Back to Journals » Open Access Surgery » Volume 16
Rare Classic Presentation of Primary Hyperparathyroidism: A Case Report and Literature Review
Authors Shale WT , Jirata TD , Enawgaw MC, Metekia AG, Kelecha YL
Received 4 September 2023
Accepted for publication 15 December 2023
Published 21 December 2023 Volume 2023:16 Pages 121—130
DOI https://doi.org/10.2147/OAS.S433529
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Luigi Bonavina
Wongel Tena Shale,1 Tolasa Dibisa Jirata,2 Mekbeb Chere Enawgaw,1,3 Ararso Gonfa Metekia,2 Yosef Lemma Kelecha1
1Jimma University College of Public Health and Medical Sciences, Department of Surgery, Jimma University, Jimma, Oromia, Ethiopia; 2Jimma University College of Public Health and Medical Sciences, Department of Orthopedic Surgery, Jimma University, Jimma, Oromia, Ethiopia; 3Jimma University College of Public Health and Medical Sciences, Department of Surgery, Urology Unit, Jimma University, Jimma, Oromia, Ethiopia
Correspondence: Wongel Tena Shale, Jimma, Oromia, Ethiopia, Tel +251911636127 ; +251913862475, Email [email protected]
Background: The endocrine disorder known as hyperparathyroidism (HPT) is characterized by excessive parathyroid hormone (PTH) secretion, and when it is accompanied with hypercalcemia it is called primary HPT (PHPT). PHPT is characterized by the hyper-functioning of one or more parathyroids. The most common form of PHPT is sporadic parathyroid adenoma.
Case Presentation: A 33-year-old male presented to our emergency room with the classic presentation of PHPT affecting the skeletal, gastrointestinal, genitourinary, and neurologic systems including, multiple long bone fractures and bilateral nephrolithiasis with preserved renal function. Diagnosis was made by elevated serum PTH and calcium levels, as well as, bone imaging. Neck ultrasound and fine needle aspiration cytology (FNAC) of the neck mass were done to localize the lesion. After pre-operative optimization, bilateral neck exploration and en-bloc resection of the left inferior parathyroid with the left lobe of the thyroid was done. In the post-operative period, he developed persistent hypotension and severe hypocalcemia.
Conclusion: Although it is uncommon in the industrialized countries, the traditional PHPT presentation with “Bones, Stones, abdominal groans, and psychic moans” is nevertheless common in impoverished nations like Ethiopia. Surgery is the mainstay of treatment but complications such as hungry bone syndrome can increase the post-operative morbidity following parathyroidectomy.
Keywords: primary hyperparathyroidism, PHPT, bones, stones, abdominal groans, psychic overtones, brown tumor, hungry bone syndrome, case report
Introduction
The endocrine disorder known as hyperparathyroidism (HPT) is characterized by excessive parathyroid hormone (PTH) secretion. It might be primary, secondary, or tertiary depending on the cause. In the current WHO classification, the term “parathyroid hyperplasia” is now used primarily in the setting of secondary hyperplasia which is most often caused by chronic renal failure.1 Increased PTH secretion and hypercalcemia are the results of primary HPT (PHPT), which is characterized by the hyper-functioning of one or more parathyroids.2 It affects 0.3% of the general population and has a population incidence of 21.6 cases per 100,000 person-years, making it the third most prevalent endocrine condition. The most common form of PHPT is sporadic parathyroid adenoma, but it can also be brought on by diffuse parathyroid gland hyperplasia or, in extremely rare cases, parathyroid carcinoma. But it can also be linked to uncommon genetic syndromes and metabolic disorders.3,4 There is not much research demonstrating PHPT’s prevalence in Ethiopia. However, studies show that black people are more likely to be affected. The frequency of PHPT was highest among blacks in a cohort of 15,234 patients with chronic hypercalcemia, 13,327 (87%) of whom had PHPT as indicated by increased or inappropriately normal parathyroid hormone levels.5
Continuous exposure to high PTH levels in PHPT results in increased bone remodeling, with a predominance of bone resorption. Patients with PHPT may exhibit fragility fractures and/or the hallmark radiological characteristics of osteitis fibrosa cystica when the condition is symptomatic.6 Patients with recurrent kidney stones should be screened for PHPT, which is evident in approximately 3% to 5% of patients with kidney stones.7,8 There is a paucity of research on the identification and treatment of psychiatric aftereffects in patients with PHPT; yet, data indicate that 25% of PHPT patients have psychiatric symptoms.9 In general, nonspecific symptoms, such as fatigue, poor appetite, weight loss, nausea, emesis, and abdominal pain, are experienced by patients with hyperparathyroidism.10 Nevertheless, Stones, bones, groans, psychic moans, and fatigue overtones—the classic PHPT manifestations—rarely occur in the developed world nowadays. Meanwhile in developing countries, the disease still continues to manifest as a florid skeletal and renal disease.11 Here, we describe a case report of a 33-year-old male patient who presented with the rare classic presentation of PHPT secondary to parathyroid adenoma. The manuscript was reported in accordance with SCARE criteria.12
Case Presentation
A 33-year-old male presented to our emergency room with bilateral pathologic femur and humeral fractures, bilateral nephrolithiasis with bilateral hydroureteronephrosis due to distal ureteric obstruction. The patient had bone pain in the upper and lower limbs that lasted for 5 months, as well as extremity swelling. He has been unable to support weight for three months. He also experienced moderate, aching flank pain on both sides. He alleges that he experienced dyspeptic symptoms and was constipated. He had just experienced a change in his mood and had been depressed. Two years prior, he had an anterior neck swelling that grew progressively. He also got easily fatigued. There was no family history of a disease similar to his.
