Back to Journals » Blood and Lymphatic Cancer: Targets and Therapy » Volume 14
Refractory Burkitt Lymphoma: Diagnosis and Interventional Strategies
Authors Malfona F , Testi AM , Chiaretti S, Moleti ML
Received 29 November 2023
Accepted for publication 23 February 2024
Published 13 March 2024 Volume 2024:14 Pages 1—15
DOI https://doi.org/10.2147/BLCTT.S407804
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Wilson Gonsalves
Francesco Malfona, Anna Maria Testi, Sabina Chiaretti, Maria Luisa Moleti
Department of Translational and Precision Medicine, ‘Sapienza’ University, Rome, Italy
Correspondence: Maria Luisa Moleti, Hematology Institute, Via Benevento 6, Rome, 00161, Italy, Tel +39 3384714201, Fax +39 0644641984, Email [email protected]
Abstract: Despite excellent results in frontline therapy, particularly in pediatric age, refractory Burkitt lymphoma still remains a therapeutic challenge, with dismal outcome. The prognosis is very poor, ranging from less than 10% to 30– 40%, with longer survival only in transplanted patients. On account of the paucity of data, mostly reporting on small series of patients, with heterogeneous characteristics and salvage treatments, at present it is impossible to draw definitive conclusions on the treatment of choice for this difficult to treat subset of patients. New insights into Burkitt lymphoma/leukemia cell biology have led to the development of new drugs, currently being tested, directed at different specific targets. Herein, we describe the results so far reported in refractory Burkitt lymphoma/leukemia, with standard treatments and hematopoietic stem cell transplant, and we review the new targeted drugs currently under evaluation.
Keywords: Burkitt lymphoma, target therapy, relapse, refractory, outcome
Introduction
Historically recognized as a model for research advances regarding pathogenesis, cytogenetics and molecular genetics, as well as the first intensely chemo-sensitive tumor to be described, Burkitt Lymphoma/leukemia (BLL) was initially described as a tumor affecting the jaws of African children,1 and is nowadays considered one of the most curable non-Hodgkin lymphomas (NHL), with cure rates outreaching 90% and 70% in pediatric and adult age, respectively.2–7 However, several patient subsets still deserve therapy optimization. A small fraction of patients displays therapy resistance or recurrence, and older patients, or those with multiple comorbidities, are often ineligible for pediatric inspired highly intensive chemotherapy. Furthermore, although BLL has excellent outcome in high-income countries, it only represents a minority of annually diagnosed NHL/leukemias in the western world, while it is the most common childhood cancer in sub-Saharan Africa, where its prognosis is poor and has been virtually unchanged over the last 40 years.8
In western countries, due to the excellent results achieved in BLL, few patients display recurrence or refractoriness after first-line treatment. Insufficient data have been reported on relapsed and refractory (R/R) BLL in children and even less in adults, regarding small series, mostly including various histologic subtypes, with heterogeneous salvage regimens. The prognosis for R/R BLL is very poor, with survival ranging from less than 10% up to 36%, being higher in responding patients submitted to autologous (auto) or allogeneic (allo) hematopoietic stem cell transplant (HSCT). Innovative therapies are currently under investigation, including new compounds directed against specific targets, such as B-cell receptor signaling inhibitors, proteasome inhibitors, next‐generation monoclonal antibodies, and novel approaches of immunotherapy [chimeric antigen receptor T cells (CAR‐T) and bispecific antibodies].
Herein, we review the results reported so far for the treatment of BLL patients R/R to frontline treatment, with a focus on the novel therapies under evaluation in this challenging setting.
Epidemiology
BLL is a mature B-cell neoplasm, characterized by a highly aggressive disease course and specific epidemiological and clinical features across different continents, although the translocation and dysregulation of the proto-oncogene MYC represent the common genetic hallmark of all BLL variants.9 BLL is diagnosed more commonly in childhood, with a peak incidence at the age of 6–7 years, representing in western countries around 40% of all NHL in children <14 years, 20% in adolescents, and less than 5% in adults.10 In equatorial Africa, where the disease distribution overlaps that of malaria,11 BLL has the highest incidence rate in pediatric cancer with 5–10 cases per 100,000 people/year,12 almost all cases being associated with Epstein–Barr virus (EBV) infection, that has a causative role (endemic BLL variant).8–12 In western countries, BLL is associated only in 10–20% of cases with EBV infection. The immunodeficiency-related variant is most diagnosed in patients affected by HIV infection with EBV found in less than 40%.13 Transplant recipients14 and people affected by inherited immune deficiencies are also rarely diagnosed with immunodeficiency-related BLL.
Given the epidemiology of the disease, about 90% of patients affected by BLL, as well as most R/R patients, live in low-middle-income countries (L-MIC), where the real incidence of BLL is likely even much higher due to under-diagnosis/registration.15
Diagnosis and Molecular Genetics
The histologic description of BLL has been practically unchanged over the years and is defined as a complete effacement of the involved tissue by a proliferation of medium-sized, monomorphic cells with basophilic cytoplasm and prominent vacuoles, showing a germinal center B-cell phenotype (CD10+, BCL6+, BCL2–) with Ki67 >95%, associated with interspersed tingible body macrophages giving a “starry sky” appearance at low magnification. However, since the first descriptions of t(8;14), with MYC gene involvement, molecular and genomic studies continuously add new insights to better define the biological nature and the different subtypes of BLL. These advances open new horizons for the new therapeutic strategies for first-line and, more importantly, salvage target therapies. Translocations involving MYC gene at chromosome 8q24 represent the hallmark of the disease, with 80% of cases carrying a t(8;14) in which MYC is fused with the immunoglobulin heavy chain locus (IGH). The remaining cases show a MYC fusion with immunoglobulin light chain locus [t(2;8) or t(8;22)]. A rare subtype of B-cell lymphoma with 11q chromosomal aberration (without MYC rearrangements), formerly known as Burkitt-like lymphoma with 11q aberration, has recently been demonstrated to have a spectrum of genomic imbalances more similar to high grade B-cell lymphoma (HGBL) and is considered accordingly in the revised 5th edition of WHO classification.9 Gene expression profile studies have elucidated differences among large B-cell lymphoma (LBCL), HGBL and BLL,16 with the latter characterized by dysregulated germinal center B-cell gene expression, higher expression of MYC pathway downstream genes and decreased expression of MHC complex class I.
