Back to Journals » Journal of Inflammation Research » Volume 17
Risk Factors for Systemic Inflammatory Response Syndrome After Percutaneous Transhepatic Cholangioscopic Lithotripsy
Authors Cheng L, Niu J, Cheng Y, Liu J, Shi M, Huang S, Ding X, Li S
Received 30 December 2023
Accepted for publication 9 April 2024
Published 25 April 2024 Volume 2024:17 Pages 2575—2587
DOI https://doi.org/10.2147/JIR.S453653
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Tara Strutt
Lve Cheng,* Junwei Niu,* Yao Cheng,* Jie Liu, Mengjia Shi, Shijia Huang, Xiong Ding, Shengwei Li
Department of Hepatobiliary Surgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiong Ding; Shengwei Li, Department of Hepatobiliary Surgery, Second Affiliated Hospital of Chongqing Medical University, 76 Linjiang Road, Yuzhong, Chongqing, 400010, People’s Republic of China, Tel +86-13677662766 ; +86-13508312245, Email [email protected]; [email protected]
Background: There is a lack of validated predictive models for the occurrence of systemic inflammatory response syndrome (SIRS) after percutaneous transhepatic cholangioscopic lithotripsy (PTCSL) for the treatment of hepatolithiasis. This is the first study to estimate the incidence of SIRS after PTCSL.
Methods: A retrospective analysis of 284 PTCSL sessions for the treatment of hepatolithiasis at our institution between January 2019 and January 2023 was performed. The development of SIRS after PTCSL was the primary study endpoint. Independent risk factors for SIRS after PTCSL were identified using univariate and multivariate logistic regression analyses. A nomogram prediction model was constructed using these independent risk factors, and the predictive value was assessed using receiver operating characteristic (ROC) curves.
Results: The incidence of SIRS after PTCSL was 20.77%. According to multivariate analysis, the number of PTCSL sessions (odds ratio [OR]=0.399, 95% confidence interval [CI]=0.202– 0.786, p=0.008), stone location (OR=2.194, 95% CI=1.107– 4.347, p=0.024), intraoperative use of norepinephrine (OR=0.301, 95% CI=0.131– 0.689, p=0.004), intraoperative puncture (OR=3.476, 95% CI=1.749– 6.906, P< 0.001), preoperative gamma-glutamyltransferase (OR=1.002, 95% CI=1.001– 1.004, p=0.009), and preoperative total lymphocyte count (OR=1.820, 95% CI=1.110– 2.985, p=0.018) were found to be independent risk factors for the development of SIRS after PTCSL. These six independent risk factors were used to construct a nomogram prediction model, which showed satisfactory accuracy with an area under the ROC curve of 0.776 (95% CI: 0.702– 0.850).
Conclusion: The number of PTCSL sessions, stone location, intraoperative use of norepinephrine, intraoperative puncture, preoperative gamma-glutamyltransferase, and preoperative total lymphocyte count may predict the occurrence of SIRS after PTCSL. This prediction model may help clinicians identify high-risk patients in advance.
Keywords: percutaneous transhepatic cholangioscopic lithotripsy, hepatolithiasis, systemic inflammatory response syndrome, risk factors, nomogram
Introduction
Hepatolithiasis is a common benign disease of the biliary tract in Southeast Asia.1,2 It is the leading cause of death from nonneoplastic biliary disease.1,2 The prolonged presence of stones can lead to biliary sepsis, biliary cirrhosis, and cholangiocarcinoma.1,2 However, due to the complexity of the anatomy of the bile ducts and the specificity of the location of these stones, there is a high rate of stone retention and recurrence after hepatectomy.3–5 It has been reported that 10–15% of cases of hepatolithiasis cannot be completely resolved by surgery.6,7 If a patient has a recurrence of a stone and is in need of further surgery, the procedure is difficult, risky and has a long recovery time.8 Therefore, a safe and reproducible minimally invasive surgical procedure to is needed to overcome the shortcomings of traditional surgeries.