Past Medical and Surgical illness: He had no significant past medical or surgical illness.
Physical examination: His vital signs on presentation were Blood pressure: 110/70mmHg, Pulse rate: 92 beats/min with regular rhythm, respiratory rate: 20 breaths/min and Temperature: 36.6°C. He had 3*4 cm measuring left lower anterior neck mass with a firm to hard consistency and it was mobile but not with deglutition. He had bilateral edematous and deformed proximal thigh with palpable crepitus from the fractures and tenderness on palpation. He also had swelling and tenderness on the right distal arm and the left proximal arm. He also had restriction of movement; otherwise the neurovascular assessment was intact. He was investigated with laboratory tests (Table 1).
 |
Table 1 Laboratory Test Results of the Patient with PHPT Before Surgery |
Diagnostic Imaging
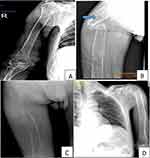
Bilateral femur shaft fracture and incomplete bilateral humerus bone fracture with osteoporosis was seen on plain x-rays of the extremities. Brown tumor was also appreciated (Figure 1).
Abdominopelvic ultrasound showed bilateral diffuse renal parenchymal disease with bilateral multiple nephrolithiasis (largest is ~0.5cm) and mild hydroureteronephrosis secondary to distal ureteric obstruction.
Neck U/S revealed well defined hypoechoic vascular mass originating from inferior pole of the left lobe of thyroid gland. The index was parathyroid adenoma.
Fine needle aspiration cytology: cellular yield composed of tight cluster sheets and papillary fragment having pleomorphism. Round to oval cells having scant cytoplasm, fine chromatin, multiple prominent nucleoli, binucleation and multinucleation in hemorrhagic background were seen. The impression was anterior neck/Parathyroid Carcinoma.
Therapeutic Intervention
After applying traction and splints to the fracture sites and receiving one unit of whole-blood transfusion, the patient was brought to the operating room where a left en-bloc resection of the parathyroid tumor with the left lobe of the thyroid was performed. At the left inferior parathyroid, there was a 3x2x2cm firm gray-white mass that was adherent to the left-thyroid lobe and trachea (Figure 2).
The mass was sent for biopsy, and the results revealed a confined mass with banded follicular cells and thick colloid around a mass of oncocytic cells with abundant, eosinophilic granular cytoplasm and indications of endocrine atypia. The final diagnosis was parathyroid adenoma (Figure 3).
The patient was stable during surgery. His vital signs, ECG monitoring, and fluid balance were all within the normal range. He was smoothly extubated after being roused from anesthesia. After that, he was moved to the Post anesthesia care unit (PACU). Despite a normal pulse rate (72–88 beats per minute), which was full in volume, his blood pressure began to decline eight hours after the operation. We administered two crystalloid fluid boluses, but his blood pressure did not normalize. Temperature, oxygen saturation, and respiratory rate were all within normal limits. His neurologic evaluation was intact. After being admitted to the surgical ICU, the patient was given a low dose of adrenaline infusion (0.25 microgram/kg/min), and his blood pressure rose to the normal range. We opted to start him on steroids after trying unsuccessfully to wean him off of pressure. On the third postoperative day, he was transported out of the surgical ICU with normal vital signs when the steroid and adrenaline were eventually stopped. The serum total calcium level was 2.41 mmol/L (normal range: 2.15–2.5 mmol/L). The serum parathyroid hormone level could not be determined due to lack of laboratory reagent.