Subsequent integrated whole-genome and RNA sequencing studies demonstrated that several other mutated genes cooperate with MYC dysregulation.17,18 TCF3 is an essential gene expressed during the germinal center transit. This gene, or its negative regulator ID3, has been shown to be mutated in 70% of sporadic and immunodeficiency-related BLL, leading to constitutive B-cell receptor (BCR) and phosphatidylinositol 3-kinase (PI3K) pathway activation. Mutations of the CCND3 gene, coding for cyclin D3, have been found in one third of cases. Moreover, PI3K isoform mutations have also been detected in R/R BLL patients,19 as well as coding and non-coding alterations in other genes. TP53 mutations, occurring in about 30% of patients, also cooperate to enhance therapy resistance.20 Recently, EBV infection has gained an important role that supersedes the distinction among geographical variants, describing a virus-driven versus a mutational-driven lymphomagenesis model. Regardless of epidemiological subtypes, EBV-positive BLL harbors higher levels of IGH somatic hypermutation and fewer cooperative mutations.21 EBV status distinction is therefore recommended by the 5th WHO classification.9 Moreover, the analysis of IGH translocation breakpoint revealed that EBV-positive tumors more commonly show an upstream breakpoint, whereas EBV-negative tumors have a breakpoint within the MYC gene (commonly intron 1). Clustering analysis of whole genome and transcriptome sequencing data recently revealed distinct genetic subgrouping shared by adult and pediatric BLL, named after the more frequent copy number aberrations and mutations observed: a DGG-BL subgroup harboring DDX3X, GNA13 and GNAI2; an IC-BL group (ID3 and CCND3); and a Q53-BL group (quiet TP53).22
Current Frontline Treatment
The treatment of BLL in patients aged <18 years has been highly successful in western countries over the last 30 years and is based on the use of 2 main chemotherapy backbones, derived from French–American–British/Lymphomes malins B (FAB/LMB84, LMB89 and LMB96) consecutive clinical trials,23–25 and from the Berlin–FrankfurtMünster (BFM) NHL90 and NHL95 regimens.26,27 These two regimens were globally very similar, relying on short intensive multiagent chemotherapy, consisting of non-cross-resistant drugs and intrathecal prophylaxis. Although slight differences were present, the two regimens achieved cure rates exceeding 85%, and approaching 95–100% in early stages. The addition of the anti-CD20 monoclonal antibody rituximab to the LMB backbone significantly increased the outcome in patients with high-risk disease, with a 3-year event-free survival (EFS) of 93.9%.4 Moreover, the addition of rituximab yielded superimposable outcome with less treatment-related toxicity in the favorable prognosis subset, with a significant reduction in anthracyclines cumulative dose.28
The outcome of pediatric regimens paved the way to an intensified approach for adult BLL that represented a turning point for this population. In Europe, with the adapted LMB95 and BFM-NHL pediatric schemas, OS of more than 50% was achieved for the first time in adult patients.29,30 In the GMALL-B-ALL/NHL2002 the addition of rituximab to the BFM schema and the methotrexate dose-adjustment for older patients yielded LFS and OS up to 75% and 80% respectively.5,29–31
In the USA, another three different regimens are mostly employed in adults, similarly based on short-intensive multiagent chemotherapy, and nowadays coupled with rituximab. The regimen published by Magrath et al in the late 1990s, consisting of alternating cycles of cyclophosphamide, doxorubicin, vincristine, high dose methotrexate (CODOX-M) and ifosfamide, high dose cytarabine, etoposide (IVAC) with intrathecal prophylaxis, demonstrated cure rates approaching 80–85%.3,32,33 Inferior outcome was reported for hyper-CVAD (cyclophosphamide, vincristine, adriamycin, dexamethasone) and R-hyper-CVAD, an ALL-adapted schema led by MD Anderson Cancer Center (MDACC), with high toxicity and a 5-year relapse-free survival (RFS) of 52%.34 The use of dose adjusted continuous-infusion chemotherapy with etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin together with rituximab (da-EPOCH-R) and intrathecal prophylaxis, showed effective with EFS of 100% and 82.1% in low- and high-risk patients, but the 4-year EFS was only 45% for patients with CNS involvement.6,7
Despite the similar good results obtained in adults with all these different protocols,35,36 older patients displayed a significantly inferior outcome, confirming that this population remains the most challenging to treat.
Outcome in Refractory/Relapsed Patients
Chemo-Immunotherapy Regimens
Modern multiagent immune-chemotherapy approaches leave a small group of patients progressing after frontline treatment. This subset often shows disease progression during treatment or shortly after treatment conclusion. Late relapses, defined as relapse >6 months after therapy completion, represent less than 10% of cases, while after 12 months are extremely uncommon.37–41 Although R/R patients represent an increasingly smaller percentage, thanks to the continuous advancements in frontline treatment, nowadays frontline therapy failure is still a catastrophic event lacking a defined effective treatment approach.35 Since BLL progression is usually an early event, patients may not result eligible for further highly intensive salvage chemotherapy, but even when a similar regimen is feasible, the outcome is unfavorable, due to acquired chemo-refractoriness.42 The rarity of R/R BLL does not permit comprehensive outcome analysis, and the few published patient series are not commonly focused on a single histologic subtype, or an identical salvage therapy. In the last two decades, mostly retrospective relatively small series have been reported for children, usually with other R/R B-NHL, and even fewer data are available for adults.