Percutaneous transhepatic cholangioscopic lithotripsy (PTCSL) is derived from percutaneous nephrolithiasis.9–11 PTCSL has gradually become one of the most important tools used to treat hepatolithiasis. PTCSL is a less invasive treatment than traditional surgery and can preserve more liver parenchyma to maintain liver function. It is particularly suitable for patients who have a history of biliary surgery or scattered stones and who cannot tolerate traditional surgery.12
Despite adequate preoperative preparation, PTCSL, as an invasive technique, is inevitably associated with surgical risks and complications. Although PTCSL has a low rate of postoperative complications, infection-related complications such as fever, systemic inflammatory response syndrome (SIRS), and sepsis may still occur postoperatively.13–15 Intraoperative PTCSL may damage bile duct cells. As a consequence, large numbers of bacteria and endotoxins are released into the blood circulation. Biliary hypertension caused by prolonged biliary flushing can increase the absorption of pathogens with the perfusate and cause an inflammatory response or even sepsis in severe cases.16,17 This can affect patient prognosis. SIRS is a step in the sepsis cascade, and studies have shown that SIRS is strongly associated with poor prognosis.18 Thus it is important to assess the risk factors associated with the occurrence of SIRS after PTCSL.
However, there is a paucity of clinical studies on this topic. This study is the first analysis of the factors that influence the occurrence of SIRS after PTCSL. A nomogram prediction model was developed to predict the risk of SIRS after PTCSL.
Materials and Methods
Study Subjects
In this retrospective study, the clinical data of 284 PTCSL sessions were collected at the Second Affiliated Hospital of Chongqing Medical University from January 2019 to January 2023. All patients provided written informed consent. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University (No. 2018–207).
The inclusion criteria were as follows: (a) confirmed diagnosis of hepatolithiasis with or without choledocholithiasis based on imaging; (b) primary stones or recurrent stones after traditional open surgery; (c) non-acute septic biliary infection or cholangitis with symptomatic improvement after treatment; and (d) Child-Pugh grade A or B.
The exclusion criteria were as follows: (a) had a combined malignant tumour; (b) had a serious bleeding tendency or coagulation dysfunction; (c) had combined postoperative pneumonia, hypovolaemia, or uncontrolled pain; (d) had a preoperative combination of liver abscess, infection in other parts of the body, or immune system disease; and (e) had Child-Pugh grade C.
Observation Indicators
The following baseline information was collected: age, gender, body mass index (BMI), hypertension, diabetes, cirrhosis, preoperative history of acute cholangitis, the number of PTCSL sessions, type of previous surgery, type of hepatolithiasis, and stone location. The preoperative laboratory indicators were as follows: albumin, globulin, alanine transferase, aspartate transferase, alkaline phosphatase, gamma-glutamyl transferase (GGT), total bilirubin, direct bilirubin, indirect bilirubin, total bile acids, prealbumin, haemoglobin, leukocytes, neutrophil percentage, neutrophil count, total lymphocyte count (TLC), and platelets. The NLR, PLR, SII, and PNI were calculated from preoperative peripheral blood samples. The intraoperative indicators were as follows: the number of puncture channels, puncture method (ultrasound or X-ray), perfusion volume, operative time, intraoperative bleeding, American Society of Anaesthesiologists (ASA) score, intraoperative use of norepinephrine solution, intraoperative puncture, and postoperative stone residue.
Standard Definition
The diagnostic criteria for SIRS are as follows: SIRS can be diagnosed if two or more of the following indicators are met: (a) shortness of breath, respiratory rate >20 breaths/min, or PaCO2<32 mmHg; (b) temperature >38 °C or <36 °C; (c) heart rate >90 /min; and (d) leukocyte count >12×109/L or <4×109/L or immature granulocytes >10%.
