Back to Journals » Research and Reports in Urology » Volume 15
Retzius-Sparing Robotic-Assisted Prostatectomy: Technical Challenges for Surgeons and Key Prospective Refinements
Authors Ferretti S, Dell'Oglio P , Ciavarella D, Galfano A, Schips L, Marchioni M
Received 1 August 2023
Accepted for publication 30 November 2023
Published 12 December 2023 Volume 2023:15 Pages 541—552
DOI https://doi.org/10.2147/RRU.S372803
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Simone Ferretti,1 Paolo Dell’Oglio,2– 4 Davide Ciavarella,1 Antonio Galfano,2 Luigi Schips,1 Michele Marchioni1
1Department of Medical, Oral and Biotechnological Sciences, G. d’Annunzio University of Chieti, Urology Unit, Chieti, Italy; 2Department of Urology, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy; 3Department of Urology, Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, the Netherlands; 4Interventional Molecular Imaging Laboratory, Department of Radiology, Leiden University Medical Center, Leiden, the Netherlands
Correspondence: Michele Marchioni, Department of Medical, Oral and Biotechnological Sciences, G. d’Annunzio University of Chieti, Urology Unit, Chieti, Italy, Tel +393296544866, Fax +390871357756, Email [email protected]
Abstract: Robotic-assisted radical prostatectomy (RARP) is the gold standard for localized prostate cancer. Several RARP approaches were developed and described over the years, aimed at improving oncological and functional outcomes. In 2010, Galfano et al described a new RARP technique, known as Retzius-sparing RARP (RS-RARP), a posterior approach through the Douglas space that spares the anterior support structures involved with urinary continence and sexual potency. This approach has been used increasingly in many centers around the world comparing its results with those of the most used standard anterior approach. Several randomized controlled trials, systematic reviews and meta-analyses demonstrated an important advantage relative to standard anterior RARP in terms of early urinary continence recovery, with comparable perioperative and long-term oncological outcomes. Several surgeons are concerned regarding RS-RARP because it appears to increase the risk of positive surgical margins (PSMs). However, this statement is based on low-certainty evidence. Indeed, the available studies compared the results of surgeons who had an initial experience with posterior RARP with those who had a solid experience with anterior RARP. Recent evidence strongly suggests that RS-RARP is feasible and safe not only in low- and intermediate-risk prostate cancer patient but also in challenging scenario such as high-risk setting, salvage prostatectomy and after transurethral resection of the prostate.
Keywords: radical prostatectomy, Retzius-sparing, urinary continence, positive surgical margins, high-risk prostate cancer
Introduction
Robotic-assisted radical prostatectomy (RARP) has drastically changed the surgical treatment of prostate cancer (PCa) patients over the past two decades, quickly becoming the gold standard.1,2 This type of minimally invasive surgery allows excellent oncological results, with the purpose of improving the patients’ quality of life (QoL), specially functional (ie, urinary continence and erectile function) and oncological (ie, positive surgical margins, biochemical recurrence) outcomes.3
The introduction of robotic surgery allowed to achieve the highest precision levels, respecting the anatomy of periprostatic tissues and improving the steps of radical prostatectomy (ie, bladder neck preservation, nerve-sparing dissection, prostate apex management).4–6 Over the years, many RARP techniques have been described, but the transperitoneal and extraperitoneal approaches remain the most used today.5 In 2010, Galfano et al described a novel technique, known as Retzius-sparing RARP (RS-RARP) or Bocciardi approach. This technique consists into a posterior approach through the Douglas space, sparing the support structures surrounding the prostate that have a crucial role in the mechanism of continence.7 After 10 years, in 2020, RS-RARP was included for the first time in European Association of Urology (EAU) guidelines as a valid alternative surgical approach to standard RARP for localized PCa patients.
The aim of this non-systematic review is to focus on the RS-RARP approach and evaluate its applications and new challenges scenarios.
Materials and Methods
This is a nonsystematic review of the literature. We research on PubMed English articles published between January 2010 and December 2022, using the key-words “Retzius-sparing radical prostatectomy”, “Robot-assisted radical prostatectomy”, “RS-RARP”, and “Prostate cancer” alone or in combination. The identified articles were then screened, selected by the authors to be included and discussed in the present review.
Surgical Technique
Robotic-assisted radical prostatectomy (RP) represents one of the most important example of “precision-surgery”, thanks to its many advantages, such as a better and stable view of the surgical field and anatomical structures, thanks to highly magnified 3D imaging system, that provides magnification up to x12, and precise controlled Endowrist instruments, which duplicates the dexterity of the surgeon’s forearm and wrist at the operative site, thus providing 7 degrees of freedom. It allows to refine and improve the fundamental steps of open RP: bladder neck preservation, nerve sparing, prostate apex management and preservation of pubo-prostatic ligaments. These improvements led to a “tailored-surgery”, based on the characteristics of the patient and tumor,8 attempting to improve the oncological and functional outcomes and approaching the Trifecta outcomes (urinary continence, potency and undetectable PSA), the standard metrics to assess the results of RARP.9,10
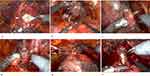
The standard anterior RARP approach provides access to the Retzius space, with the possibility of damaging all the structures involved in urinary continence and sexual potency such as endopelvic fascia, neurovascular bundles, puboprostatic ligaments, pudendal arteries, and the Santorini plexus.7 This approach begins with the incision of the parietal peritoneum laterally umbilical ligaments with the access to the space of Retzius. The bladder neck is incised (Figure 1a), and the prostate dissection moves posteriorly up to identify the seminal vesicles and the vasa deferentia. The vasa deferentia are transected and their distal part used for lifting the prostate facilitates the isolation of the seminal vesicles (Figure 1b). The Denonvilliers fascia is then incised and after the control of the prostatic pedicles (Figures 1c and d) and the Santorini plexus, the dissection of the prostate moves from posterolateral side to the apex (Figure 1e), the urethra is transected (Figure 1f) and the vesico-urethral anastomosis is performed.