The incidence of BLL progression and relapse, so far reported in children, ranges from 4% to 15%.23,25,41 The outcome in the small reported pediatric series is dismal, with 10–31% survival and no standard therapeutic option.43 Different regimens were employed, including high dose methotrexate and/or cytarabine, platinum and, recently, in association with rituximab (Table 1). Among salvage regimens, R-ICE (rituximab, ifosfamide, carboplatin, etoposide) is the most employed, showing 60–70% complete/partial response.44,45
 |
Table 1 Relapsed/Refractory BLL: Results with Current Salvage Therapies in Children and Adults |
At diagnosis, well defined factors are significantly associated with risk of progression, such as age, LDH level, stage and primary CNS or bone marrow involvement, initial high-risk therapy arm and addition of rituximab in frontline therapy.23,25,38,39 At progression, factors associated with survival are less clear, due to the limits of retrospective reports, including different treatments and histologic subtypes of aggressive B-NHL. Time to progression <6 months, bone marrow involvement and LDH at progression >2 times upper limit have been variably associated with an even poorer outcome.38–40
The FAB/LMB cooperative group reported the outcome of R/R patients who had been enrolled in previous frontline trials. A proportion of patients, ranging from 4% to 9%, experienced progression or relapse in different trials, even when rituximab was added to the LMB backbone. In the LMB96 trial,39 104 R/R B-NHL children, including 72 R/R BLL, were treated according to the physician’s choice, with a 2-year OS of 23.3%, worse in patients with primary refractory disease versus relapse after at least 6 months from first CR (15.4% vs 34.5%, p=0.002). Patients with bone marrow progression displayed 2-year OS of 7%. For patients with R/R disease after LMB2001 protocol,40 auto HSCT was recommended in consolidation once in second CR (CR2). Among 33 R/R children (4.3% of enrolled patients), 27 had BLL with 11 having BM involvement. Most patients received a salvage regimen containing rituximab; the overall CR rate was 47%. Eleven BLL patients were consolidated with auto and 6 with allo HSCT. Patients who obtained CR2 before consolidation had the best 5-year OS of 75%, while patients consolidated after a nonresponse had 0% OS, regardless of the HSCT type. Globally, R/R BLL patients had 5-year OS of 29.6%, comparable to the OS of patients in the pre-rituximab era. Recently, the outcome of 157 R/R BLL children enrolled in consecutive BFM-NHL 86, 90, 95 and 04 protocols have been reported.41 Progression rate was similar among the studies, in the range of 8%, but OS for R/R patients was significantly better since the introduction of rituximab in the 2000s (before 2000 11% vs after 2000 27%; p<0.001). In the latter cohort, 71/75 patients were treated with curative intent, 20 consolidated with auto and 26 with allo HSCT. This study reported a benefit in survival for patients consolidated with allo HSCT (58% vs 25%, respectively). Moreover, 15 patients treated according to R-VICI schedule (rituximab, vincristine, idarubicin, ifosfamide, carboplatinum, dexamethasone), followed by allo HSCT, retained a significant improvement in survival (67% vs 19%) compared to all other reinduction schemas and transplant approach. An international retrospective study on R/R pediatric NHL was recently reported, focusing on the re-induction treatment, HSCT and risk factors. The 8-year survival probability was 28±3% for 254 BLL.51
The only prospective pediatric clinical trial investigating salvage chemo-immunotherapy so far reported, enrolled 14 children with sporadic R/R BLL without CNS involvement, and 6 with LBCL who received a salvage treatment with rituximab, ifosfamide, carboplatin and etoposide (R-ICE).44 Among BLL patients, 4 CR were documented, all consolidated with HSCT (3 auto and 1 allo), with achievement of long-term remission in 3/4 patients. All non-responding patients died at a median time of 2.5 months.
In the adult setting, given the paucity of data and the absence of prospective trials, it is even more difficult to establish the outcome of BLL patients progressing after frontline treatment. Recently, a retrospective survey on 641 newly diagnosed adult BLL patients in a real-life setting, conducted among 30 US centers treated with R-chemotherapy, reported 14% primary refractory disease, 7% partial response (PR) or stable disease (SD) (the majority of whom subsequently died of disease progression), 12% relapse, 90% of whom within 12 months from diagnosis. Disease progression was not associated to a defined frontline regimen. The 87 primary refractory patients were treated with different salvage regimens (R-ICE the most used), followed by allo HSCT in 7. Ten were alive at last FU.2 This data is in line with that of a European survey, that reported a relapse rate of 6% among 105 BLL patients treated with different backbones, with no differences between regimens. All relapsed patients died, as well as 21 out of 22 patients with refractory disease.35 The MDACC reported on 35 adult BLL patients who were R/R to frontline hyper-CVAD (R-hyper-CVAD in 24), 28 of whom salvaged with different regimens. The cohort was enriched in patients with late relapses who displayed improved overall response rate (ORR) compared to early R/R patients (61% vs 0%, respectively), with a consequent median OS of 5 months vs 1.4 months in the two groups.50 Dismal prognosis was recently confirmed by a Canadian multicenter survey on 74 BLL adults, mainly refractory, which showed that only 58% of patients underwent salvage therapy with curative intent; the consequent 2-year OS was 17%. The remainder of patients underwent palliative care.52
Table 1 summarizes the results of outcome with current salvage therapies in R/R BLL, in children and adults.
Transplant
As for the investigation of more appropriate salvage chemotherapy, the reported data on consolidation with auto or allo HSCT in R/R BLL mostly come from retrospective studies, usually collecting different NHL subtypes, sometimes with the exclusion of CNS or BM involvement, and usually including both pediatric and adult patients. Collectively, in the R/R setting, HSCT showed very poor outcome in BLL patients, with higher relapse rate and treatment-related mortality (TRM) compared to other histological subtypes. Historical surveys from the European Society for Blood and Marrow Transplantation (EBMT) reported 3-year OS of 37% for adult BLL patients achieving a CR2 after salvage chemotherapy and consolidated with high-dose therapy (HDT) and auto HSCT (OS for patients not in CR at the time of HDT was 7%), similarly to those undergoing allo HSCT (4-year OS 37%), who experienced not insignificant TRM up to 30%.54 The Center for International Bone and Marrow Transplantation Research (CIBMTR) evaluated 241 BLL patients transplanted between 1985 and 2007, including both children and adults. The majority (67%) were not in first CR. For these R/R patients the 5-year non-relapse mortality was 12% with auto HSCT, 30% with allo HSCT; the 5-year PFS and OS were 27% and 31% for auto HSCT, and 19% and 20% for allo HSCT, respectively.48 In the recent retrospective international study, Burkhardt et al reported the HSCT impact in 254 pediatric R/R BLL.51 The OS was 3% in nontransplanted patients, compared with 44% for autotransplanted and 46% for allografted patients, who experienced higher TRM (14% vs 8%). A high rate of failure was recorded both in auto (47%) and allo HSCT (39%).
Other transplant strategies have been evaluated. The tandem myeloablative conditioning (MAC) auto/reduced intensity conditioning (RIC) allo strategy aimed at achieving the maximum response, with a MAC auto HSCT, prior to a RIC allo HSCT, reducing the TRM and maintaining the graft versus lymphoma effect. A Phase II study on adult poor risk NHL patients, with various histologic subtypes, showed 72% PFS for the 29 patients completing the tandem program.55 The same strategy was adopted in a multicenter prospective study, showing 70% 10-year EFS for the 10 R/R NHL patients who received the tandem strategy.49 However, in both studies, about one third of patients did not complete the planned therapy. After these initial results, and a subsequent promising study adding yttrium‐90 ibritumomab tiuxetan to tandem auto/allo HSCT,53 no further experience with tandem auto/allo in R/R BLL has been reported in literature.