Surgical Techniques
Preoperatively, patients with acute cholangitis require antibiotic therapy. In the absence of bile culture results, β-lactam antibiotics were used; in the presence of positive bile culture results, appropriate antibiotics based on susceptibility testing were used until the patient’s infection symptoms had recovered. A single dose of the same antibiotic was given 30 minutes before surgery. A single dose of β-lactam antibiotic prophylaxis 30 minutes before surgery was given to patients without preoperative acute cholangitis. Intraoperative biliary irrigation was performed with a saline solution in 3-litre bags connected to a choledochoscope by an infusion set suspended at a height of 2.0 m, with the irrigation speed set to the maximum. After induction of general anaesthesia, the surgical procedure followed the standardised PTCSL protocol: for patients treated with one-step PTCSL, the stone location was clarified based on preoperative imaging, and the puncture pathway was planned.19 The target bile duct was punctured with a coarse 18G needle (9,013,606, Zhengzhou Dior Medical Technology Company, China) under ultrasound guidance (DC-7T, Shenzhen Mindray Bio-Medical Electronics Company, China). Then the sinusoid was dilated from 8-Fr to 18-Fr by a fascial tearing device (9,013,606, Zhengzhou Dior Medical Technology Company, China) after placement of a guide wire (10S13508, Hunan Epte Medical Equipment Company, China). Finally, a 16-Fr or 18-Fr protective sheath (G06444, Cook Medical Holdings LLC, America) was placed into the intrahepatic bile ducts to create a channel. A combination of a rigid choledochoscope (8968.405, Richard Wolf Company, Germany) and an electronic choledochoscope (EyeMax CDS11001, Nanjing Micro-Tech Company, China) was used to search for intrahepatic bile duct stones. For stones smaller than the diameter of the sheath, a basket (VDK-BAS-18-70-15-N4-D,Jiangsu Vedkang Medical Technology Company, China) was used to remove the stones. For larger stones, holmium laser lithotripsy (DHL-1-D, Wuxi Dahua Laser Device Company, China) was used to remove the stones. Postoperative support drains (9,013,606, Zhengzhou Dior Medical Technology Company, China) were removed at regular intervals. Patients treated with two-step PTCSL underwent puncture and drainage of the target bile ducts under ultrasound or X-ray guidance before surgery. Approximately two weeks after the puncture, the procedure was performed in the same way as the one-step procedure. All surgical treatments and postoperative care were performed under the guidance of the same surgeon Yao Cheng, who has over 10 years of experience performing surgery, to ensure the quality and safety of the procedures.
Statistical Analysis
The obtained data were analysed using SPSS 26.0 statistical software. Continuous variables with a normal distribution are described by the mean ± standard deviation (SD); nonnormally distributed continuous variables are described by the median (interquartile range); and categorical variables are described by the frequency (percentage). The chi-square test was used to compare categorical variables. Student’s t-test was used to compare continuous variables with a normal distribution between the two groups. The Mann–Whitney U-test was used for nonnormally distributed variables. Those with significance in the univariate logistic analyses were analysed by multivariate logistic stepwise regression using the Forward: LR method. R software was used to construct a nomogram for predicting the occurrence of SIRS after PTCSL. Receiver operating characteristic (ROC) curves were plotted to calculate the area under the curve (AUC) to evaluate the predictive ability of the model. Additionally, calibration and decision curves were plotted for assessment of the model discrimination, calibration, and net clinical benefit of the model. P<0.05 was regarded as statistically significant.
Results
A total of 284 PTCSL sessions were included in this study, and the incidence of SIRS after PTCSL was 20.77%. Treatment with β-lactam antibiotics is usually needed for approximately one week in patients who develop postoperative SIRS, and these patients usually have recovery of their infectious marker levels and clinical symptoms. There were no patients who experienced adverse outcomes such as MODS, biliary sepsis, or death.
The number of PTCSL sessions, stone location, hypertension, operation time, perfusion volume, intraoperative use of norepinephrine solution, intraoperative puncture, preoperative GGT, preoperative total bile acid, preoperative platelets, and preoperative PNI were significantly different between the two groups (p < 0.05). There was no significant difference (p > 0.05) in the other clinical indices between the two groups (Table 1).