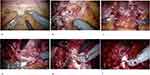
In 2010, Galfano et al described a novel approach, named Retzius-sparing RARP (RS-RARP) or Bocciardi technique, that allows to spare those structures responsible for the mechanism of continence.7 The surgical technique involves the positioning of the patient in the standard 30° Trendelenburg position, six laparoscopic trocars, 30° lens. After incision of the parietal peritoneum at the level of the vesico-rectal space (Douglas space) (Figure 2a), the seminal vesicles and the vasa deferentia are identified, isolated and retracted with two trans-abdominal stitches in order to increase the surgical field (Figure 2b and c). Posterior prostatic dissection occurs in an antegrade way. Specifically, the Denonvilliers fascia is incised, and the posterior plane is developed up to the prostatic apex (Figure 2d). Thereafter the prostate is pushed downwards, the lateral surface of the prostate is identified, the bladder neck is then identified and incised (Figure 2e). Two short cardinal stitch are located at the level of the mucosa of the bladder in order to easily identify the bladder neck orifice during the uretro-vesical anastomosis. Anterior prostatic dissection also occurs in an antegrade way, up to the prostatic apex, with or without incision of Santorini plexus. The urethra is dissected (Figure 2f), and a standard Van Velthoven vesicourethral anastomosis is performed.7
Standard RARP vs Retzius-Sparing RARP
Oncological and Functional Outcomes
The functional outcomes were immediately encouraging. In a prospective, non-controlled study, Galfano et al analyzed 200 patients undergoing RS-RARP.11 Immediate urinary continence (0–1 safety pad/day 1 week after catheter removal) was achieved in 90% of cases and in 96% 1 year after surgery. In a selected cohort, 64 patients were treated with bilateral nerve-sparing technique: 40 and 80% have first sexual intercourse at 1 month and 1 year after surgery, respectively. However, the authors reported an overall positive surgical margins (PSMs) of 25.5%, mostly localized at prostatic apex in the most aggressive forms of disease.11 Over time, several studies tried to validate the feasibility and effectiveness of this approach compared to the standard one (Table 1), especially by analyzing the oncological outcomes (positive surgical margins and biochemical recurrence) (Table 2) and the functional outcomes (early and long-term urinary continence recovery) (Table 3).12–16
 |
Table 1 Studies’ Characteristics |
 |
Table 2 Positive Surgical Margin Rates Among the Studies |
 |
Table 3 Urinary Continence Rates Among the Studies |
In 2014, Lim et al relied on a cohort of 50 patients who underwent RS-RARP with 6 months follow-up and compared perioperative outcome with a database of 581 patients who underwent standard RARP.12 A total of 70% of RS-RARP group were completely dry and 92% had 0 pad per day usage, at 1 month after surgery. Whereas, early continence was significantly inferior in the standard RARP groups, especially in the unmatched cohort (only 36% were completely dry).12 There were no significant differences in terms of PSM rates (14% RS group vs 14% matched group and 11.3% unmatched group), with anterior localization of PSM greater in the RS-RARP group compared with either the matched or unmatched standard RARP groups. A subgroup analysis of pT2 and pT3 tumors showed no significant differences in the PSM rates for the two approaches.12
The excellent continence recovery after RS-RARP was confirmed by Dalela et al (ref). The authors in their trial compared functional outcomes in 120 patients with low and intermediate-risk PCa randomized in RS-RARP group and standard RARP.13 They reported urinary continence rates (0–1 safety pad per day) of 71% in posterior approach group vs 48% in the anterior group, at 1 week after catheter removal (p = 0.01). Meanwhile, continence rates at 1.2 and 3 months after surgery were 83%, 88 and 95% for RS-RARP vs 67%, 72%, and 86% for standard RARP, respectively. The median 24-h pad weights test showed a reduced urine loss in patients undergoing RS (5 g) compared to the standard one (25 g).13 In a post-hoc analysis, Menon et al14 confirmed that the time to continence recovery is significantly quicker with the posterior approach. However, it did not translate into a persistent advantage in continence beyond 6 months compared to the anterior approach (98.3% vs 93.3%). Sexual function, oncologic outcomes and complications were comparable in the 2 groups:14 one year after surgery 86.5% of men could achieve erection in the posterior group vs 69.2% in the anterior one, with no tendency toward earlier return of erectile function compared to 3-months follow-up (43.7% vs 36.7%).14 Furthermore, no statistically significant differences between the 2 groups were found in PMS rates (11.7% for posterior and 8.3% for anterior RARP) in patients with pT3a or greater disease. Finally, the probability of BCR free-survival was 0.93 (95% CI: 0.85–1.0) for anterior RARP compared to 0.84 (95% CI: 0.68–1.0) for posterior RARP.14
