID Screen® PPR Competition (IDvet Innovative Diagnostics, Montpellier, France) according to the manufacturer’s OIE reference lab recommended technique. The kit micro-plates were coated with PPRV recombinant nucleoprotein. The reagents were allowed to come to room temperature (21 ± 5°C) before use and homogenized by the vortex. First, 25µL of dilution buffer 13 was added to all wells. Next, 25µL of the positive control was distributed to wells A1 and B1, and 25µL of the negative control was added to wells C1 and D1. Then, 25µL of each serum to be tested was added to the rest wells and incubated for 45 ± 4 minutes at 37± 3°C using a micro-plate incubator shaker (AQS manufacturing, LTD, UK). Each well was washed three rounds, using 300 µL of prepared wash solution. Next, 100µL of the conjugate 1× diluted in dilution buffer 4 was added to each well. After incubating for 30 ± 3 minutes at 21± 5°C, each well was washed three rounds with 300µL of the wash solution. Then, 100µL of the substrate solution was added to each well and incubated for 15 ± 2 minutes at 21± 5°C in the dark. Finally, 100µL of H2SO4 (stop solution) was added to all wells; the OD was read at 450 nanometers using the ELISA reader (Highland Park, USA). The cut-off points were calculated as competition percentages from the OD values using the following formula.22
Competition percentage = (OD Sample)/(OD Negative Control) x 100
If the percentage of competition (S/N %) was less or equal to 50% the sample was taken as positive, samples presenting the S/N% greater than 60% were negative and those showing S/N% between the negative and positive values were said to be uncertain (50%< S/N% ≤60%).
Questionnaire Survey
Verbal consent was obtained from the interviewees (respondents) who were willing to give information. Interviews were performed in Afan Oromo and Amharic. At the start of our research, we attempted to obtain an ethical approval letter from the office responsible for such tasks. Unfortunately, the director in charge of providing the letter had passed away from Covid-19, and as a result, all the staff members were forced to isolate themselves. The office was closed for more than two months, causing possible delays in our research. Therefore, to avoid any further delays, we decided to acquire the ethical approval letter from the district where the research was being carried out.
Verbal consensus was taken after being approved from Dera district Fishery and livestock development office Ethics Committee coordinator Mr Usman Yasin Ahmed. Verbal consensus was taken because 95% of the farmer in Ethiopia neither read nor write the local language and as a result consent was taken to ensure that farmers who cannot read and write are able to access and understand key information, receive the same level of support as their literate counterparts, and be active participants in decision-making processes. We preferred verbal consent because it is important to be able to conduct interviews quickly and efficiently. Additionally, taking verbal consent provides a platform for these farmers to express their opinions while expanding their knowledge base through education.
The objective of the survey was also explained to them. The survey was conducted by administering the semi-structured questionnaires to 225 household representatives (143 males and 82 females) for identifying risk factors. The contents of the questionnaire were mainly focused to collect information about age, vaccination history, housing system, grazing and watering management, animal marketing, movement of animals, raising system, and flock size. Interviewing and sampling were done in collaboration with the two district veterinary offices; the veterinary assistant and Asella regional laboratory field workers were present at all sampling sites.
Data Analysis
Data obtained from the questionnaire and laboratory results were recorded, coded, and stored in a Microsoft Excel sheet. Microsoft Excel sheet format of the animal and flock level data was transferred to IBM SPSS version 25. Descriptive statistics were used to summarize the data and analytical statistics were used as appropriate. The units of analysis were individual sheep and goats, and flocks. Animal-level seroprevalence was calculated for all categories of assumed risk factors as the number of PPR-infected individuals divided by the number of individuals sampled and for flock-level sero-prevalence, the number of positive flocks for at least one sheep/goat divided by the total number of flocks tested. Univariable analysis was performed and factors with p-value <0.25 were taken forward for multivariable analysis to correct for confounding effects. Associations between PPR sero-positivity and risk factors for all units of analysis were investigated by using multivariable logistic regression. The strength of the association between PPR seropositivity and the potential risk factors (explanatory variables) was assessed using the adjusted odds ratios (AOR). For all analyses, the confidence level is at 95%, and P ≤ 0.05 was set for significance. The collinearity of the variables was analyzed using Spearman-correlation coefficient. Interaction between the factors taken to multivariable analysis was tested and turned with no interaction.