Presently, considering the emerging role of new targeted cellular therapies, HSCT will probably no longer be considered as a game changer for patients with aggressive BLL in disease progression. However, transplant continue to be a choice for selected patients. Particularly, in the cellular therapy era, allo HSCT could be reserved for patients who experience disease progression or relapse after CAR T-cell therapy. Allo HSCT may also have a role for patients after response to emerging treatment modalities.
Table 1 summarizes the results of outcome with HSCT in R/R BLL, in children and adults.
Novel Therapies
The data reported above depict an urgent need for novel therapies in R/R setting, where patients display high chemo-resistance, heavy pretreatment and often ineligibility to a highly intensive salvage chemotherapy, or unsuitability for HDT because of BM involvement or stem cells mobilization failure.
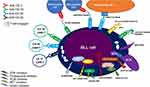
Many novel therapies are under evaluation for aggressive B-cell lymphomas, particularly in the adult population. Novel therapies for BLL comprehend the use of BCR inhibitors, proteasome inhibitors, or next generation monoclonal antibodies associated to chemotherapy in immune-chemotherapy approaches, bispecific antibodies, chimeric antigen receptor T (CAR T) cells, and finally novel compounds with different targets, such as MYC or newer discovered pathogenic pathways (Figure 1). Table 2 summarizes selected published and actively recruiting clinical trials with novel compounds in R/R BLL.
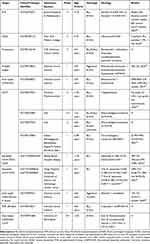 |
Table 2 Selected Actively Recruiting Clinical Trials Employing Novel Agents for Relapsed/Refractory BLL |
New Immuno-Chemotherapy Approaches
Several attempts to improve R/R patients’ outcome by adding novel targeted agents or small molecule compounds to a chemotherapy backbone have been tested.
The BCR signaling inhibitor Ibrutinib shows activity in in vitro BLL models, and is under investigation in non-germinal center type diffuse large B-cell lymphoma (DLBCL).58 Ibrutinib was added to R-ICE, or R-VICI chemotherapy, in a randomized clinical trial, including patients with R/R aggressive B-NHL (21 BLL).56 No significant difference in EFS was found between ibrutinib-R-chemo versus R-chemo, with median EFS 6.1 months and 7 months, respectively; a higher rate of major bleedings was reported in ibrutinib arm (17% vs 7%).
The next generation monoclonal antibody obinutuzumab was reported to significantly enhance cell death against both R-sensitive and R-resistant BLL cell lines.65 This led to the incorporation of obinutuzumab to salvage regimens like ICE chemotherapy. An update of the ongoing clinical trial (NCT02393157) enrolling children, adolescents and young adults with R/R B-NHL, based on obinotuzumab-ICE salvage therapy, showed long term remission for 2 out of 6 BLL patients (1 CR, 1 PR), both receiving HSCT as consolidation.57
Furthermore, the MDACC is employing the proteasome inhibitor bortezomib in association with clofarabine, etoposide, cyclophosphamide, liposomal vincristine and dexamethasone in adults with R/R ALL, lymphoblastic lymphoma, BLL and aggressive B-NHL (NCT03136146). Results are awaited.
Bispecific Antibodies: Blinatumomab
Blinatumomab is a bispecific CD3/CD19 T-cell engager antibody, which is approved for the treatment of R/R and minimal residual disease positive CD19+ B-ALL. The strong BLL cells CD19 positivity led to the investigation of blinatumomab in BLL. Given early in vitro evidence of T-lymphocyte cytokine secretion with subsequent anticancer activity, blinatumomab was, therefore, employed in R/R B-NHL patients with variable results.66 The GMALL group reported the outcome of 3 young adults with R/R BLL treated with blinatumomab single agent with the following schedule: 28 µg/daily for the first 4 days, then 52 days at 112 µg/daily.67 Responders were offered a 4-week schedule as consolidation and maintenance for a total of 6 cycles. One out of the first 3 patients enrolled, obtained a CR, followed by HDT and total body irradiation, auto HSCT, after which the patient further relapsed, and was re-salvaged with blinatumomab, obtaining a third CR. In parallel, the California Cancer Consortium is randomly employing blinatumomab (NCT02568553) with a very similar schedule, together with lenalidomide 20 mg/daily for adult R/R B-NHL, including BLL (1 BLL enrolled as of January 2024).59 The updated results on the first 18 patients enrolled showed 83% ORR with 50% CR rate in patients treated with blina/lena combination, with a median PFS of 8.3 months. The safety profile was acceptable, with no grade 3–4 cytokine release syndrome (CRS). Less optimistic results were recently retrospectively collected, where CR rate was registered in 5 out of 9 R/R BLL adults treated with blinatumomab monotherapy as 28-day cycles for a median number of 3 cycles.68 However, median PFS was 2 months, and 8/9 patients subsequently relapsed and died. Neurotoxicity and CRS were acceptable, while infections occurred in 4/9 patients.
Although the published data are based on smaller case series to gain conclusions, blinatumomab seems to have less activity in BLL compared with CD19-positive ALL. Further investigation is warranted, in consideration of different possible schedules and pharmacokinetics, also considering the upcoming subcutaneous administration. Different bispecific monoclonal antibodies are once again changing the therapeutic landscape of R/R B-NHL, and their investigation in BLL patients is strongly awaited. An effort in this direction might come from an ongoing global clinical trial enrolling pediatric and young adults with a R/R BLL with three arm randomization design, including the anti-CD3/anti-CD20 monoclonal antibody odronextamab, a combination of the conjugated anti-CD19 monoclonal antibody loncastuximab tesirine with chemotherapy or an anti-CD19 CAR-T (Glob-NHL, NCT05991388).
Chimeric Antigen Receptor T (CAR-T) Cells
In the last 5 years, the approved T cell products, genetically modified to express a chimeric antigen receptor targeting CD19 (CD19 CAR-T cells), have revolutionized the salvage approach to R/R CD19-positive ALL, as well as aggressive and indolent B-NHL. However, although higher response rates have been documented with CAR-T products – even in heavily pretreated patients – compared to any other R/R approved treatment, a large proportion of patients still show recurrence or progression. Furthermore, a slower response kinetics has been reported for lymphomas compared with ALL patients. Ongoing research is addressing these questions, spanning from the possibility of tumor immune-escape to a lack of CAR-T cell persistence.61 In any case, R/R BLL was not included in the major clinical studies that led to CD19 CAR-T cells approval. Therefore, the outcome with CAR-T in this subset arises from very recent, small case series but represents an active field of ongoing investigation.
