Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 16
Risk Factors Associated with Unsuppressed Viral Load in People Living with HIV Receiving Antiretroviral Treatment in Jawa Barat, Indonesia
Authors Wisaksana R, Hartantri Y , Hutajulu E
Received 8 April 2023
Accepted for publication 18 December 2023
Published 24 January 2024 Volume 2024:16 Pages 1—7
DOI https://doi.org/10.2147/HIV.S407681
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Rudi Wisaksana, Yovita Hartantri, Elisabeth Hutajulu
Department of Internal Medicine, Faculty of Medicine, Universitas Padjadjaran/ Hasan Sadikin General Hospital, Bandung, Indonesia
Correspondence: Elisabeth Hutajulu, Tel +62 81220648061, Fax +62 22 2032216, Email [email protected]
Introduction: The success rate of antiretroviral therapy (ART) was sufficiently high globally that by 2030, Human Immunodeficiency Virus (HIV) infection would no longer become a significant health issue. The evaluation of the success rate of ART in Indonesia is still fairly low, with estimates that 6.1% of the total population living with HIV. This is affected by treatment failure and limitations of viral load (VL) testing. We investigated the risk factors for failure of VL suppression in HIV patients on ART.
Methods: An institution-based cross-sectional study was conducted among 1203 subjects, medical records of adult HIV patients in Jawa Barat, who followed the Viral Load Months Program from the government in 2020. Data were taken using total sampling technique and analyzed with multivariate logistic regression model using SPSS version 21 software.
Results: Of the 1203 subjects, 5.2% had unsuppressed VL and 94.8% had suppressed VL. The results showed that a nevirapine-based regimen was the main factor increasing unsuppressed risk (Prevalence Odds Ratio (POR) 3.75 (95% CI: 1.41– 9.99, p-value 0.008)). The other factors are ART duration < 5 years (POR 2.46; 95% CI: 1.22– 4.97, p-value 0.012), WHO clinical stage III–IV (POR 2.30; 95% CI: 1.30– 4.08, p-value 0.004), and loss to follow-up history (POR 2.28, 95% CI: 1.03– 5.07). Meanwhile, the zidovudine-based regimen reduced the risk of failure (POR 0.34 (95% CI: 0.12– 0.96)).
Conclusion: Several factors could contribute to unsuppressed VL in HIV patients receiving ART, which warrants further investigations.
Keywords: viral load, HIV, risk factors, unsuppressed, antiretroviral therapy, failure
Introduction
Human Immunodeficiency virus infection remains a global health problem and becomes the tenth leading cause of death. The second highest number of HIV cases occurred in Asia and had become a worldwide concern, including in Indonesia.1 United Nations AIDS (UNAIDS) have updated the 2030 sustainable Development Goals (SDGs) to achieve 95-95-95 HIV treatment and have less than 200,000 new cases.2,3
Antiretroviral therapy is a lifesaving treatment for patients with HIV/AIDS. The treatment aims to suppress viral load, restore immune function, improve quality of life, and reduce morbidity and mortality. ART failure is defined as clinical, immunological, and virological failure. Viral load monitoring has become the standard diagnosis of failure ART.4,5 However in Indonesia, limited access to routine viral load monitoring makes the challenge to identify early regimen failure. Delayed or failure to identify ART failure may lead to drug resistance, toxicity, high viral load, high risk of second-line failure, and risk of morbidity and mortality.6
UNAIDs 90-90-90 evaluation in Indonesia was far below the target, estimated that only about 51% of the population at risk underwent HIV tests and knowing their status, and about 17% among them underwent the viral load test. For the third 90, there were 89.1% with viral load suppressed.7 Evaluation of antiretroviral therapy (ART) is not regularly done in all healthcare facilities because of the unavailability of viral load tests and limited cost. This study aims to determine risk factors for failure of viral load suppression in HIV patients receiving ART in West Java. This result of the study can be considered a priority for routine viral load tests, as early detection of virological failure.
Materials and Methods
This was a cross-sectional analytic study conducted in Jawa Barat, Indonesia. The study evaluated all HIV patients receiving antiretroviral therapy who followed the HIV load examination months program of the Indonesia Ministry of Health from July to December 2020. The study was conducted upon approval from the Padjadjaran University Ethics committee stated by letter No. LB.02.01/X.6.5/224/2021.