Results
In this study, 662 small ruminants (319 sheep and 343 goats) were sampled; among which 68 of them were sero-positive. An overall sero-prevalence of 10.3% (95% CI = 8.2–12.8) was estimated (Table 1). A flock with at least one positive animal was considered a positive flock for PPR; 100% (95% CI = 94–100) flock-level sero-prevalence was found.
 |
Table 1 Univariable Logistic Regression Analysis of Individual Animal-Level Sero-Prevalence and Possible Risk Factors |
Risk Factors for Individual Sero-Positivity of Small Ruminants
Among sampled sheep (n = 319), 70.2% were females (n = 224) and 29.8% were males (n = 95). Similarly, 70.3% female (n = 241) and 29.7% male (n = 102) goats were sampled. The age distribution (including both sheep and goats) was 52% more than six months to two years of age (>0.5–2) (n = 345) and 48% more than two years old (>2) (n = 317).
Based on this study result, the highest district-based sero-prevalence was found in Dera (11.2%; P = 0.217) compared to the Gerar Jarso district 8%. At the Kebele level, the highest sero-prevalence was recorded in Amoma Gendo (17%; P = 0.076) and the lowest was in Gerar Geber (6%). The higher (15.2%; P = 0.000) and the lower (5%) sero-positivity were detected in goats and sheep, respectively. The disease was more prevalent in females (12.7%; P = 0.003) than in males (4.6%). Sheep and goats greater than two years old showed a higher prevalence of PPR (14%; COR = 2.16) than those more than six months to two years old (7%). In newly introduced animals 25% (P = 0.000) sero-prevalence was estimated and 7.4% was recorded in permanently stayed small ruminants. Small ruminants sampled from communal grazing and watering system were in higher prevalence of the PPR antibody (11.5%; COR = 9.98) than those found in private (1.3%). The study also showed that larger flock sizes had higher sero-positivity (14.6%; P = 0.001) than the smaller flock sizes (6.6%) (Table 1).
The univariable logistic regression analysis on individual animal-level risk factors showed that the district (P = 0.217), species (P = 0.000), sex (P = 0.003), age (P = 0.004), new animal introduction (P = 0.000), grazing and watering management (P = 0.023), and raising the two species together (P = 0.000) had a statistically significant effect on sero-prevalence of PPR (P < 0.05). Accordingly, they were selected for the final model (Table 2).
 |
Table 2 Multivariable Logistic Regression Analysis in Animal-Based Sero-Prevalence of PPR in Association with Potential Risk Factors |
In the final model analysis result, the factor species (P = 0.000), flock size (P = 0.019), sex (P = 0.003), age (P = 0.001), new animal introduction (P = 0.000), grazing and watering management (P = 0.024), and rearing of the two species together (P = 0.000) were shown as statistically significantly (P < 0.05) associated with the sero-prevalence of PPR. Goats were 4 times (OR = 4.0) more likely sero-positive to PPR antibody compared to sheep. The larger flock sizes had the odds of 2 times being sero-positive than the smaller flock size (Table 2).
As shown in Table 2, female sheep and goats were more likely to be sero-positive to PPR by 3 times (OR = 3.2) than males. Small ruminants with an age of greater than two years had almost 3 times more odds (OR = 2.7) of sero-positivity for the disease than those aged greater than six months to two years. The newly introduced small ruminants were statistically, strongly significant with p-value=0.00 and had the odds of 4-times (OR = 4.4) sero-positivity than animals being born at home.
As illustrated in Table 2, sheep and goats kept under communal grazing and watering system were nearly 12 times (OR = 11.5) more likely sero-positive than privately managed small ruminants. Small ruminants reared together were almost 9 times (OR = 9.4) at a higher chance of being seropositive compared with separately reared small ruminants (Table 2).