Little is known about CAR-T efficacy in pediatric R/R BLL patients. A Phase 2 multicenter study (BIANCA, NCT03610724), which has recently completed enrollment, is investigating the use of tigenlecleucel (tisa-cel) in children and young adults treated with ≥1 prior line and no active CNS involvement. A preliminary update reported 8 patients (3 BLL) enrolled, all safely infused after bridging chemotherapy with a safety profile comparable to that reported for other B-NHL.60 The ZUMA-4 (NCT02625480) trial is evaluating the use of brexucabtagene autoleucel (brexu-cel, KTE-X19) in children, adolescents and young adults with with refractory BLL, or patients who underwent at least 2 therapy lines or in relapse after allo HSCT.
Based on the experience of CAR-T in ALL and B-NHL, tumor immune-escape is a demonstrated mechanism of progression that has been addressed by targeting multiple tumor antigens, with variable efficacy. Chinese investigators reported early efficacy of a sequential approach consisting of the infusion of CD19-, CD22- and CD20-directed CAR-T cells in 5 children with R/R BLL.69 Three patients achieved CR with the first infusion, whereas the remaining 2 needed CD22- and CD20-directed products. This approach was later applied to 23 children (10 with CNS disease).62 Within 3 months, regardless of the infusions number, 21 patients achieved CR (91%), with an 18-month PFS and OS rate of 78% and 83%. Although very promising, additional questions remain regarding the feasibility of this approach, exposing patients to multiple infusions with subsequent potential toxicity, longer hospitalization, and requiring highly qualified facilities. The costs of this sequential procedure also represent an issue.
In the adult setting, given the demonstrated feasibility that led to the FDA and EMA authorization of different CD19 CAR-T products for ALL, DLBCL and high-grade B-cell lymphoma, their employment in the context of R/R BLL is also now under investigation. A multicenter survey aiming at assessing real-world outcome of patients treated with CD19 CAR-T cells in the USA recently reported a total of 13 heavily pretreated BLL patients treated with axicabtagene ciloleucel (axi-cel, n=8), tisa-cel (n=3) and lisocabtagene maraleucel (liso-cel, n=2).70 The CR rate was 53.8%, with no grade ≥3 CRS, and 2 patients experiencing grade ≥3 immune-effector cells associated neurotoxicity syndrome (ICANS). However, only 4 patients showed durable remission; 2 of them underwent allo HSCT (both eventually progressed). Brexu-cel is under investigation in a multicenter basket study including adult patients with R/R BLL, or Richter syndrome (ZUMA-25, NCT05537766). Furthermore, recently, second generation CAR-T cells targeting both CD19 and CD22 were designed for a sequential infusion approach in 28 adults, subdivided into two cohorts. Responding patients in the second cohort were consolidated with auto HSCT preceded by carmustine+etoposide+cytarabine+melphalan (BEAM)-conditioning chemotherapy.63 CR rates were 33% vs 84.6% in the two cohorts, respectively, with a 1-year PFS and OS both at 55.6% in the whole cohort.
Altogether, this early evidence might suggest that a CAR-T approach to BLL might be limited more than other B-NHL, because of its highly aggressive nature, by the aphaeretic procedures and manufacture time needed for obtaining a CAR-T product. Moreover, targeting more than one antigen seems to improve outcome in this setting. To address these issues, off-the-shelf allogeneic cellular products with immediate availability might be a game changer in R/R BLL; however, results are still far too immature to assess comparisons, so the main questions in this field remain unanswered.
Other Novel Compounds
New compelling genomic evidence in BLL biology provides the rationale for investigation of novel compounds with different targets, all sharing the common goal of disrupting the MYC pathway, by directly addressing MYC protein, or downstream MYC-dependent upregulated proteins, such as TCF3, ID3, cyclin D3 and PI3K specific isoforms. Nonetheless, epigenetic modifiers show potential activity against a Burkitt cells transcriptional program. Although MYC inhibition has obvious potential therapeutic benefits, MYC inhibitors are still yet to be identified. However, there is evidence that the activity of histone deacetylase inhibitors, bromodomain and extraterminal motif (BET) inhibitor is mediated by transcription down-regulation of genes, such as MYC, BCL2 and CDK6, all involved in cell cycle progression, apoptosis and cell proliferation.71 In particular, BET inhibition resulted in MYC down-regulation in MLL-rearranged ALL cell lines treated with JQ1, the second BET inhibitor described.72 These effects appear to be more pronounced in MYC-disregulated neoplasia. BET inhibitors are currently under investigation for B-NHL and other hematologic malignancies in Phase I trials, often in combination with standard anti-lymphoma agents. Romidepsin, a histone deacetylase inhibitor, was combined with alisertib, an inhibitor of Aurora A kinase (protein responsible for chromosome segregation during mitosis), in 25 patients with R/R aggressive NHL (2 BLL) (NCT01897012); however, PFS and OS was very poor; progression was detected in 23 patients with a median time of 5 months.64
Additional mutations, recently defined in BLL, present the rationale for further therapeutic investigation, such as the evidence of TCF3 and ID3 mutations in up to 70% of BLL cases, which lead to constitutive PI3K pathway activation.18 Copanlisib, a PI3K alfa and delta isoforms inhibitor, recently approved for relapsed follicular lymphoma, is under investigation from the US NCI in association with da-EPOCH-R in patients with progressed BLL, or aggressive B-NHL (NCT04933617). Wilke et al recently demonstrated that the in vitro and in vivo inhibition of serine hydroxymethyltransferase 2 (SHMT2), promoting the degradation of TCF3, blocks the tonic BCR signaling responsible for Burkitt cell survival.73 Moreover, CCND3 is mutated in a fraction of patients, and is activated by TCF3 and interacts with CDK6, which can be targeted by novel CDK6 inhibitors.19
Moreover, a Phase 2 clinical trial is enrolling patients with R/R BLL (or patients at diagnosis refusing conventional therapy) and high-grade B-cell lymphoma to a treatment with devimistat (CPI-613), a lipoic acid analogue, which is demonstrated to induce cell death by impairing ATP production via glycolytic metabolism, highly active in Burkitt cells.74
These agents might find their place not only in improving R/R BLL outcome, but their integration with frontline therapies in the setting of older patients, and those ineligible to intensive chemotherapy, offer the potential to reduce chemotherapy intensity.