In another study, Qiu et al (ref) relied on 110 patients treated with RS-RARP of standard RARP and evaluated early urinary continence recovery, defined as 0–1 safety pad used within 1 week after catheter removal.15 The authors described an immediate continence rate of 69.1% in the first group compared with 30.9% in the second group (risk ratio [RR] = 2.24, 95% confidential interval [CI]: 1.48–3.51, p = 0.000). After 12-month follow-up, better continence recovery was observed in posterior vs anterior approach (HR = 1.51, 95% CI: 1.01–2.24, p = 0.007).15
In their prospective clinical trial, Asimakopoulos et al evaluated urinary continence recovery in 102 patients undergoing RS-RARP and standard RARP.16 Immediate urinary continence, defined as 0–1 safety pad per day 1 week after catheter removal, was 30% (95% CI: 17–47%) for the standard RARP and 51.3% (95% CI: 35–68%) for the RS-RALP (p = 0.05). The median time to continence was 1 day and 21 days, respectively (p = 0.02).16 The analysis of PSM rates reported 10% in standard approach vs 28.2% in RS approach (p = 0.05), but a sub-analysis revealed that this difference was due to the higher rate of pT3 disease in RS-RARP and not related to the extent of neurovascular tissue dissection. Furthermore, the univariate and multivariate regression analysis showed that age and the surgical approach were the only factors significantly associated with immediate continence recovery.16
To date, eight systematic reviews and meta-analyses investigated the role of RS-RARP.22–29 It merit mention the systematic review by Checcucci et al that reported comparable surgical outcomes and no significant difference in overall complications.22 RS-RARP showed higher PSM rate (24% vs 15%), mainly in anterior tumors, but when the analysis was stratified for pT stage, there were similar rates for pT3 disease.22 PSM exposes to a greater risk of BCR and consequently to salvage treatments that could worsen functional outcomes, even if no difference in BCR at 1 year was reported (13.3% in the RS-RARP group and 16.7% in the standard RALP group).22 The authors showed a statistically significant advantage of urinary continence (0–1 safety pad per day) in RS-RARP group at 1 month (OR 2.54, 95% CI: 1.16–5.53; P = 0.02), 3 months (OR 3.86, 95% CI: 2.23–6.68; P < 0.001), 6 months (OR 3.61, 95% CI: 1.88–6.91; P = 0.001), and 12 months (OR 7.29, 95% CI: 1.89–28.13; P = 0.004). Unfortunately, the data regarding erectile dysfunction and potency are lacking.22 All these analyses confirm that RS-RARP leads to a faster recovery in continence, with the same risk of complications, Phukan et al reported that early continence is significantly better in Bocciardi approach compared to standard one, even if this benefit seems to reduce with time, with no statistical significance at 6 (RR 1.24, 95% CI 0.99–1.54, p 0.06) and 12 months (RR 1.13, 95% CI 0.93–1.39, p 0.22).23 Albisinni et al confirmed the improvement of immediate urinary continence for patients undergoing RS-RALP, with continence rates ranging from 51% to 71% compared to standard RALP (21–48%).24 However, in the following 6 months follow-up this benefit was progressively lost. Moreover, the authors reported no significant differences in terms of QoL between the 2 techniques in the various phases of the follow-up.24 In a recent meta-analysis, Barakat et al showed higher PSM rates after RS-RARP in pT2 and pT3 cohorts, although not statistically significant in the second case (RR = 1.39; 95% CI 1.01–1.91 vs RR = 1.36; 95% CI 0.74–2.50).25 The authors confirmed that immediate continence recovery was higher with RS-RARP (RR = 1.81; 95% CI 1.26–2.60) compared to standard RALP, with progressive improvement over time at 3 (RR = 1.57; 95% CI 0.69–3.58) and 6 months (RR = 1.22; 95% CI 0.89–1.66), but no significant difference at 12-month follow-up (RR = 1.14; 95% CI 0.98–1.32). The data regarding erectile dysfunction show similar results in both groups.25
In the Cochrane review, Rosenberg et al analyzed 5 randomized controlled trials, which included 502 patients. They reported an improvement in urinary continence at 1 week (RR: 1.74) and 3 months (RR: 1.33) post catheter removal in RS-RARP compared to standard RARP. No statistically significant difference was found in continence recovery at 12 months post-operatively (RR: 1.01). Low-certainly evidence was reported about the risk of PSM in RS-RARP (RR: 1.95). The results about potency recovery (RR: 0.98) and BCR (HR: 0.45) after RS-RALP was uncertain.26
The obstacle still to overcome is PSMs, which seems to be related to the learning curve for RS-RARP. Galfano et al demonstrated an important reduction in PSM rate between the first and second 100 RS-RARP (22.4% vs 9%), for both pT2 and pT3 disease.11
RS-RARP in High-Risk Prostate Cancer
The most available studies on RS-RARP focused on low- and intermediate-risk PCa. According to European Association of Urology (EAU) guidelines, the treatment of high-risk PCa patients (PSA > 20 ng/mL, Gleason score 8–10 or clinical-stage ≥T3) involves radical prostatectomy with extended pelvic lymph node dissection as part of a multimodal treatment, including radiotherapy and androgen deprivation therapy (ADT).1 Many detractors believe that RS-RARP cannot play an important role in the setting of high-risk patients, in the absence of high-level evidence and with the risk of increasing PSM rate (Tables 1–3).