Associated Risk Factors for Sheep and Goat Sero-Positivity
In the separate univariable logistic regression analysis of sheep and goat data, the possible risk factors for PPR sero-positivity were identified. The new animal introduction was the only risk factor that had strong statistical association (P = 0.000) for being sero-positive in sheep (Table 3). While in goats, flock size (P = 0.001), sex (P = 0.003), age (P = 0.001), new animal introduction (P = 0.000), and rearing of goat and sheep together (P = 0.003) were found to be the significant risk factors (Table 4).
 |
Table 3 Variables Associated with the Sero-Positivity in Univariable Models of Sheep Data |
 |
Table 4 Variables Associated with the Sero-Positivity in Univariable Models of Goat Data |
In the final model analysis result (Table 5), flock size (P = 0.012), sex (P = 0.007), age (P = 0.000), new animal introduction (P = 0.001), and rearing the two species together (P=0.002) were the potential risk factors for the sero-positivity of goats. Hence, goats that had a large flock size were 2 times (OR = 2.4) more likely to be sero-positive for PPR antibody than the smaller flock size. Female goats had nearly 4 times (OR = 3.6) odds of being sero-positive compared to males. Goats, aged greater than two years, were almost 4 times (OR = 3.7) at a higher chance to have PPR antibodies than those aged between greater than six months and two years. Newly introduced goats were in the odds of almost 4 times (OR = 3.6) compared to those born in the flock. Goats reared with sheep were 7 times (OR = 7.1) more seropositive than those raised separately (Table 5).
 |
Table 5 Multivariable Logistic Regression of Potential Risk Factors Associated with Sero-Prevalence of Goats |
Discussions
In this study, the overall individual animal-based sero-prevalence of peste des petits ruminants was 10.3%. This finding is comparable with the result of 6.8% by Abraham et al,17 from Afar and Borena, 8% by Waret-Szkuta et al,19 in Benishangul, 7.93% and 13.62% by Fentie et al,22 in East Gojjam and North Gondar, respectively. It is also slightly in agreement with Wegayehu et al,16 who reported 5.71% from Horo Guduru Zone, Oromia region. This finding was higher than the result of Waret-Szkuta et al,19 who reported 1.7%, 4.6%, and 1.8%, from Oromia, Amhara, and Southern Nations Nationalities and Peoples Region, respectively.
It is also higher than the result reported by 2.1% by Gebre et al,23 from Bench Maji and Kefa Zones, SNNPR. However, it is lower than Delil et al24 who documented 36.6% from Awash Fentale, OIE4 who reported 27.3% and 38.3% from Itang (Gambella) and Adar (Afar), respectively, Afera et al25 who reported 47.5% from Tigray, and Gari et al26 who reported 48.43% in East Shewa and Arsi areas. This variation might be due to the geographical location and climatic differences between the localities, variation in the production system, and proportion of sample size.7,8,15,19
Species-specific prevalence was determined in this study. Goats were observed to have significantly (P < 0.005) greater sero-prevalence (15.2%) than sheep (5%). This finding was in agreement with Fentie et al,22 who documented higher sero-positivity in goats than sheep (21.57% in goats and 14.89% in sheep). Other findings as Abubakar et al and Waret-Szkuta et al7,19 (49.5% in sheep and 56.3% in goats) and Delil et al24 (7.3% sheep and 42.6% goats) were reported a higher sero-prevalence of PPR in goats than sheep. On contrary, the findings of Abraham et al17 from Afar and Borena, Saeed et al27 from Sudan, Abdalla et al28 from the Kordofan state of Sudan, and Gelana et al14 from Horo Guduru zone of Ethiopia documented higher seroprevalence in sheep than goats. The difference in prevalence might be due to the difference in the proportion of sampled animals, management system, and geospatial location of the study areas. An outbreak with high mortality in sheep was also reported that sheep possessed an innate resistance to the clinical effects of the disease, but occasional field strains could overcome this resistance and produce high mortality.25,26,29,30 Goats are affected more severely by PPR virus exposure compared to sheep, and they exhibit easily noticed clinical signs while sheep undergo a mild form of the disease as documented by Delil et al, Libeau et al and Farougou et al.24,31,32
The multivariable logistic regression analysis result showed that females were more likely to be sero-positive than males. It was also indicated that females (12.7%) had significantly higher sero-positivity than the male counterparts (4.6%). This observation agrees with the result of OIE, Gebre et al, Wondimagegn and Libeau et al.4,23,29,31 This might be due to sampling size variation and the physiological differences where females reveal some degree of predominance infection because of production and reproduction-related stresses. This could be also due to male small ruminants are not stayed long in the flock (sold or slaughtered) while the females remain for breeding.4,16,17,19,25 This result reflection disagrees with the findings of Alemu et al33 from East Amhara Region and Swai et al34 from Tanzania who stated that males had higher seroprevalence than female small ruminants.