 |
Table 1 Procedure Characteristics of Percutaneous Transhepatic Cholangioscopic Lithotripsy (PTCSL) |
After univariate logistic analysis, the results showed the following: number of PTCSL sessions (odds ratio [OR]=0.342, 95% confidence interval [CI]=0.187–0.624, p<0.001), stone location (OR=2.406, 95% CI=1.293–4.477, p=0.006), hypertension (OR=3.052, 95% CI=1.168–7.975, p=0.023), operation time (OR=1.006, 95% CI=1.001–1.010, p=0.010), perfusion volume (OR=1.067, 95% CI=1.021–1.116, p=0.004), intraoperative use of norepinephrine solution (OR=0.443, 95% CI=0.212–0.924, p=0.030), intraoperative puncture (OR=3.918, 95% CI=2.143–7.161, p<0.001), preoperative alkaline phosphatase (OR=1.002, 95% CI=1.001–1.004, p=0.003), preoperative GGT (OR=1.002, 95% CI=1.001–1.004, p<0.001), preoperative leucocyte (OR=1.122, 95% CI=1.012–1.244, p=0.029), preoperative total lymphocyte count (OR=1.840, 95% CI=1.198–2.826, p=0.005), preoperative platelet count (OR=1.004, 95% CI=1.001–1.007, p=0.007), and preoperative PNI (OR=1.049, 95% CI=1.004–1.096, p=0.033)were the potential risk factors for the development of post-operative SIRS after PTCSL (P < 0. 05) (Table 2). The results of the multivariate logistic stepwise regression analysis revealed that the number of PTCSL sessions (OR=0.399, 95% CI=0.202–0.786, p=0.008), stone location (OR=2.194, 95% CI=1.107–4.347, p=0.024), intraoperative use of norepinephrine (OR=0.301, 95% CI=0.131–0.689, p=0.004), intraoperative puncture (OR=3.476, 95% CI=1.749–6.906, p<0.001), preoperative gamma-glutamyl transferase (OR=1.002, 95% CI=1.001–1.004, p=0.009), and preoperative total lymphocyte count (OR=1.820, 95% CI=1.110–2.985, p=0.018) were the independent risk factors for the occurrence of SIRS postoperatively (Table 2).
 |
Table 2 Univariate and Multivariate Logistic Regression Analyses for Predictors of SIRS After PTCSL |
The nomogram prediction model was constructed from the results of the multivariate logistic stepwise regression (Figure 1). The area under the ROC curve of the model was 0.776 (95% CI: 0.702–0.850) (Figure 2). The bootstrap method was used for the internal validation of the dataset and for the construction of calibration curves. The calibration curves showed that the actual predicted probability curves fit well with the ideal predicted probability curves (Figure 3). This indicates that the model has good accuracy and consistency. The decision curve showed that if the threshold probability was 0.1–0.8, the net benefit of the model was greater than that of either the intervention scenario for all patients or the no-intervention scenario (Figure 4). The clinical impact curve showed a narrowing of the gap when the risk threshold was 0.4, which is a good fit (Figure 5).
 |
Figure 1 A nomogram for predicting the incidence of SIRS after PTCSL. |
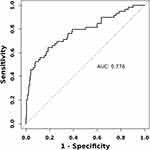 |
Figure 2 ROC curves for predicting SIRS after PTCSL. |
 |
Figure 3 Calibration curve for the prediction of SIRS after PTCSL by using the nomogram. |
 |
Figure 4 Prediction of the DCA curve of SIRS after PTCSL by using the nomogram. |
 |
Figure 5 Prediction of the clinical impact curve of SIRS after PTCSL by using the nomogram. |
Discussion
PTCSL has the same research value as percutaneous nephrolithotripsy (PCNL). However, there are no reports in the literature on the occurrence of SIRS after PTCSL up to now. Thus, this study is the first to investigate the occurrence of SIRS after PTCLS.
Hwang and Robert B used percutaneous nephrolithotomy successively to treat hepatolithiasis, their technique was based on the technique of PCNL, and the traditional technique of percutaneous hepatic cholangioscopy was continuously improved by Yanmin Liu et al9,10,20,21 PTCSL gradually became mature. PTCSL and PCNL therefore have many similarities in operation. PTCSL is an invasive procedure that requires the creation of a skin-to-intrahepatic bile duct channel rather than a natural channel. It is not possible to avoid the risks and complications of surgery. The complications of PTCSL are similar to those of PCNL because when the pressure in the bile duct exceeds 30 mmH2O during lithotripsy, the venous sinuses in the bile duct wall are opened and a variety of bacteria and endotoxins inevitably enter the bloodstream with the irrigation fluid.16
SIRS is an uncontrolled inflammatory response of the body to a variety of cytokines and inflammatory mediators.22 Numerous studies have shown that the incidence of SIRS after PCNL is 22.1%-43.0%.23–25 In our study, the incidence of SIRS after PTCSL was 20.77%. This may be due to intraoperative injury to the peribiliary vessels during puncture or sinusoidal dilatation and to the lining of the bile ducts during lithotripsy. This may result in the release of inflammatory factors into the circulation through the bile ducts, leading to the development of SIRS.