In 2019, Nyarangi-Dix et al attempted to evaluate for the first time ever the role of RS-RARP in high-risk setting and locally advanced prostate cancer patients.18 In this retrospective study on a cohort of 50 patients the authors reported 38% of early urinary continence recovery after 1 week catheter removal. This increased to 98% after 1 year of follow-up. Moreover, 41% of patients achieved an erection sufficient for penetration. Regarding oncological results, the PSM rate was 42% with 84% of pT3a disease treated.18 However, this study is limited by the small sample size and the short follow-up. Therefore, do deeply explore the safety profile of RS-RARP in terms of functional and oncological outcome in high-risk prostate cancer setting, Dell’Oglio et al evaluated 320 high-risk PCa patients treated in a single European center.20 They reported a PSM rate of 28.8%, in line with those reported by the largest case series for high-risk patients treated with standard anterior approach (range between 25.3% and 34.8% REF).30–33 Freedom from BCR and additional treatments at 4 years was 63.6%.20 Of note, the authors demonstrated a faster and better urinary continence recovery in short term and similar results in long-term relative to standard RARP. Specifically, the immediate urinary continence recovery was 53%, 84% and 85% respectively after 1 and 2 years.20 Moreover, the results on the safety of technique were in line with the standard RARP (4%–14.3% perioperative complications): the authors reported 4% of intraoperative complications and 14% of postoperative complications.
Subsequently, in a multicentric study, Galfano et al analyzed 579 cases of high-risk PCa undergoing RS-RARP performed by 9 expert surgeons (>100 cases of RS-RARP performed).19 Comparing the data obtained with those of high-risk patients undergoing standard RARP in high volume centers, the authors reported similar PSM rates (31% vs 29%), but higher BCR rates (27% vs 19%), probably due to a higher rate of locally advanced tumors.19 High PSA, large prostate volume and long surgical time were independent predictors of PSM. The faster recovery of urinary continence (no or 1 safety pad/day) after RS-RARP was confirmed with 66% after 1 week and 89% after 1 year.19
Raheem et al evaluated the predictors of BCR after RS-RARP in a cohort of 359 patients.17 These patients were stratified in National Comprehensive Cancer Network (NCCN) prostate cancer risk classification groups:34 very low- (7%), low- (11.4%), intermediate (35.9%), high- (27.6%) and very high-risk PCa (18.1%). The overall BCR rate and PSM rate were 14.8% and 30.6% respectively, at a median follow-up of 26 months. Among the high-risk group, the PSM rate was 41.2%, the BCR rate was 22% and the 3-year BCR free-survival rate was 72%.17 When PSMs were stratified according to pathological stage, the authors reported 14.6% in patients with pT2 disease, 40.8% with pT3a disease, 67.4% with pT3b disease and 100% with pT4 disease. The sub-cohort of patients with pT2 had a higher BCR free-survival rate of 89.5% compared with pT3a (75.2%) and ≥pT3b (65.2%) (p < 0.001).17 Furthermore, patients with PSMs had lower BCR free-survival rate (76.3%) compared with patients with negative surgical margins (84%) (p = 0.009). The study of preoperative clinical variables described how PSA, percentage of maximum core involvement on biopsy and clinical stage ≥T3a were predictors of BCR, whereas tumor volume and pathological GS were the main pathological risk factors.17
RS-RARP After Transurethral Resection of the Prostate (TURP)
Radical prostatectomy after TURP is more challenging and associated with worse functional and oncological outcomes. These findings applied to open and anterior standard robot-assisted RP.35–38
Recently, Tappero et al reported for the first time ever that previous TURP unfavorably impact also on functional and oncological outcomes of patients treated with RS approach39 In a series of 1386 RS-RARP patients treated at a single European referral center, 99 (7%) had previous TURP. The rates of immediate continence recovery were 40 vs 67% in previous TURP group vs no-TURP patients (p < 0.001). At 12 months from RS-RARP, the rates of continence recovery were 68 vs 94% in previous TURP vs no-TURP patients (p < 0.001). Patients with a history of previous TURP had a significantly lower likelihood to achieve both immediate (OR: 0.32, p < 0.001) and 12-months continence recovery (HR: 0.54, p < 0.001). Despite its inner higher complexity, RS-RARP in patients with previous TURP is not associated with increased PSMs and with higher cost in terms of complications.39
Salvage RS-RARP
The standard of care for localized PCa is represented by radical prostatectomy (RP) or definitive radiotherapy.1 About 40% of patients with intermediate- and high-risk PCa undergo definitive radiotherapy.40 However, radiation failure may occur in a subset of these patients (20–60%),41 which may experience a BCR, defined by a rise in their prostate-specific antigen (PSA) level of 2 ng/mL above the postradiotherapy nadir.1 Unlike the management of BCR after RP, the correct management after radiotherapy remains unclear due to a lack of data in this patients setting.
Though potentially curative, salvage prostatectomy has not been frequently performed due to high rates of intra- and postoperative complications (rectal injury, urinary incontinence, anastomotic stricture, positive surgical margins and BCR).42 The advent of robotic surgery seems to be able to reduce these high complication rates (39–47%), making salvage prostatectomy safer, although urinary incontinence remains an unsolved problem.43 RS-RARP has been shown to improve urinary continence outcomes, especially in early return to continence,13,15 therefore this suggests a theoretical advantage of the anterior approach to reduce complication rates.