In considering the two species’ sex, female goats had more sero-prevalence (19.1%) than female sheep (5.8%) as described in Table 5 and Table 4, respectively. This finding is in line with OIE and Libeau et al,4,31 who reported a higher PPR antibody sero-prevalence in female goats compared with female sheep. A similar comparison was observed in male goats, which recorded, a slightly higher sero-prevalence (5.9%) compared with male sheep (3.1%). This could also be due to the fact that male small ruminants usually not allowed to stay long in the flock. This increased PPR prevalence in goats in both sexes might be the difference in the proportion of sampling and variation in recovery rate on sheep and goats. Sheep’s recovery is usually higher than goats’.26
From the multivariable logistic regression, the chance of being sero-positive was higher in the age group >2 years (adults). The adult small ruminants had higher sero-prevalence (14%) than the younger age group (7%). The reports of Abubakar et al, Waret-Szkuta et al, Abdalla et al, Wondimagegn and Alemu et al7,19,28,29,33 documented high prevalence in adults, which agreed with this result. In contrast, Farougou et al32 from Niger and Afera et al25 reported high sero-prevalence in young (>0.5–2) small ruminants. Libeau et al31 reported that the natural infection of the PPR virus at a very young age might have the probability to carry antibodies for 1–2 years following exposure and remains positive for a long time.
In this research, we also found that communal grazing and watering systems were significantly associated with the transmission of PPR virus infection among sheep and goats than private grazing management. In agreement with this result, Abubakar et al7 from Pakistan documented that continued year-round circulation of the virus was enhanced by frequent animal-to-animal contact on the grazing pasture. Wegayehu et al16 also indicated communal sharing of grazing land probably increases small ruminant vulnerability to infection. Even though the aerosol route is the main transmission way for PPRV, oral transmission is possible by contact with secretions or excretions (saliva, feces, urine, and vaginal, nasal or ocular discharges) of infected animals from the communal pasture.15,17,22
The introduction of new small ruminants appears to be a risk factor for seropositive status, in the multivariable logistic regression analysis. Bello et al35 from Nigeria, Clarke et al9 from Bangladesh, and De Nardi et al36 from Algeria stated that the introduction of new animals purchased from the live animal market was a means of disease transmission. Wondimagegn and Alemu et al29,33 also reported that newly introduced small ruminants were in a higher chance of being sero-positive than the home-born ones. This might be due to the absence of practicing isolation and quarantine of newly bought animals before mixing into the flock.23 In contrast to this result, Saeed et al27 reported that newly introduced animals had lower sero-positivity in Sudan.