Conclusions
Prognosis for BLL displaying progression after the firstline treatment remains dismal, even in western countries where treatment facilities and therapeutic innovation are available for all patients. All efforts must be addressed to maximize the results of frontline treatment. In this regard, novel therapies will be extremely important, consenting an increase in the efficacy of firstline therapy, while reducing the chemotherapeutic burden. These new therapeutic approaches are even more necessary for the R/R BLL patients, who are heavily pretreated, frequently not eligible for further aggressive therapy and whose tumor cells rapidly acquire mechanisms of resistance to the available chemotherapeutic agents. Due to the paucity of BLL R/R patients, the evaluation of the results with both standard and innovative therapeutic approaches is very difficult. Cooperative prospective studies, enrolling adequate numbers of patients, including children, adolescents and adults, are necessary to understand which of the newly explored ways should be pursued in the future.
Author Contributions
Maria Luisa Moleti and Francesco Malfona wrote the paper. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Burkitt D, O’Connor GT. Malignant lymphoma in African children. I. A clinical syndrome. Cancer. 1961;14:258–269.
2. Evens AM, Danilov A, Jagadeesh D, et al. Burkitt lymphoma in the modern era: real-world outcomes and prognostication across 30 US cancer centers. Blood. 2021;137(3):374–386. doi:10.1182/blood.2020006926
3. Mead GM, Sydes MR, Walewski J, et al. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt’s lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol. 2002;13(8):1264–1274. doi:10.1093/annonc/mdf253
4. Minard-Colin V, Aupérin A, Pillon M, et al. Rituximab for High-Risk, Mature B-Cell Non-Hodgkin’s Lymphoma in Children. N Engl J Med. 2020;382(23):2207–2219. doi:10.1056/NEJMoa1915315
5. Ribrag V, Koscielny S, Bosq J, et al. Rituximab and dose-dense chemotherapy for adults with Burkitt’s lymphoma: a randomised, controlled, open-label, Phase 3 trial. Lancet. 2016;387(10036):2402–2411. doi:10.1016/S0140-6736(15)01317-3
6. Dunleavy K, Pittaluga S, Shovlin M, et al. Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med. 2013;369(20):1915–1925. doi:10.1056/NEJMoa1308392
7. Roschewski M, Dunleavy K, Abramson JS, et al. Multicenter Study of Risk-Adapted Therapy With Dose-Adjusted EPOCH-R in Adults With Untreated Burkitt Lymphoma. J Clin Oncol. 2020;38(22):2519–2529. doi:10.1200/JCO.20.00303
8. Gopal S, Gross TG. How I treat Burkitt lymphoma in children, adolescents, and young adults in sub-Saharan Africa. Blood. 2018;132(3):254–263. doi:10.1182/blood-2018-04-844472
9. Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: lymphoid Neoplasms. Leukemia. 2022;36(7):1720–1748. doi:10.1038/s41375-022-01620-2
10. Hochberg J, El-Mallawany NK, Abla O. Adolescent and young adult non-Hodgkin lymphoma. Br J Haematol. 2016;173(4):637–650. doi:10.1111/bjh.14074
11. Chene A, Donati D, Orem J, et al. Endemic Burkitt’s lymphoma as a polymicrobial disease: new insights on the interaction between Plasmodium falciparum and Epstein-Barr virus. Semin Cancer Biol. 2009;19(6):411–420. doi:10.1016/j.semcancer.2009.10.002
12. Goldman S, Cairo MS. Diagnosis and management of mature B-cell lymphomas in children, adolescents, and young adults. Best Pract Res Clin Haematol. 2023;36(2):101463. doi:10.1016/j.beha.2023.101463
13. Atallah-Yunes SA, Murphy DJ, Noy A. HIV-associated Burkitt lymphoma. Lancet Haematol. 2020;7(8):e594–e600. doi:10.1016/S2352-3026(20)30126-5
14. Afify Z, Orjuela-Grimm M, Smith CM, et al. Burkitt lymphoma after solid-organ transplant: treatment and outcomes in the paediatric PTLD collaborative. Br J Haematol. 2023;200(3):297–305. doi:10.1111/bjh.18498
15. Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443–459. doi:10.3322/caac.21357
16. Dave SS, Fu K, Wright GW, et al. Molecular diagnosis of Burkitt’s lymphoma. N Engl J Med. 2006;354(23):2431–2442. doi:10.1056/NEJMoa055759
17. Love C, Sun Z, Jima D, et al. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet. 2012;44(12):1321–1325. doi:10.1038/ng.2468
18. Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490(7418):116–120. doi:10.1038/nature11378
19. Panea RI, Love CL, Shingleton JR, et al. The whole-genome landscape of Burkitt lymphoma subtypes. Blood. 2019;134(19):1598–1607. doi:10.1182/blood.2019001880
20. Schmitz R, Ceribelli M, Pittaluga S, et al. Oncogenic mechanisms in Burkitt lymphoma. Cold Spring Harb Perspect Med. 2014;4(2):a014282. doi:10.1101/cshperspect.a014282
21. Grande BM, Gerhard DS, Jiang A, et al. Genome-wide discovery of somatic coding and noncoding mutations in pediatric endemic and sporadic Burkitt lymphoma. Blood. 2019;133(12):1313–1324. doi:10.1182/blood-2018-09-871418
22. Thomas N, Dreval K, Gerhard DS, et al. Genetic subgroups inform on pathobiology in adult and pediatric Burkitt lymphoma. Blood. 2023;141(8):904–916. doi:10.1182/blood.2022016534
23. Patte C, Auperin A, Gerrard M, et al., FAB/LMB96 International Study Committee. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. 2007;109(7):2773–2780. doi:10.1182/blood-2006-07-036673
24. Cairo MS, Gerrard M, Sposto R, et al. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109(7):2736–2743. doi:10.1182/blood-2006-07-036665
25. Cairo MS, Sposto R, Gerrard M, et al. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (≥ 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin’s lymphoma: results of the FAB LMB 96 study. J Clin Oncol. 2012;30(4):387–393. doi:10.1200/JCO.2010.33.3369
26. Reiter A, Schrappe M, Tiemann M, et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: a report of the Berlin-Frankfurt-Münster Group Trial NHL-BFM 90. Blood. 1999;94(10):3294–3306.
27. Woessmann W, Seidemann K, Mann G, et al. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM Group Study NHL-BFM95. Blood. 2005;105(3):948–958. doi:10.1182/blood-2004-03-0973
28. Goldman S, Barth M, Hochberg J, et al. Reduced burden of oncologic therapy in children, adolescents and young adults with good risk (GR) CD20 + mature B‐cell lymphoma. Br J Haematol. 2018;182(Suppl. 1):11.