Multivariate logistic stepwise regression analysis revealed that the number of PTCSL sessions, stone location, intraoperative use of norepinephrine solution, intraoperative puncture, preoperative GGT, and preoperative TLC were independent risk factors for the development of SIRS after PTCSL. PTCSL is a percutaneous transhepatic puncture of the hepatic bile duct to create a corresponding sinusoidal channel and then, after gradual dilatation of the sinusoidal channel, a sheath is placed through which choledochoscopy can be repeated to remove stones with reproducibility. A PTCD tube may be left in place until the patient’s condition stabilises, and then a PTCSL may be used to remove the stones again in some patients who are unable to have the stones removed with one surgery due to complicated stone distribution, intraoperative biliary bleeding, or poor general condition and who cannot tolerate prolonged surgery. Most of the patients in our study did not receive PTCSL for the first time. In our study, the incidence of postoperative SIRS was greater in patients treated for the first time with PTCSL. This may be because the prolonged presence of biliary stones in these patients has the potential to cause occult biliary infections or even preexisting bacterial colonisation of the biliary tract, which increases the chances of bacterial entry into the bloodstream during sinus dilatation and biliary flushing. In patients undergoing PTCSL for the first time, intraoperative gradual dilatation of the sinus tract destroys the surrounding granulation tissue, and excessive traction on the sinus tract wall when the choledochoscope enters the angulated bile ducts during lithotripsy tends to cause sinus tract laceration. Patients who undergo another PTCSL usually require time between surgeries, during which time the surrounding fibrous tissue proliferates and wraps around the sinus to make the sinus tract firmer and the procedure more tolerable for the patient. In addition, the removal of stones during the previous operation relieved the partial obstruction of the biliary tract, and the use of an indwelling drain to drain the bile effectively controlled the biliary tract infection, thus reducing the risk of postoperative SIRS. Hepatolithiasis usually occurs in the left or right posterior lobe of the liver and is often associated with choledocholithiasis. Patients with bilateral bile duct stones are more prone to cryptogenic biliary tract infection because of the large stone burden, which is usually associated with narrowing or dilatation of the intrahepatic bile ducts. According to the results of multifactorial analyses, the location of the stones influences the safety of the procedure. Patients with widely distributed stones have a severe biliary inflammatory response and poor-quality intrahepatic bile ducts, which undoubtedly increases the risk of intraoperative injury and postoperative infection. Second, widely distributed stones are more difficult and time-consuming to remove, and most of these patients need to be treated with holmium laser lithotripsy, in which pathogens and endotoxins on the surface of the stone are released into the irrigation fluid after the stone is broken up and these pathogens and endotoxins are then absorbed into the bloodstream. Therefore, for hepatolithiasis, we should pay attention to the location of the stone before surgery and consider the location of the stone along with the findings on imaging to develop an optimal surgical program and minimise intraoperative damage to the bile ducts during the procedure.26 The results of this study showed that intraoperative use of a norepinephrine solution may act as a protective factor and reduce the incidence of postoperative SIRS. The reason may be that during lithotripsy, a small amount of biliary exudate or diffuse mucosal bleeding occurs due to biliary inflammation or stone friction, and the norepinephrine solution acts uniformly on the intrahepatic bile ducts, causing constriction of the blood vessels to reduce the absorption of harmful substances. In clinical work, some patients with two-step PTCSL had to be repunctured because the biliary drainage tube had fallen out, which was not addressed in time, and the original sinus tract could not be found. In our study, when intraoperative puncture was performed at the same time as stone extraction, puncture damage to tissues and blood vessels resulted in an increased risk of infection. Therefore, in patients who cannot tolerate surgery, the first step is to try to drain the bile by puncture, reduce the pressure on the bile duct and control the inflammation of the bile duct with antibiotics, and then, once the condition has stabilised, to try lithotripsy as a second step. We found that the occurrence of SIRS after PTCSL may be influenced by both preoperative GGT and preoperative TLC. GGT is widely distributed in the biliary system, is mostly excreted through the bile ducts, and is an important serum marker for the assessment of biliary obstruction. In our study, patients with higher preoperative GGT levels had a greater risk of postoperative SIRS after PTCSL, which may be related to prolonged preoperative biliary obstruction and poor bacterial growth in the bile. Animal studies have shown that the GGT concentration can predict the occurrence of SIRS-related death.27 Lymphocytes play an important role in the regulation of the immune response and are involved in the pathophysiological processes of inflammation.28 This finding implies that the lymphocyte count has great potential for predicting postoperative SIRS. Multifactorial analysis revealed that preoperative TLC served as an independent risk factor, suggesting that lymphocytes may play an important role in the development of postoperative SIRS. Therefore, preoperative GGT and TLC can be used as important reference indicators for the clinical prediction of postoperative SIRS. Patients with hepatolithiasis often experience impaired bile drainage due to stone obstruction, frequently leading to latent biliary infections and cholangitis. Effective biliary drainage and antibiotic therapy are paramount in managing hepatolithiasis complicated by cholangitis.29 In cases where preoperative leucocyte levels indicate a high likelihood of infection, extended preoperative antibiotic therapy becomes essential to mitigate the infection and ameliorate patient symptoms, thereby ensuring surgical safety. However, it’s crucial to emphasize that while antibiotics play a pivotal role in infection control, they are not a substitute for surgical intervention aimed at alleviating biliary obstruction. For patients who exhibit inadequate response to initial antibiotic therapy, prompt surgical intervention should be considered once the patient’s systemic condition permits, with the objective of relieving biliary pressure.29,30 PTCSL present a dual advantage by facilitating both stone removal to alleviate obstruction and bile drainage through the establishment of a sinusoidal passage.