The result of multivariable logistic regression showed that flock size was statistically significant (P = 0.019). The large flock size had the odds of 2 times being sero-positive. This finding is in agreement with the works of OIE, Gelana et al, Gebre et al and Gari et al4,14,23,26 while the report of Saeed et al27 stands in contrast to this result. The overcrowding or the stocking density of small ruminants might have increased the spread of the virus due to direct contact with the animals.15
Dera district had slightly higher seroprevalence (11.2%) than Gerar Jarso (8%) district. The seroprevalence result variation between the districts might be due to the contagious nature of the disease in wide geographic areas and infecting perhaps most of the susceptible animals in affected villages.8,29,33 The sample size difference, geographical and seasonal effects, host population density, disease control programs, and the social environment that can influence the contact rates and husbandry practices may explain the differences with other areas.4,25,28,31
In the agro-ecology-based sero-prevalence, lowland areas were statistically significant (P = 0.025) and had the highest sero-prevalence. The finding of this study agrees with the report of Waret-Szkuta et al, Fentie et al, Afera et al and Abdalla et al.19,22,25,28 The documents29,33 reported higher seroprevalence of PPR in lowland areas of Somali and Eastern Amhara region, respectively. This might be due to different production systems with exchanges and movements in areas of lowland being more frequent and involving larger numbers of animals. Abdalla et al28 from Sudan and Bello et al35 from Nigeria documented that lowland agroecology was the risk factor for PPR sero-positivity. In Ethiopia, small ruminants mainly thrive on free-range grazing lands, shrubs, and forest grounds. Agro-climatic conditions influence the availability of these resources and the movement of animals becomes necessary to ensure the provision of fodder and water. This is particularly important during the dry season and in low-altitude areas where resources are scarce. However, in the report of Wegayehu et al,16 the highest PPR prevalence was found in the midland agro-ecology from Horo Guduru Zone, Western Ethiopia.
Conclusion and Recommendations
The present study confirmed the presence of PPR antibodies in sheep and goats in our study areas (Dera and Gerar Jarso districts). The fact that antibodies of PPR virus were detected in studied Kebeles and districts suggests, the infection has been circulating/endemic. The disease was found to be more prevalent in Dera than Gerar Jarso district. This study has indicated that sero-prevalence of PPRV in goats was higher than that of sheep. Flock size, species sex, age, the introduction of a new animal to the flock, utilization of communal grazing and watering system, and mixed species rearing were found to be the higher disease predictors or risk factors for the occurrence and distribution of PPR infection.
Based on the concluding remarks, the following recommendations are forwarded:
- Mass vaccination programs should be mandatory.
- Further studies are needed, to establish the disease situation in other animals in the study area.
Disclosure
The authors report no conflicts of interest in this work.
References
1. CSA (Central Statistical Authority). The Federal Democratic Republic of Ethiopia, Central Statistical Agency, Report on livestock and livestock characteristics, Agricultural Sample Survey. In: Statistical Bulletin 589 Volume II (March). Addis Ababa, Ethiopia; 2021:1–199.
2. Husen M, Aliyi F, Damtew S, et al. Prevalence of small ruminant helminthiasis in and around Tullo district in western Harerghe zone, eastern Ethiopia. Austin J Vet Sci Anim Husb. 2018;5(1):1038.
3. FAO, OIE. Peste Des Petits Ruminants Global Eradication Program. Contributing to Food Security, Poverty Alleviation and Resilience. Rome: Food and Agriculture Organization of the United Nations and the World Organization for Animal Health; 2016.
4. OIE. Peste Des Petits Ruminants (Infection with Peste Des Petits Ruminants virus).OIE Terrestrial Manual. World Organization for Animal Health. 2019. 1–16.