Mason et al described, in a literature review, how the salvage RS approach can improve continence while maintaining oncological integrity.44 Three retrospective studies comparing salvage RS-RARP with standard salvage RARP were analyzed (Schuetz et al,45 Madi et al,46 Kowalczyk et al47). They reported lower complication rates in RS approach compared to standard one (10% vs 26%), with no rectal injury probably due to direct visualization of the rectum that this technique allows. RS-RARP has been shown to improve earlier return to continence, significant improvement in immediate and long-term continence, and many patients are immediately continental upon catheter removal:44 Schuetz et al reported immediate continence (no safety pad per day) 14% in salvage RS-RARP group and 0% in standard approach, with an improvement at 12 months follow-up (28% vs 0%).45 Madi et al reported continence rate (0–1 safety pad per day) in salvage RS of 25% after catheter removal, improved up to 80% at 3 months follow-up, compared to standard approach.46 Kowalczyk et al reported early urinary continence in salvage RS, as both definition 0 safety pad per day and 0–1 safety pad per day with 54% and 78%, respectively.47 Potency outcomes are poorly recorded throughout the salvage literature, remain consistently poor regardless of the type of surgery. RS-RARP approaches have been linked to higher PSM rates, although it is unclear whether these are clinically significant.44 Indeed, even with this increase in PSM, the rates of BCR and add-on treatment were not significantly different, suggesting that these margins may not have clinical consequences.44 Further studies regarding this technique in the salvage setting are needed to ensure oncological control.
Learning Curve in RS-RARP
More than a decade after the introduction of RS-RARP, its benefits in terms of better urinary continence outcomes, especially immediate continence, have been highlighted in numerous systemic reviews and meta-analyses.22–25 However, there is still a reluctant opinion from some experts to perform RS-RARP, who argue that the technique is technically difficult with a higher risk of PSMs than the standard approach.
In 2020, Galfano et al provided the first report on the learning curve of RS-RALP, relying on a multi-institutional database.48 The study evaluated the effect of surgical experience on perioperative, functional and oncological outcomes during the first 50 cases of RS-RARP performed by surgeons naïve to this new approach48 The authors reported a statistically significant improvement in terms of console time, complications and immediate continence recovery during the learning process. Conversely, the authors failed to observe a statistically significant improvement in terms of PSMs with the increasing surgical experience, strongly suggesting that 50 cases are not enough to reduce the PSM rates.48 This is crucial in order to understand that the available evidence suggesting differences in PSMs rate between RS-RARP and standard RARP might be related to the learning curve associated with RS-RARP approach.48
Anceschi et al demonstrated that the learning curve in naïve surgeons is equivalent between standard RARP and RS-RARP.49 Urinary continence (94.5% vs 90%, p 0.125), sexual function (34.6% vs 32.5%, p 0.861), PSM (21.6% vs 16%, p 0.225), 1-year BCR (2.87% vs 2.46%, p 0.783) and 1-year trifecta rates (34.3% vs 34.5%, p 0.354) were comparable between mentors group and trainers group. Supervised trainee surgeons duplicate the results of their mentors during the learning process in high-volume centers, regardless of the surgical technique considered. For both trainee and expert surgeons, each incremental RARP procedure is associated with an increase of 0.1% in trifecta outcomes, highlighting the role of experience consolidation as a major factor in improving the quality of RARP composite outcome.49
The study by Olivero et al also evaluated the oncological and functional results after RS-RARP performed by young surgeons at the beginning of their robotic experience.50 They reported no difference in oncological outcomes between the learning curve group and experts one (PSM rates 19.9% vs 25.4%, p = 0.13, BCR 4.8% vs 5.6%, p = 0.59). The immediate urinary continence was comparable between two groups (82.2% vs 83.9%, p = 0.68) and at 1-year follow-up (90.1% vs 94.2%, p = 0.27). Only the surgical time was in favor of experienced surgeons.50 Elliot et al evaluated the results of a single experienced robotic surgeon who transitioned from standard RALP to RS-RARP.51 A longer operating room time has been documented. Complication rates were similar. No differences in early oncological outcomes, including PSM and BCR rates, and need for adjuvant or salvage treatments. Time to urinary continence and immediate continence rate were higher in the RS-RARP group.51
RS-RARP can be safely adopted by experienced standard RARP surgeons without compromising early oncological outcomes and with the benefit of improved early continence recovery. Moreover, the RS approach can be considered feasible, safe and attractive for surgeons properly trained, even for those who are at the beginning of their robotic experience.