Based on the results of the above studies, a nomogram prediction model with good predictive efficacy was constructed. We believe that the utilization of this prediction model enables the early identification of high-risk patients during the preoperative phase, thereby enhancing future clinical decision-making support.
The recent Sepsis-3 Task Force recommended the use of the Sequential (Sepsis-related) Organ Failure Assessment (SOFA) score and the quick SOFA (qSOFA) score to screen patients at high risk of developing septic shock.31,32 However, we deliberately used the concept of SIRS because SOFA scoring is too cumbersome, qSOFA lacks the necessary sensitivity, and sepsis is uncommon in the postoperative period.33,34 The SIRS criteria are still widely used in clinical practice because they are easy to measure, reproducible and sensitive compared to the first two scores.35,36 We used the SIRS criteria to identify patients at risk of developing sepsis. SIRS can be associated with noninfectious causes and is not specific for infection; therefore, we excluded other potential causes of fever and tachycardia that would have occurred postoperatively.
This study has several limitations. First, surgical time and perfusion volume were statistically significant only in the univariate analyses and were not independent prognostic factors. This may be because the time from the establishment of the puncture or sinus dilation to the end of the procedure could not be accurately recorded from the chart review. The specific perfusion volume could not be recorded at the time of data collection and could only be assessed by the number of 3-litre saline bags used intraoperatively. Second, we were unable to directly evaluate the intrabiliary pressure. Third, we did not include certain specific inflammatory markers, such as C-reactive protein or procalcitonin, in the study due to incomplete data. Finally, based on this study, we believe that these indicators can predict the occurrence of postoperative SIRS after PTCSL, but their impact on the specific mechanisms of postoperative SIRS occurrence needs to be further investigated in the future. Future multicentre studies with larger sample sizes are needed to elucidate the clinical significance of this model.
Conclusion
This study shows for the first time that the number of PTCSL sessions, stone location, intraoperative use of norepinephrine, intraoperative puncture, preoperative GGT, and preoperative TLC are independent risk factors for the development of SIRS after PTCSL. A nomogram prediction model was established to predict the occurrence of SIRS after PTCSL. The results of the study showed that the nomogram may help clinicians identify high-risk patients.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. For further information, please contact the corresponding author, Shengwei Li.