5. Megersa B, Biffa D, Belina T, et al. Serological investigation of peste des petits ruminants (PPR) in small ruminants managed under pastoral and agro-pastoral systems in Ethiopia. Small Ruminant Res. 2011;97(1–3):134–138. doi:10.1016/j.smallrumres.2011.03.003
6. Ruget AS, Tran A, Waret-Szkuta A, et al. Spatial multicriteria evaluation for mapping the risk of occurrence of Peste des Petits Ruminants in Eastern Africa and the Union of the Comoros. Front Veterinary Sci. 2019;6:455. doi:10.3389/fvets.2019.00455
7. Abubakar M, Javed Arshed M, Hussain M, et al. Evidence of peste des petits ruminants in serology of sheep and goats from Sindh, Pakistan. Transbound Emerg Dis. 2011;58(2):152–156. doi:10.1111/j.1865-1682.2010.01193.x
8. Balamurugan V, Hemadri D, Gajendragad MR, et al. Diagnosis and control of peste des petits ruminants: a comprehensive review. Virus Dis. 2014;25(1):39–56. doi:10.1007/s13337-013-0188-2
9. Clarke BD, Islam MR, Yusuf MA, et al. Molecular detection, isolation and characterization of Peste‐des‐petits ruminants virus from goat milk from outbreaks in Bangladesh and its implication for eradication strategy. Transbound Emerg Dis. 2018;65(6):1597–1604. doi:10.1111/tbed.12911
10. Hailegebreal G. Seroprevalence of Peste des Petits ruminants in selected districts of Siltie and Gurage zones, south region, Ethiopia. J Animal Veterinary Adv. 2019;18(2):30–34. doi:10.36478/javaa.2019.30.34
11. Gizaw F, Merera O, Zeru F, et al. Sero-Prevalence and Socioeconomic Impacts of Peste Des Petits Ruminants in Small Ruminants of Selected Districts of Afar, Ethiopia. J Veterinary Sci Technol. 2018;9(1). doi:10.4172/2157-7579.1000513
12. Ishag OM, Saeed IK, Ali YH. Peste des petits ruminants outbreaks in White Nile State, Sudan: research communication. Onderstepoort J Veterinary Res. 2015;82(1):1–4. doi:10.4102/ojvr.v82i1.897
13. Munir M, Zohari S, Berg M. Genome organization of peste des petits ruminants virus. In: Molecular Biology and Pathogenesis of Peste Des Petits Ruminants Virus. Springer, Berlin, Heidelberg: Springer Science & Business Media; 2013: 1–22.
14. Gelana M, Gebremedhin EZ, Gizaw D. Seroepidemiology of Peste des Petits ruminants in sheep and goats in the selected district of Horu Guduru Zone, Western Ethiopia. Res Vet Sci. 2020;132:527–534. doi:10.1016/j.rvsc.2020.07.019
15. OIE. Peste Des Petits Ruminants: Aetiology, Epidemiology, Diagnosis, Prevention and Control References. OIE Technical Disease Cards, World Organisation for Animal health. 2020. 1–16.
16. Wegayehu T, Adamu H, Petros B. Prevalence of Giardia duodenalis and Cryptosporidium species infections among children and cattle in North Shewa Zone, Ethiopia. BMC Infect Dis. 2013;13(1):1–7. doi:10.1186/1471-2334-13-419
17. Abraham G, Sintayehu A, Libeau G, et al. Antibody seroprevalences against peste des petits ruminants (PPR) virus in camels, cattle, goats and sheep in Ethiopia. Prev Vet Med. 2005;70(1–2):51–57. doi:10.1016/j.prevetmed.2005.02.011
18. Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research.
19. Waret-Szkuta A, Roger F, Chavernac D, et al. Peste des Petits Ruminants (PPR) in Ethiopia: analysis of a national serological survey. BMC Vet Res. 2008;4(4):34. doi:10.1186/1746-6148-4-34
20. Elbers ARW, Stegeman JA, De Jong MF, et al. Estimating sample sizes for a two‐stage sampling survey of seroprevalence of pseudo rabies virus (PRV) ‐infected swine at a regional level in the Netherlands. Veterinary Quarterly. 1995;17(3):92–95. doi:10.1080/01652176.1995.9694540
21. Thrusfield M. Veterinary Epidemiology.
22. Fentie T, Teshome Y, Ayele B, et al. Sero-epidemiological study of Peste des petits ruminants in small ruminants in Amahara region, Ethiopia. Comp Clin Path. 2018;27(4):1029–1036. doi:10.1007/s00580-018-2697-2
23. Gebre T, Deneke Y, Begna F. Seroprevalence and Associated Risk Factors of Peste Des Petits Ruminants (PPR) in Sheep and Goats in Four Districts of Bench Maji and Kafa Zones, South West Ethiopia. Global Veterinaria. 2018;20(6):260–270.