A Look into the Near Future
Thanks to the technological improvements in the robotic field, increasingly pushed towards a surgery as minimally invasive as possible without reducing oncological results, in 2018 the da Vinci single-port system (SP) was approved for urological patients.52 Consequently, new studies have been published on its use also in the urological field, such as for example in radical prostatectomy, where; however, the real benefits are still to be investigated. The da Vinci SP shares different elements with its precursors, multiport da Vinci systems, as well as the same operative interface that guarantees high definition three-dimensional visualization, with magnification and scaled movement, and tremor reduction. It uses a single port of 27 mm that allows the introduction of an 8-mm articulating flexible camera and three articulating 6-mm instruments. The camera can rotate in all directions, so the use of a 0° lens provides new visualization angles, while the instruments maintain a fixed position. The first studies mainly focused on the anterior approach, however the first experiences with the posterior approach have also been published. Ng et al reported their experience with SP-RARP performed in cadaveric model, describing additional benefit in viewing the operating field, in particular during the posterior dissection, bladder dissection, and anastomosis.53 Agarwal et al in their cohort of 49 patients undergoing SP-RARP, seven cases were treated with RS approach. They reported no post-operative complication with all patients complete continent at 1 week catheter removal and 1 patient with PSM.54
Conclusion
Current literature confirms that RS-RARP is a reliable and safe technique, to be used as a valid alternative to the standard RARP, not only in the setting of low- and intermediate-risk patients, but also in high-risk disease. The posterior approach is associated with earlier continence recovery relative to standard RARP approach. However, this advantage is affected by the history of previous TURP. Moreover, RS-RARP appears to increase the risk of positive surgical margins (PSMs). However, the latter seems to be related to the learning process of this harder procedure. Data regarding erectile function recovery is still unclear and lacks comparative studies.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–262. doi:10.1016/j.eururo.2020.09.042
2. Montorsi F, Wilson TG, Rosen RC, et al. Best practices in robot-assisted radical prostatectomy: recommendations of the Pasadena consensus panel. Eur Urol. 2012;62(3):368–381. doi:10.1016/j.eururo.2012.05.057
3. Autorino R, Porpiglia F, Dasgupta P, et al. Precision surgery and genitourinary cancers. Eur J Surg Oncol. 2017;43(5):893–908. doi:10.1016/j.ejso.2017.02.005
4. Ma X, Tang K, Yang C, et al. Bladder neck preservation improves time to continence after radical prostatectomy: a systematic review and meta-analysis. Oncotarget. 2016;7(41):67463–67475. doi:10.18632/oncotarget.11997
5. Martini A, Falagario UG, Villers A, et al. Contemporary techniques of prostate dissection for robot-assisted prostatectomy. Eur Urol. 2020;78:583–591.
6. Sridhar AN, Abozaid M, Rajan P, et al. Surgical techniques to optimize early urinary continence recovery post robot assisted radical prostatectomy for prostate cancer. Curr Urol Rep. 2017;18(9):71. doi:10.1007/s11934-017-0717-4
7. Galfano A, Ascione A, Grimaldi S, Petralia G, Strada E, Bocciardi AM. A new anatomic approach for robot-assisted laparoscopic prostatectomy: a feasibility study for completely intrafascial surgery. Eur Urol. 2010;58(3):457–461. doi:10.1016/j.eururo.2010.06.008
8. Walz J, Epstein JI, Ganzer R, et al. A critical analysis of the current knowledge of surgical anatomy of the prostate related to optimisation of cancer control and preservation of continence and erection in candidates for radical prostatectomy: an update. Eur Urol. 2016;70(2):301–311. doi:10.1016/j.eururo.2016.01.026
9. Ou YC, Yang CK, Wang J, et al. The trifecta outcome in 300 consecutive cases of robotic-assisted laparoscopic radical prostatectomy according to D’Amico risk criteria. Eur J Surg Oncol. 2013;39(1):107–113. doi:10.1016/j.ejso.2012.10.003
10. Borregales LD, Berg WT, Tal O, et al. Trifecta’ after radical prostatectomy: is there a standard definition? BJU Int. 2013;112(1):60–67. doi:10.1111/bju.12002
11. Galfano A, Di Trapani D, Sozzi F, et al. Beyond the learning curve of the Retzius-sparing approach for robot-assisted laparoscopic radical prostatectomy: oncologic and functional results of the first 200 patients with ≥ 1 year of follow-up. Eur Urol. 2013;64(6):974–980. doi:10.1016/j.eururo.2013.06.046
12. Lim SK, Kim KH, Shin T-Y, et al. Retzius-sparing robot-assisted laparoscopic radical prostatectomy: combining the best of retropubic and perineal approaches. BJU Int. 2014;114(2):236–244. doi:10.1111/bju.12705
13. Dalela D, Jeong W, Prasad M-A, et al. A pragmatic randomized controlled trial examining the impact of the retzius-sparing approach on early urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2017;72(5):677–685. doi:10.1016/j.eururo.2017.04.029