Ethics Approval and Informed Consent
Written informed consent was obtained from all participants. Individuals cannot be identified based on the data presented. We declare to ensure the confidentiality of patient data. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Review Board of The Second Hospital of Chongqing Medical University (No.2018-207).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception and design, data acquisition, or data analysis and interpretation, participated in the drafting of the article or critically revising it for important intellectual content, agreed to submit to the current journal, gave final approval for the version to be published, and agreed to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81701950, 82172135), Natural Science Foundation of Chongqing (Grant No. CSTC2021JCYJ-MSXMX0294, CSTB2022NSCQ-MSX0058), Medical Research Projects of Chongqing (Grant No. 2018MSXM132, 2023ZDXM003, 2024JSTG028), and Kuanren Talents Program of the second affiliated hospital of Chongqing Medical University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kim HJ, Kim JS, Joo MK, et al. Hepatolithiasis and intrahepatic cholangiocarcinoma: a review. World J Gastroenterol. 2015;21(48):13418–13431. doi:10.3748/wjg.v21.i48.13418
2. Tazuma S, Unno M, Igarashi Y, et al. Evidence-based clinical practice guidelines for cholelithiasis 2016. J Gastroenterol. 2017;52(3):276–300. doi:10.1007/s00535-016-1289-7
3. Uchiyama K, Kawai M, Ueno M, et al. Reducing residual and recurrent stones by hepatectomy for hepatolithiasis. J gastro surg. 2007;11(5):626–630. doi:10.1007/s11605-006-0024-8
4. Liu FB, Yu XJ, Wang GB, et al. Preliminary study of a new pathological evolution-based clinical hepatolithiasis classification. World J Gastroenterol. 2015;21(7):2169–2177. doi:10.3748/wjg.v21.i7.2169
5. Peng L, Xiao J, Liu Z, et al. Laparoscopic left-sided hepatectomy for the treatment of hepatolithiasis: a comparative study with open approach. Int j Surg. 2017;40:117–123. doi:10.1016/j.ijsu.2017.02.068
6. PengJX, WangLZ, Diao J-F, et al. Major hepatectomy for primary hepatolithiasis: a comparative study of laparoscopic versus open treatment. Surg Endosc. 2018;32(10):4271–4276. doi:10.1007/s00464-018-6176-2
7. Huang L, Lai J, Liao C, et al. Classification of left-side hepatolithiasis for laparoscopic middle hepatic vein-guided anatomical hemihepatectomy combined with transhepatic duct lithotomy. Surg Endosc. 2023;37(7):5737–5751. doi:10.1007/s00464-023-10198-4
8. Williams E, Beckingham I, El Sayed G, et al. Updated guideline on the management of common bile duct stones (CBDS). Gut. 2017;66(5):765–782. doi:10.1136/gutjnl-2016-312317
9. Hwang MH, Mo LR, Yang JC, et al. Percutaneous transhepatic cholangioscopic ultrasonic lithotripsy (PTCS-USL) in the treatment of retained or recurrent intrahepatic stones. Gastroin endo. 1987;33(4):303–306. doi:10.1016/S0016-5107(87)71604-6
10. Nadler RB, Rubenstein JN, Kim SC, et al. Percutaneous hepatolithotomy: the Northwestern University experience. J Endourol. 2002;16(5):293–297. doi:10.1089/089277902760102776
11. Yao D, Wu S. Application of laparoscopic technique in the treatment of hepatolithiasis. Surgical Laparo End Percu Tech. 2020;31(2):247–253. doi:10.1097/SLE.0000000000000871
12. Yin Z, Shen B. Comparative study of the effect of U100 laser and pneumatic ballistic combined with percutaneous transhepatic cholangioscopic lithotomy in the treatment of intra-and extrahepatic bile duct stones and its effect on liver function. Pakistan J Med Sci. 2022;38(6):1686–1690. doi:10.12669/pjms.38.6.5903
13. Chen MF, Jan YY. Percutaneous transhepatic cholangioscopic lithotripsy. Br J Surg. 1990;77(5):530–532. doi:10.1002/bjs.1800770519
14. Ou Y, Li J, Liang, C. Risk factors analyses associated with postoperative infection in choledochoscopy for intrahepatic bile duct stones (IHDs): a single-center retrospective study in real-world setting. Surg Endosc. 2024; 38(4):2050–2061. doi:10.1007/s00464-024-10737-7
15. Huang MH, Chen CH, Yang JC, et al. Long-term outcome of percutaneous transhepatic cholangioscopic lithotomy for hepatolithiasis. Am J Gastroenterol. 2003;98(12):2655–2662. doi:10.1111/j.1572-0241.2003.08770.x
16. Dellinger EP, Kirshenbaum G, Weinstein M, et al. Determinants of adverse reaction following postoperative T-tube cholangiogram. Ann Surg. 1980;191(4):397–403. doi:10.1097/00000658-198004000-00002
17. Lau WY, Fan ST, Yip WC, et al. Optimal irrigation pressures in operative choledochoscopy. Aust NZ j surg. 1988;58(1):63–66. doi:10.1111/j.1445-2197.1988.tb00970.x
18. Clere-Jehl R, Mariotte A, Meziani F, et al. JAK-STAT targeting offers novel therapeutic opportunities in sepsis. Trends Mol Med. 2020;26(11):987–1002. doi:10.1016/j.molmed.2020.06.007
19. Tao H, Wang P, Sun B, et al. One-step multichannel percutaneous transhepatic cholangioscopic lithotripsy applied in bilateral hepatolithiasis. World J Surg. 2020;44(5):1586–1594. doi:10.1007/s00268-020-05368-7
20. Liu YM, Wang CZ. Improved percutaneous transhepatic cholangioscopy in the treatment of intrahepatic stone. J Surg Concepts Pract. 2004; 9(6): 485–486. Chinese.