24. Delil F, Asfaw Y, Gebreegziabher B. Prevalence of antibodies to peste des petits ruminants virus before and during outbreaks of the disease in Awash Fentale district, Afar, Ethiopia. Trop Anim Health Prod. 2012;44(7):1329–1330. doi:10.1007/s11250-012-0110-8
25. Afera B, Hussien D, Amsalu K. Seroprevalence of Peste des petits ruminants in goats of southern parts of Tigray region. Global Veterinaria. 2014;12(4):512–516.
26. Gari G, Serda B, Negesa D, et al. Serological Investigation of Peste Des Petits Ruminants in East Shewa and Arsi Zones, Oromia Region, Ethiopia. Vet Med Int. 2017;2017:1–5. doi:10.1155/2017/9769071
27. Saeed FA, Abdel-Aziz SA, Gumaa MM. Seroprevalence and associated risk factors of Peste des petits ruminants among sheep and goats in Kassala state, Sudan. Open J Animal Sci. 2018;8(4):381–395. doi:10.4236/ojas.2018.84029
28. Abdalla AS, Majok AA, El Malik KH, et al. Sero-prevalence of peste des petits ruminant’s virus (PPRV) in small ruminants in Blue Nile, Gadaref and North Kordofan States of Sudan. J Public Health Epidemiol. 2012;4(3):59–64. doi:10.5897/JPHE11.213
29. Wondimagegn D Sero-Epidemiology and Spatial Distribution of Peste des Petits Ruminants Virus Antibodies in Some Selected pastoral Areas of Somali Regional State, Ethiopia. MSc Thesis, Addis Ababa University, College of Veterinary Medicine and Agriculture, veterinary medicine international. 2016;7(2).
30. Yalew S, Woldemichal G, Mamo M. Seroprevalence of Peste Des Petits Ruminant’s Virus Antibody in Assosa Zone, Benishangulgumuz Region, Ethiopia. ARC J Animal Veterinary Sci. 2019;5(3):29–33.
31. Libeau G, Prehaud C, Lancelot R, et al. Development of a competitive ELISA for detecting antibodies to the peste des petits ruminants virus using a recombinant nucleoprotein. Res Vet Sci. 1995;58(1):50–55. doi:10.1016/0034-5288(95)90088-8
32. Farougou S, Gagara M, Mensah GA. Prevalence of peste des petits ruminants in the arid zone in the Republic of Niger. Onderstepoort J Veterinary Res. 2013;80(1):6. doi:10.4102/ojvr.v80i1.544
33. Alemu B, Gari G, Libeau G, et al. Molecular detection and phylogenetic analysis of Peste des petits ruminants virus circulating in small ruminants in eastern Amhara region, Ethiopia. BMC Vet Res. 2019;15(1):1–9. doi:10.1186/s12917-019-1828-6
34. Swai ES, Kapaga A, Kivaria F, et al. Prevalence and distribution of Peste des petits ruminant’s virus antibodies in various districts of Tanzania. Vet Res Commun. 2009;33(8):927–936. doi:10.1007/s11259-009-9311-7
35. Bello MB, Kazeem HM, Oladele SB, et al. Seroprevalence of Peste des petits ruminants among unvaccinated small ruminants in Sokoto State, northwestern Nigeria. Comp Clin Path. 2018;27:1141–1146. doi:10.1007/s00580-018-2711-8
36. De Nardi M, Lamin Saleh SM, Batten C, et al. First evidence of peste des petits ruminants (PPR) virus circulation in Algeria (Sahrawi territories): outbreak investigation and virus lineage identification. Transbound Emerg Dis. 2012;59(3):214–222. doi:10.1111/j.1865-1682.2011.01260.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.