All HIV patients over 18 years of age on ART for at least 6 months who followed the Viral load examinations program were included in the study. A schematic diagram of the inclusion/exclusion criteria is given in Figure 1. Information on age, sex, body mass index, World Health Organization (WHO) clinical stage, loss to follow-up history, ART duration, type of current antiretroviral regimen (divided into nevirapine-based and zidovudine-based), and health care type were abstracted from the medical record. The program followed 4353 patients, but 1203 of them met the inclusion and exclusion criteria.
 |
Figure 1 Schematic diagram of inclusion and exclusion criteria. |
Age was measured by years up to the time of the study done and was categorized into three groups: 18–24 years old, 25–64 years old, and ≥65 years old. Body mass index was defined as ratio of body weight (kg) to the squared of body height (m2) and was categorized into two groups: underweight (<18.5 kg/m2) and normal (≥18.5 kg/m2). Health care facility was defined as a health care facility that provides ART and was categorized into three groups: primary health care, type C-D, and type A-B. HIV/AIDS clinical stage was defined according to WHO during the ART program and was categorized into two groups: stage I–II and stage III–IV. ART duration was categorized into two groups: ≤5 years dan >5 years. Viral suppression was defined as viral load <1000 copies/mL and an unsuppressed viral load as viral load >1000 copies/mL according to WHO guidelines.4 Loss to follow-up history was defined as ART stopped in >1 month, following the previous study.8
The data were collected using Microsoft Excel 2011 spreadsheet. Then, they were cleaned and analyzed using Statistical Product and Service Solution (SPSS) for windows 22.0. Data were presented as frequency and corresponding percentage. Bivariate analysis was performed for the independent variables and the outcome variable (virological suppression status) and prevalence odds ratios (POR) and 95% confidence intervals were calculated. POR is equivalent to the odds ratio (OR), therefore both can be calculated using the same formula. The only difference between POR and OR is the former used in cross-sectional studies. Multivariate logistic regression was used to identify risk factors associated with unsuppressed viral load. Variables were entered into the multivariate analysis based on previous literature and statistical significance in bivariate analysis (p-value <0.25).
Results
Table 1 shows the characteristics of the 1203 patients whose data were available for analysis. The median age of subjects was 36 (minimum 18, maximum 77) years old. There were 854 (71%) male patients, 1081 (89.9%) patients who came from tertiary and secondary hospitals and most did not have a loss to follow-up history (94.8%). For the body mass index category, there were 299 (24.9%) underweight patients (<18.5 kg/m2).
 |
Table 1 Baseline Characteristics |
The majority of the patients received a fixed drug combination of Tenofovir, Lamivudine, and Efavirenz (TDF/3TC/EFV) in 67.9%. There were 806 (67%) patients with ART duration of more than 5 years and 661 (54.9%) patients with WHO clinical stage III–IV. Of the total sample (1203 patients), 1141 (94.8%) patients had suppressed viral load and 62 (5.2%) patients had unsuppressed viral loads.
Bivariate analysis of the factors (Table 2) showed ART duration <5 years (prevalence odds ratio (POR) 0.039; 95% confidence interval (CI) 1.91 (1.03–3.57)) and WHO clinical stage III–IV (POR 0.009); 95% CI 2.08 (1.19–3.64) were associated with the risk of unsuppressed viral load. Table 3 shows multivariate logistic regression revealed that nevirapine-based (aPOR 0.008); 95% CI 3.76 (1.41–9.99), ART duration <5 years (aPOR 0.012); 95% CI 2.46 (1.22–4.97), WHO clinical stage III–IV (aPOR 0.004); 95% CI 2.30 (1.30–4.08), and loss to follow-up history (aPOR 0.042); 95% CI 2.28 (1.03–5.07) were associated with risk of viral load suppression failure. While zidovudine-based factors were found to be protective of viral load suppression failure (aPOR 0.041); 95% CI 0.34 (0.12–0.96).
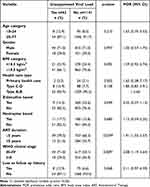 |
Table 2 Bivariate Analysis Factors Associated with Unsuppressed Viral Load |
 |
Table 3 Multivariate Analysis Factors Associated with Unsuppressed Viral Load |
Discussion
This study aimed to evaluate antiretroviral therapy in HIV patients who followed the viral load examination months program by the Ministry of Health. Indonesian Health facilities for HIV counseling and testing services were increasing and continuing to grow in 2019. As test coverage increased, the number of people living with HIV/AIDS (PLWHA) receiving testing, support, and treatment also increased. We took data from the main e-database called HIV AIDS Information System (SIHA) which is the primary source of information on all HIV patients used across all the health centers and hospitals in Indonesia. According to the SIHA report in 2019, there were 48% of PLWHA knew their status, 26% of PLWHA received ART, and only 9% of PLWHA underwent the viral load test. Nationally, among those received ART and underwent viral load evaluation, there were 91% of PLWHA who had viral load suppression (viral load <1000).9 Indonesian Ministry of Health also reported in 2020 that only about 6.8% of PLWHA underwent viral load testing, and among them, 89.1% achieved viral load suppression, slightly below the UNAIDS 2020 target.7 Our study showed the viral load suppression rate was 94.8%, slightly below third 95 targets UNAIDS.3 Our study’s main result showed that zidovudine-based regimen, nevirapine-based regimen, ART duration <5 years, WHO clinical stage III–IV, and loss to follow-up history were risk factors associated with unsuppressed viral load.