14. Menon M, Dalela D, Jamil M, et al. Functional recovery, oncologic outcomes and postoperative complications after robot-assisted radical prostatectomy: an evidence-based analysis comparing the retzius sparing and standard approaches. J Urol. 2018;199(5):1210–1217. doi:10.1016/j.juro.2017.11.115
15. Qiu X, Li Y, Chen M, et al. Retzius-sparing robot-assisted radical prostatectomy improves early recovery of urinary continence: a randomized, controlled, single-blind trial with a 1-year follow-up. BJU Int. 2020;126(5):633–640. doi:10.1111/bju.15195
16. Asimakopoulos AD, Topazio L, De Angelis M, et al. Retzius-sparing versus standard robot-assisted radical prostatectomy: a prospective randomized comparison on immediate continence rates. Surg Endosc. 2019;33(7):2187–2196. doi:10.1007/s00464-018-6499-z
17. Abdel Raheem A, Chang KD, Alenzi MJ, et al. Predictors of biochemical recurrence after Retzius-sparing robot-assisted radical prostatectomy: analysis of 359 cases with a median follow-up period of 26 months. Int J Urol. 2018;25(12):1006–1014. doi:10.1111/iju.13808
18. Nyarangi-Dix JN, Görtz M, Gradinarov G, et al. Retzius-sparing robot-assisted laparoscopic radical prostatectomy: functional and early oncologic results in aggressive and locally advanced prostate cancer. BMC Urol. 2019;19(1):113. doi:10.1186/s12894-019-0550-9
19. Galfano A, Tappero S, Eden C, et al. Multicentric experience in Retzius-sparing robot-assisted radical prostatectomy performed by expert surgeons for high-risk prostate cancer. Minerva Urol Nephrol. 2022;74(5):607–614. doi:10.23736/S2724-6051.22.04857-1
20. Dell’Oglio P, Tappero S, Longoni M, et al. Retzius-sparing robot-assisted radical prostatectomy in high-risk prostate cancer patients: results from a large single-institution series. Eur Urol Open Sci. 2022;38:69–78. doi:10.1016/j.euros.2022.02.007
21. Abdollah F, Dalela D, Sood A, et al. Functional outcomes of clinically high-risk prostate cancer patients treated with robot-assisted radical prostatectomy: a multi-institutional analysis. Prostate Cancer Prostatic Dis. 2017;20(4):395–400. doi:10.1038/pcan.2017.26
22. Checcucci E, Veccia A, Fiori C, et al. Retzius-sparing robot-assisted radical prostatectomy vs the standard approach: a systematic review and analysis of comparative outcomes. BJU Int. 2020;125(1):8–16. doi:10.1111/bju.14887
23. Phukan C, Mclean A, Nambiar A, et al. Retzius sparing robotic assisted radical prostatectomy vs. conventional robotic assisted radical prostatectomy: a systematic review and meta-analysis. World J Urol. 2020;38(5):1123–1134. doi:10.1007/s00345-019-02798-4
24. Albisinni S, Dasnoy C, Diamand R, et al. Anterior vs. Retzius-sparing robotic assisted radical prostatectomy: can the approach really make a difference? Minerva Urol Nephrol. 2022;74(2):137–145. doi:10.23736/S2724-6051.21.04623-1
25. Barakat B, Othman H, Gauger U, Wolff I, Hadaschik B, Rehme C. Retzius sparing radical prostatectomy versus robot-assisted radical prostatectomy: which technique is more beneficial for prostate cancer patients (MASTER Study)? A systematic review and meta-analysis. Eur Urol Focus. 2022;8(4):1060–1071. doi:10.1016/j.euf.2021.08.003
26. Rosenberg JE, Jung JH, Edgerton Z, et al. Retzius-sparing versus standard robotic-assisted laparoscopic prostatectomy for the treatment of clinically localized prostate cancer. Cochrane Database Syst Rev. 2020;8(8):CD013641. doi:10.1002/14651858.CD013641.pub2
27. Tai T-E, Wu -C-C, Kang Y-N, Wu J-C. Effects of Retzius sparing on robot-assisted laparoscopic prostatectomy: a systematic review with meta-analysis. Surg Endosc. 2020;34(9):4020–4029. doi:10.1007/s00464-019-07190-2
28. Xu J-N, Xu Z-Y, Yin H-M. Comparison of retzius-sparing robot-assisted radical prostatectomy vs. conventional robot-assisted radical prostatectomy: an up-to-date meta-analysis. Front Surg. 2021;8:738421. doi:10.3389/fsurg.2021.738421
29. Chung DY, Jung HD, Kim DK, et al. Outcomes of Retzius-sparing versus conventional robot-assisted radical prostatectomy: a KSER update series systematic review and meta-analysis. PLoS One. 2022;17:e0268182. doi:10.1371/journal.pone.0268182
30. Kumar A, Samavedi S, Bates AS, et al. Safety of selective nerve sparing in high risk prostate cancer during robot-assisted radical prostatectomy. J Robot Surg. 2017;11(2):129–138. doi:10.1007/s11701-016-0627-3
31. Abdollah F, Sood A, Sammon JD, et al. Long-term cancer control outcomes in patients with clinically high-risk prostate cancer treated with robot-assisted radical prostatectomy: results from a multi-institutional study of 1100 patients. Eur Urol. 2015;68(3):497–505. doi:10.1016/j.eururo.2015.06.020
32. Abdollah F, Dalela D, Sood A, et al. Intermediate-term cancer control outcomes in prostate cancer patients treated with robotic-assisted laparoscopic radical prostatectomy: a multi-institutional analysis. World J Urol. 2016;34:1357–1366. doi:10.1007/s00345-016-1781-y