29. Diviné M, Casassus P, Koscielny S, et al. Burkitt lymphoma in adults: a prospective study of 72 patients treated with an adapted pediatric LMB protocol. Ann Oncol. 2005;16(12):1928–1935. doi:10.1093/annonc/mdi403
30. Hoelzer D, Ludwig WD, Thiel E, et al. Improved outcome in adult B-cell acute lymphoblastic leukemia. Blood. 1996;87(2):495–508.
31. Hoelzer D, Walewski J, Döhner H, et al. Improved outcome of adult Burkitt lymphoma/leukemia with rituximab and chemotherapy: report of a large prospective multicenter trial. Blood. 2014;124(26):3870–3879. doi:10.1182/blood-2014-03-563627
32. Magrath I, Adde M, Shad A, et al. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol. 1996;14(3):925–934. doi:10.1200/JCO.1996.14.3.925
33. Corazzelli G, Frigeri F, Russo F, et al. RD-CODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and ‘unclassifiable’ highly aggressive B-cell lymphoma. Br J Haematol. 2012;156(2):234–244. doi:10.1111/j.1365-2141.2011.08947.x
34. Thomas DA, Faderl S, O’Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106(7):1569–1580. doi:10.1002/cncr.21776
35. Oosten LEM, Chamuleau MED, Thielen FW, et al. Treatment of sporadic Burkitt lymphoma in adults, a retrospective comparison of four treatment regimens. Ann Hematol. 2018;97(2):255–266. doi:10.1007/s00277-017-3167-7
36. Chamuleau MED, Stenner F, Chitu Ir DA, et al. R-CODOX-M/R-IVAC versus DA-EPOCH-R in patients with newly diagnosed Burkitt lymphoma (HOVON/SAKK): final results of a multicentre, phase 3, open-label, randomised trial. Lancet Hematol. 2023. doi:10.1016/S2352-3026(23)00279-X
37. Kim H, Park ES, Lee SH, et al. Clinical outcome of relapsed or refractory burkitt lymphoma and mature B-cell lymphoblastic leukemia in children and adolescents. Cancer Res Treat. 2014;46(4):358–365. doi:10.4143/crt.2013.047
38. Jourdain A, Auperin A, Minard-Colin V, et al. Outcome of and prognostic factors for relapse in children and adolescents with mature B-cell lymphoma and leukemia treated in three consecutive prospective “Lymphomes Malins B” protocols. A Société Française des Cancers de l’Enfant study. Haematologica. 2015;100(6):810–817. doi:10.3324/haematol.2014.121434
39. Cairo M, Auperin A, Perkins SL, et al. Overall survival of children and adolescents with mature B cell non-Hodgkin lymphoma who had refractory or relapsed disease during or after treatment with FAB/LMB 96: a report from the FAB/LMB 96 study group. Br J Haematol. 2018;182(6):859–869. doi:10.1111/bjh.15491
40. Rigaud C, Auperin A, Jourdain A, et al. Outcome of relapse in children and adolescents with B-cell non-Hodgkin lymphoma and mature acute leukemia: a report from the French LMB study. Pediatr Blood Cancer. 2019;66(9):e27873. doi:10.1002/pbc.27873
41. Woessmann W, Zimmermann M, Meinhardt A, et al. Progressive or relapsed Burkitt lymphoma or leukemia in children and adolescents after BFM-type first-line therapy. Blood. 2020;135(14):1124–1132. doi:10.1182/blood.2019003591
42. Reutter K, Sandmann S, Rohde J, et al. Reconstructing clonal evolution in relapsed and non-relapsed Burkitt lymphoma. Leukemia. 2021;35(2):639–643. doi:10.1038/s41375-020-0862-5
43. Moleti ML, Testi AM, Foà R. Treatment of relapsed/refractory paediatric aggressive B-cell non-Hodgkin lymphoma. Br J Haematol. 2020;189(5):826–843. doi:10.1111/bjh.16461
44. Griffin TC, Weitzman S, Weinstein H, et al. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20+) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2009;52(2):177–181. doi:10.1002/pbc.21753
45. Osumi T, Mori T, Fujita N, et al. Relapsed/refractory pediatric B-cell non-Hodgkin lymphoma treated with rituximab combination therapy: a report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Pediatr Blood Cancer. 2016;63(10):1794–1799. doi:10.1002/pbc.26105
46. Atra A, Gerrard M, Hobson R, et al. Outcome of relapsed or refractory childhood B-cell acute lymphoblastic leukaemia and B-cell non-Hodgkin’s lymphoma treated with the UKCCSG 9003/9002 protocols. Br J Haematol. 2001;112(4):965–968. doi:10.1046/j.1365-2141.2001.02647.x
47. Anoop P, Sankpal S, Stiller C, et al. Outcome of childhood relapsed or refractory mature B-cell non-Hodgkin lymphoma and acute lymphoblastic leukemia. Leuk Lymphoma. 2012;53(10):1882–1888. doi:10.3109/10428194.2012.677534
48. Maramattom LV, Hari PN, Burns LJ, et al. Autologous and allogeneic transplantation for burkitt lymphoma outcomes and changes in utilization: a report from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2013;19(2):173–179. doi:10.1016/j.bbmt.2012.11.016
49. Satwani P, Jin Z, Martin PL, et al. Sequential myeloablative autologous stem cell transplantation and reduced intensity allogeneic hematopoietic cell transplantation is safe and feasible in children, adolescents and young adults with poor-risk refractory or recurrent Hodgkin and non-Hodgkin lymphoma. Leukemia. 2015;29(2):448–455. doi:10.1038/leu.2014.194
50. Short NJ, Kantarjian HM, Ko H, et al. Outcomes of adults with relapsed or refractory Burkitt and high-grade B-cell leukemia/lymphoma. Am J Hematol. 2017;92(6):E114–E117. doi:10.1002/ajh.24720
51. Burkhardt B, Taj M, Garnier N, et al. Treatment and Outcome Analysis of 639 Relapsed Non-Hodgkin Lymphomas in Children and Adolescents and Resulting Treatment Recommendations. Cancers. 2021;13(9):2075. doi:10.3390/cancers13092075
52. Manji F. Outcomes in Relapsed/Refractory Burkitt Lymphoma: a Multi-Centre Canadian Experience. Blood. 2021;138(Supplement 1):2525. doi:10.1182/blood-2021-146036
53. Gardenswartz A, Mehta B, El‐Mallawany N, et al. Safety and efficacy of myeloablative conditioning autologous stem cell transplantation, targeted immunotherapy, and reduced intensity conditioning allogeneic stem cell transplantation in children, adolescents, and young adults with relapsed/refractory mature B‐cell Non Hodgkin lymphoma. Br J Haematol. 2024;182(Suppl. 1):23.