We found that the majority of the total study population (90.8%) was in productive age, which is the range of ages where the people are usually occupied with jobs (25–64 years old), with a median age of 36 years old. This result is consistent with the PLWHA population in Indonesia according to SIHA, the study by Ssekalembeet al in Kediri, and a previous study in Jawa Barat by Wisaksana et al. This population is the most susceptible to HIV infection by sexual transmission. As highlighted by SIHA, the highest risk factor for HIV transmission is sexual transmission (homosexual 22.2% and heterosexual 16.5%) in Indonesia.2,7,8
Nevirapine-based regimens were the strongest factor in unsuppressed viral load as patients with nevirapine-based regimens were found to be greater three times at risk of facing suppression failure compared to theirs. Similarly, a study conducted in Kenya by Kimulwo et al showed a greater risk of virological failure among HIV patients receiving nevirapine because of poor adherence.10 Nevirapine, a class of nonnucleoside reverse transcriptase inhibitors (NNRTI) has side effects (usually nausea, rash, weakness, fever, and headache) that often cause poor adherence.11
The result showed that the ART duration <5 years increased the risk of unsuppressed viral load. This failure suppression may be associated with drug resistance, incomplete virological response, or virological rebound.12 Rosenblum et al study showed decreased virological failure risk with longer ART duration because HIV patients had better adherence. Another study by Boakye et al in Ghana stated that shorter duration of ART (6 months–2 years) was associated with failure of suppressing viral load.13,14
Our study revealed that patients with WHO clinical stage III–IV had two times higher odds to develop unsuppressed viral load. Similarly, studies were conducted in Western Ethiopia, Zimbabwe, Mozambique, and Swaziland. Virological failure could be due to delayed ART initiation causing high viral load and low CD4 and increasing opportunistic infections with pill burden that affected patient adherence.6
Suboptimal adherence and poor adherence lead to viral rebound episodes (Bulage et al, 2017; O’Connor et al, 2017).15,16 Our study showed that loss to follow-up history had a higher risk for unsuppressed viral load. Consistently with a previous study conducted in Jawa Barat by Wisaksana et al, that found treatment discontinuation history >1 month significantly increased virological failure (OR 12.92; 95% CI 4.57–36.56, p-value <0.001).8 This result was also shown by Demsie DG.et al study in Ethiopia with AOR 5.4 (95% CI 2.61–10.45, p-value 0.002).17
We found that the zidovudine-based regimen had a protective effect and decreased virological failure risk by above three times. This may be due to the usage of zidovudine in our center being limited to patients without severe malnutrition or comorbidities. This finding is in agreement with other reports in Nigeria that zidovudine was superior to tenofovir (HR 1.47, 95% CI 1.21–1.79) (Scarsie KKet al, 2016).18 Dafin et al found that the virological failure was higher in the tenofovir population, this was associated with higher resistance risk NNRTIs and K65R mutation crossed reaction in the tenofovir population.19
The finding of the study had several limitations. We excluded more than the total sample we can analyze, the result may not be generalized to the Jawa Barat population. We also did not evaluate previous therapy, viral load at initiation, and comorbidities that may affect the risk of unsuppressed viral load. Despite its limitation, however, we believe that this study provides important information which would be useful for the next study and viral load program priority in our province.
Conclusion
Multivariate analysis showed that nevirapine-based, ART duration <5 years, WHO clinical stage III–IV, and loss to follow-up history were significantly associated with unsuppressed viral load, while the zidovudine-based regimen had a protective effect. Special attention should be given to HIV patients receiving ART who have those risk factors for early recognition and management of treatment failure. We suggest further study in multiple ART centers by cohort method to determine the predictor of unsuppressed viral load in HIV patients receiving ART.
Ethical Consideration
Ethical clearance was obtained from the instutional review board (Ref. No. LB.02.01/X.6.5/224/2021) of Padjadjaran University College of Medicine. Informed consent was waived by the review committee as the data source of patients’ medical record numbers was anonymously registered using codes without personal identity, such as name or address. The patients were not subject to any harm as far as confidentiality was kept. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board.