33. Mazzone E, Dell’Oglio P, Rosiello G, et al. Technical refinements in superextended robot-assisted radical prostatectomy for locally advanced prostate cancer patients at multiparametric magnetic resonance imaging. Eur Urol. 2021;80(1):104–112. doi:10.1016/j.eururo.2020.09.009
34. Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(5):479–505. doi:10.6004/jnccn.2019.0023
35. Gupta NP, Singh P, Nayyar R. Outcomes of robot-assisted radical prostatectomy in men with previous transurethral resection of prostate. BJU Int. 2011;108(9):1501–1505. doi:10.1111/j.1464-410X.2011.10113.x
36. Zugor V, Labanaris AP, Porres D, Witt JH. Surgical, oncologic, and short-term functional outcomes in patients undergoing robot-assisted prostatectomy after previous transurethral resection of the prostate. J Endourol. 2012;26(5):515–519. doi:10.1089/end.2011.0205
37. Hung C-F, Yang C-K, Ou Y-C. Robotic assisted laparoscopic radical prostatectomy following transurethral resection of the prostate: perioperative, oncologic and functional outcomes. Prostate Int. 2014;2(2):82–89. doi:10.12954/PI.14046
38. Pompe RS, Leyh-Bannurah S-R, Preisser F, et al. Radical prostatectomy after previous TUR-P: oncological, surgical, and functional outcomes. Urol Oncol. 2018;36(12):527.e21–527.e28. doi:10.1016/j.urolonc.2018.08.010
39. Tappero S, Vecchio E, Palagonia E, et al. Retzius-sparing robot-assisted radical prostatectomy after previous trans-urethral resection of the prostate: assessment of functional and oncological outcomes. Eur J Surg Oncol. 2023;49(8):1524–1535. doi:10.1016/j.ejso.2023.03.218
40. Mahal BA, Butler S, Franco I, et al. Use of active surveillance or watchful waiting for low-risk prostate cancer and management trends across risk groups in the United States, 2010–2015. JAMA. 2019;321(7):704–706. doi:10.1001/jama.2018.19941
41. Zietman AL, Coen JJ, Dallow KC, Shipley WU. The treatment of prostate cancer by conventional radiation therapy: an analysis of long-term outcome. Int J Radiat Oncol Biol Phys. 1995;32(2):287–292. doi:10.1016/0360-3016(95)00123-G
42. Bianco FJ, Scardino PT, Stephenson AJ, Diblasio CJ, Fearn PA, Eastham JA. Long-term oncologic results of salvage radical prostatectomy for locally recurrent prostate cancer after radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62(2):448–453. doi:10.1016/j.ijrobp.2004.09.049
43. Eandi JA, Link BA, Nelson RA, et al. Robotic assisted laparoscopic salvage prostatectomy for radiation resistant prostate cancer. J Urol. 2010;183(1):133–137. doi:10.1016/j.juro.2009.08.134
44. Mason JB, Hatch L, Dall C, Kowalczyk KJ. Salvage retzius-sparing radical prostatectomy: a review of complications, functional outcomes, and oncologic outcomes. Curr Oncol. 2022;29(12):9733–9743. doi:10.3390/curroncol29120764
45. Schuetz V, Reimold P, Goertz M, et al. Evolution of salvage radical prostatectomy from open to robotic and further to retzius sparing surgery. J Clin Med. 2021;11(1):202. doi:10.3390/jcm11010202
46. Madi R, Sayyid RK, Hiffa A, Thomas E, Terris MK, Klaassen Z. Early experience with salvage retzius-sparing robotic-assisted radical prostatectomy: oncologic and functional outcomes. Urology. 2021;149:117–121. doi:10.1016/j.urology.2020.12.029
47. Kowalczyk KJ, Madi RH, Eden CG, et al. Comparative outcomes of salvage retzius-sparing versus standard robotic prostatectomy: an international, multi-surgeon series. J Urol. 2021;206(5):1184–1191. doi:10.1097/JU.0000000000001939
48. Galfano A, Secco S, Dell’Oglio P, et al. Retzius-sparing robot-assisted radical prostatectomy: early learning curve experience in three continents. BJU Int. 2021;127(4):412–417. doi:10.1111/bju.15196
49. Anceschi U, Galfano A, Luciani L, et al. Analysis of predictors of early trifecta achievement after robot-assisted radical prostatectomy for trainers and expert surgeons: the learning curve never ends. Minerva Urol Nephrol. 2022;74(2):133–136. doi:10.23736/S2724-6051.22.04805-4
50. Olivero A, Galfano A, Piccinelli M, et al. Retzius-sparing robotic radical prostatectomy for surgeons in the learning curve: a propensity score-matching analysis. Eur Urol Focus. 2021;7(4):772–778. doi:10.1016/j.euf.2020.03.002
51. Elliott N, Pahouja G, Felice M, et al. Transition from standard robotic prostatectomy to Retzius-sparing prostatectomy: feasibility and early outcomes. J Robot Surg. 2023;17(5):2035–2040. doi:10.1007/s11701-023-01596-w
52. Dobbs RW, Halgrimson WR, Talamini S, Vigneswaran HT, Wilson JO, Crivellaro S. Single-port robotic surgery: the next generation of minimally invasive urology. World J Urol. 2020;38(4):897–905. doi:10.1007/s00345-019-02898-1
53. C-F N, Chan ESY, Teoh JYC. The use of the da Vinci sp system for retzius-sparing radical prostatectomy in cadaveric model. Urology. 2019;125:260. doi:10.1016/j.urology.2018.11.042
54. Agarwal DK, Sharma V, Toussi A, et al. initial experience with da Vinci single-port robot-assisted radical prostatectomies. Eur Urol. 2020;77(3):373–379. doi:10.1016/j.eururo.2019.04.001
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.