54. Sweetenham JW, Pearce R, Taghipour G, et al. Adult Burkitt’s and Burkitt-like non-Hodgkin’s lymphoma--outcome for patients treated with high-dose therapy and autologous stem-cell transplantation in first remission or at relapse: results from the European Group for Blood and Marrow Transplantation. J Clin Oncol. 1996;14(9):2465–2472. doi:10.1200/JCO.1996.14.9.2465
55. Chen YB, Lane AA, Logan B, et al. Impact of conditioning regimen on outcomes for patients with lymphoma undergoing high-dose therapy with autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21(6):1046–1053. doi:10.1016/j.bbmt.2015.02.005
56. Burke GAA, Vinti L, Kabickova E, et al. Ibrutinib plus RICE or RVICI for relapsed/refractory mature B-cell non-Hodgkin lymphoma in children and young adults: SPARKLE trial. Blood Adv. 2023;7(4):602–610. doi:10.1182/bloodadvances.2022008802
57. Barth MJ, Hochberg J, Harrison L, et al. Phase 2 Trial of Obinutuzumab, a Humanized, Glycoengineered Monoclonal CD20 Antibody, in Combination with Ifosfamide, Carboplatin and Etoposide for Relapsed/Refractory Mature B-Cell Non-Hodgkin Lymphoma. Br J Haematol. 2022;182(Suppl. 1):48–49.
58. Melani C, Lakhotia R, Pittaluga S, et al. Phase 1 Study of Venetoclax, Ibrutinib, Prednisone, Obinutuzumab, and Lenalidomide in Combination with Polatuzumab (ViPOR-P) in Relapsed/Refractory B-Cell Lymphoma: preliminary Analysis of Safety and Efficacy. Blood. 2022;140(Supplement 1):6602–6603.
59. Poh C, Frankel P, Ruel C, et al. Blinatumomab/Lenalidomide in Relapsed/Refractory Non-Hodgkin’s Lymphoma: a Phase I California Cancer Consortium Study of Safety, Efficacy and Immune Correlative Analysis. Blood. 2019;134(Supplement_1):760.
60. Minard V, Maude SL, Buechner J, et al. Bianca: Phase II, single-arm, global trial to determine efficacy and safety of tisagenlecleucel in pediatric/young adult (YA) patients (Pts) with relapsed/refractory B-cell non-Hodgkin lymphoma (R/R B-NHL). J clin oncol. 2020;38(15_suppl). doi:10.1200/JCO.2020.38.15_suppl.e22504
61. Martínez-Cibrián N, Ortiz-Maldonado V, Español-Rego M, et al. The academic point-of-care anti-CD19 chimeric antigen receptor T-cell product varnimcabtagene autoleucel (ARI-0001 cells) shows efficacy and safety in the treatment of relapsed/refractory B-cell non-Hodgkin lymphoma. Br J Haematol. 2023. doi:10.1111/bjh.19170
62. Liu Y, Deng B, Hu B, et al. Sequential different B-cell antigen-targeted CAR T-cell therapy for pediatric refractory/relapsed Burkitt lymphoma. Blood Adv. 2022;6(3):717–730. doi:10.1182/bloodadvances.2021004557
63. Wu J, Cao Y, Zhang Q, et al. Chimeric Antigen Receptor-Modified T Cell Immunotherapy for Relapsed and Refractory Adult Burkitt Lymphoma. Front Immunol. 2022;13:879983. doi:10.3389/fimmu.2022.879983
64. Strati P, Nastoupil LJ, Davis RE, et al. A Phase 1 trial of alisertib and romidepsin for relapsed/refractory aggressive B-cell and T-cell lymphomas. Haematologica. 2020;105(1):e26–e28. doi:10.3324/haematol.2019.220012
65. Awasthi A, Ayello J, Van de Ven C, et al. Obinutuzumab (GA101) compared to rituximab significantly enhances cell death and antibody-dependent cytotoxicity and improves overall survival against CD20(+) rituximab-sensitive/-resistant Burkitt lymphoma (BL) and precursor B-acute lymphoblastic leukaemia (pre-B-ALL): potential targeted therapy in patients with poor risk CD20(+) BL and pre-B-ALL. Br J Haematol. 2015;171(5):763–775. doi:10.1111/bjh.13764
66. Tiwari AA, Edani T, Ayello J, et al. Blinatumomab enhanced anti-tumor activity against rituximab sensitive and resistant Burkitt Lymphoma (BL) and Primary Mediastinal B-cell Lymphoma (PMBL). Cancer Res. 2019;79(13_Supplement):2381.
67. Duell J, Zugmaier G, Eisele F, et al. Treatment of R/R Burkitt Lymphoma with blinatumomab is feasible and induced a long lasting complete remission. HemaSphere. 2019;3(S1):816–817.
68. Bohler J, Bacher U, Banz Y, et al. Blinatumomab in Relapsed/Refractory Burkitt Lymphoma. Cancers. 2022;15(1):44. doi:10.3390/cancers15010044
69. Zhang W, Yang J, Zhou C, et al. Early response observed in pediatric patients with relapsed/refractory Burkitt lymphoma treated with chimeric antigen receptor T cells. Blood. 2020;135(26):2425–2427. doi:10.1182/blood.2019002008
70. Samples LS, Sadrzadeh H, Frigault MJ, et al. Outcomes among adult recipients of CD19 CAR T-cell therapy for Burkitt lymphoma. J clin oncol. 2023;41(16 suppl):7571.
71. Doroshow DB, Eder JP, LoRusso PM. BET inhibitors: a novel epigenetic approach. Ann Oncol. 2017;28(8):1776–1787. doi:10.1093/annonc/mdx157
72. Zuber J, Shi J, Wang E, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478(7370):524–528. doi:10.1038/nature10334
73. Wilke AC, Doebele C, Zindel A, et al. SHMT2 inhibition disrupts the TCF3 transcriptional survival program in Burkitt lymphoma. Blood. 2022;139(4):538–553. doi:10.1182/blood.2021012081
74. Noy A, Pardee TS, Nikolaenko L, et al. A Phase II Clinical Trial of Cpi-613 (devimistat) in Patients with Relapsed or Refractory Burkitt Lymphoma/Leukemia or High-Grade B-Cell Lymphoma with Rearrangements of MYC and BCL2 and/or BCL6. Blood. 2019;134(Supplement 1):4087. doi:10.1182/blood-2019-131563
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.