21. Lu HW, Liu YM. A experimental study on formation of fistula after percutaneous transhepatic cholangiodrainage(PTCD)J]. China Journal of Endoscopy. 2004; 10(11):44–50. Chinese.
22. Simpson SQ. SIRS in the Time of Sepsis-3. Chest. 2018;153(1):34–38. doi:10.1016/j.chest.2017.10.006
23. He Y, D XIA, Tong Y, et al. Predictive value of CD3(+) cells and interleukin 2 receptor in systemic inflammatory response syndrome after percutaneous nephrolithotomy. Front Immunol. 2022;13:1017219. doi:10.3389/fimmu.2022.1017219
24. Xu P, Zhang S, Zhang Y, et al. Preoperative antibiotic therapy exceeding 7 days can minimize infectious complications after percutaneous nephrolithotomy in patients with positive urine culture. World j Urol. 2022;40(1):193–199. doi:10.1007/s00345-021-03834-y
25. Danilovic A, Talizin TB, Torricelli FCM, et al. One week pre-operative oral antibiotics for percutaneous nephrolithotomy reduce risk of infection: a systematic review and meta-analysis. Int Braz J urol. 2023;49(2):184–193. doi:10.1590/s1677-5538.ibju.2022.0544
26. Wen XD, Wang T, Huang Z, et al. Step-by-step strategy in the management of residual hepatolithiasis using post-operative cholangioscopy. Ther Adv Gastroenterol. 2017;10(11):853–864. doi:10.1177/1756283X17731489
27. Jaramillo C, Renaud DL, Arroyo LG, et al. Serum haptoglobin concentration and liver enzyme activity as indicators of systemic inflammatory response syndrome and survival of sick calves. J Veterinary Internal Med. 2022;36(2):812–819. doi:10.1111/jvim.16357
28. Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517(7534):293–301. doi:10.1038/nature14189
29. Miura F, Okamoto K, Takada T, et al. Tokyo guidelines 2018: initial management of acute biliary infection and flowchart for acute cholangitis. J Hepato Biliary Pancreatic Sci. 2018;25(1):31–40. doi:10.1002/jhbp.509
30. Mukai S, Itoi T, Baron TH, et al. Indications and techniques of biliary drainage for acute cholangitis in updated Tokyo Guidelines 2018. J Hepato Biliary Pancreatic Sci. 2017;24(10):537–549. doi:10.1002/jhbp.496
31. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
32. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):762–774. doi:10.1001/jama.2016.0288
33. Askim Å, Moser F, Gustad LT, et al. Poor performance of quick-SOFA (qSOFA) score in predicting severe sepsis and mortality - a prospective study of patients admitted with infection to the emergency department. Scand j tra resusci emerg med. 2017;25(1):56. doi:10.1186/s13049-017-0399-4
34. Song JU, Sin CK, Park HK, et al. Performance of the quick sequential (sepsis-related) organ failure assessment score as a prognostic tool in infected patients outside the intensive care unit: a systematic review and meta-analysis. Critical Care. 2018;22(1):28. doi:10.1186/s13054-018-1952-x
35. Serafim R, Gomes JA, Salluh J, et al. A comparison of the quick-sofa and systemic inflammatory response syndrome criteria for the diagnosis of sepsis and prediction of mortality: a systematic review and meta-analysis. Chest. 2018;153(3):646–655. doi:10.1016/j.chest.2017.12.015
36. X QIU, Lei YP, Zhou RX. SIRS, SOFA, qSOFA, and NEWS in the diagnosis of sepsis and prediction of adverse outcomes: a systematic review and meta-analysis. Exp Rev Anti-Infective Ther. 2023;21(8):891–900. doi:10.1080/14787210.2023.2237192
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