Acknowledgments
We would like to express our sincere gratitude to our colleagues in the Department of Internal Medicine, Universitas Padjadjaran/Hasan Sadikin General Hospital, Bandung, Indonesia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Global AIDS update; 2021. Available from: https://www.unaids.org/sites/default/.files/media_asset/UNAIDS_FactSheet_en.pdf.
2. Ssekalembe G, Atoillah M, Isfandiari HS. Current Status Towards 90-90-90 UNAIDS Target and Factors Associated with HIV Viral Load Suppression in Kediri City, Indonesia. Dovepress. 2020;12:47–57.
3. Global AIDS update. Fast-Track Ending the AIDS epidemic by 2030; 2014. Available from: https://www.unaids.org/en/resources/documents/2014/JC2686_WAD2014report.
4. WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection.
5. WHO. Guidelines: Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery, and Monitoring: Recommendations for a Public Health Approach.
6. Desalegn M, Seyoum D, Tola EK, G RT. Determinants of First-Line Antiretroviral Treatment Failure among Adult HIV Patients at Nekemte Specialized Hospital, Western Ethiopia: unmatched case-control Study. SAGE Open Medicine. 2021;9:11. doi:10.1177/20503121211030182
7. KEMENKES. Laporan Perkembangan HIV/AIDS & Penyakit Infeksi Menular Di Indonesia Triwulan IV Tahun 2020. In: Direktur Jenderal KR, editor. Jakarta: Kemenkes RI; 2021.
8. Wisaksana R, F A, Indrati A, et al. Virological Failure and Drug Resistance During First Line Anti-Retroviral Treatment in Indonesia. J med virol. 2013;85:1394–1401. doi:10.1002/jmv.23606
9. KEMENKES. Rencana Aksi Nasional Pencegahan Dan Pengendalian HIV AIDS Dan PIMS Di Indonesia Tahun 2020-2024. Jakarta: Kementerian Kesehatan Republik indonesia; 2020.
10. Kimulwo MJ, Okendo J, Aman RA, et al. Plasma Nevirapine Concentrations Predict Virological and Adherence Failure in Kenyan HIV-1 Infected Patients with Extensive Antiretroviral Treatment Exposure. PLoS One. 2017;14.
11. Ochieng W, Kitawi RC, Nzomo TJ, et al. Correlates of Adherence and Treatment Failure Among Kenyan Patients on Long-term Highly Active Anti-Retroviral Therapy. J Acquir Immune De c Syndr. 2016;69(2):17.
12. Hicham T, Ilyas E, Tarik H, et al. Risk Factors Associated with Unsuppressed Viral Load in HIV‐1 Infected Patients at the First Antiretroviral Therapy in Morocco. Int J Mycobacteriol. 2019;8:5. doi:10.4103/ijmy.ijmy_41_19
13. DHHS. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. New York: Department of Health and Human Services; 2021.
14. Boakye P, Safowaa A. Prevalence and predictors of viral load suppression in adults living with HIV in the Western Region of Ghana: a cross-sectional study. AIMS Public Health. 2023;10(2):469–479. doi:10.3934/publichealth.2023033
15. Bulage L, Ssewanyana I, Nankabirwa V, et al. Factors associated with virological non-suppression among HIV-positive patients on antiretroviral therapy in Uganda, August 2014-July 2015. BMC Infect Dis. 2017;17(1):326. doi:10.1186/s12879-017-2428-3
16. O’Connor J, Smith C, Lampe FC, et al. Durability of viral suppression with first-line antiretroviral therapy in patients with HIV in the UK: an observational cohort study. Lancet HIV. 2017;4(7):7.
17. Demsie DG, Bantie AT, Allene MD, Alema NM. Antiretroviral treatment failure among HIV-positive adults taking first-line therapy and associated risk factors at Adigrat General hospital, Adigrat, Ethiopia 2019: a cross-sectional study. Int J Surg Open. 2020;26(2020):6. doi:10.1016/j.ijso.2020.08.001
18. Scarsi KK, Eisen G, Darin KM, et al. Superior Effectiveness of Zidovudine Compared With Tenofovir When Combined With Nevirapine-based Antiretroviral Therapy in a Large Nigerian Cohort. Clinl Infect Dis. 2016;62(4):7.
19. Rey D, Hoen B, Chavanet P, et al. High Rate of Early Virological Failure with the Once-Daily Tenofovir/Lamivudine/Nevirapine Combination in Naive HIV-1-infected Patients. J Antimicrob Chemother. 2009;63:380–388. doi:10.1093/jac/dkn471
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