Follow-Up and Outcomes
After being transferred to the orthopedics ward on the fourteenth postoperative day (POD), the patient began to experience low-grade intermittent fever, carpopedal spasms, shortness of breath, and altered behavior. According to blood tests, the serum calcium level was 1.48 mmol/L and the serum magnesium level was 0.33 mmol/L (normal range 0.66–1.07 mmol/L). With the diagnosis of hypocalcemic tetany, he was started on intravenous calcium gluconate, and the symptoms began to improve. We entertained the possibility of hungry bone syndrome as an underlying cause of the hypocalcemia but we were unable to confirm it because serum PTH-level test was not available, therefore not performed. However, the patient rejected inpatient care and left against medical advice on the 24th POD for financial reasons. The next step was to manage the renal calculi endoscopically and correct the fractures once the patient was stabilized. About a month after the patient left the hospital, despite our best efforts to contact them by phone, we learned that the patient was deceased.
Discussion
PHPT is an endocrine disorder characterized by autonomous production of PTH. Classically characterized as hypercalcemia in the presence of elevated serum PTH concentration, it is now recognized as a spectrum ranging from inappropriately high or even normal PTH in the setting of high-normal or even normal calcium.13 With a prevalence of between 0.1 and 1.0%, it is the third most prevalent endocrine condition after diabetes mellitus and thyroid disease. Between the ages of 50 and 60, the incidence is the highest and it is predominantly seen in women.14–16 Up to 95% of cases are sporadic, with Multiple endocrine neoplasia 1 (MEN1) and MEN 2A syndromes among the remaining 5–10% familial cases. Nearly 80% of instances of PHPT are caused by an underlying solitary parathyroid adenoma. The second most frequent cause is diffuse glandular hyperplasia. Less than 1% of PHPT cases are due to parathyroid carcinoma, a relatively uncommon cause of this disorder.14,15 To our knowledge, there are 6 cases of PHPT reported from Ethiopia in the literature (Table 2).
 |
Table 2 Reported Cases of Primary Hyperparathyroidism (PHPT) in Ethiopia |
Regarding the prevalence, signs, and complications of PHPT, there are remarkable regional variations. PHPT is still an uncommonly detected, overtly symptomatic condition in underdeveloped nations, characterized by “bones, stones, abdominal groans, and psychic moans.” This may be due to the fact that screening for hypercalcemia in healthy populations is not a common practice in developing nations like Ethiopia and that access to medical care is generally poor.4 Contrary to the clinical picture seen in poor countries, the majority of the current series of PHPT patients from advanced nations are elderly females with mild to moderate hypercalcemia and very few with classic symptoms and signs.4,20 These days, routine biochemical laboratory tests that are performed for various reasons are more frequently used to diagnose individuals. Although patients frequently lack the typical symptoms, PHPT is linked to a wide range of non-specific complaints, including depression, memory loss, fatigue, insomnia, musculoskeletal issues like bone or muscle pains, and gastrointestinal manifestations like gastroesophageal reflux disease and dyspepsia. When these symptoms first appear, they might not be attributed to PHPT, but after surgery, there is frequently a noticeable improvement that betters the quality of life.21,22 Our patient demonstrated a full spectrum of the disease and the typical symptoms of primary hyperparathyroidism, which is quite rare.
In the developed nations, the first sign of primary hyperparathyroidism is often the discovery of hypercalcemia during routine biochemical testing or during the evaluation of postmenopausal women with osteoporosis.23 It is important to measure the total serum calcium level and correct it for albumin levels. Ionized calcium measurement may be helpful in some circumstances, such as with individuals who have hyperalbuminemia.24 The assessment of hypercalcemia continues with the measurement of serum PTH. The presence of both an elevated calcium level and an elevated PTH level, or a level that falls within an unexpected “normal” range, typically denotes the presence of primary hyperparathyroidism.23 Once primary hyperparathyroidism has been diagnosed, familial variants, which make up for 5% of cases, should be taken into consideration and ruled out. It is important to carefully screen patients for the presence of familial PHPT features.25 Assessment of renal function, the amount of serum 25-hydroxyvitamin D, the 24-hour urine calcium, and the calcium:creatinine clearance ratio should all be included in laboratory tests. Genetic testing may be necessary in some circumstances (such as in young individuals with a family history of a syndrome-associated malignancy) to identify the underlying cause of primary hyperparathyroidism and assess the risk of developing additional cancers.26,27
In order to rule out kidney stones or nephrocalcinosis, renal ultrasonography is advised.28 At the lumbar spine, hip, and distal portion of the forearm, bone mineral density should be assessed. Cortical bone is catabolized by PTH, and regions rich in cortical bone are preferentially affected. The distal part of the forearm is most significantly affected. Once patients have severe metabolic bone problems such ostitis cystica fibrosa or a pathologic fracture, plain x-rays can be beneficial.29 Neck imaging is mostly utilized to locate affected parathyroid glands during surgical planning, and it is rarely used for diagnosis. The most popular methods are ultrasonography and sestamibi scanning. Sestamibi scanning’s key benefit is its potential to locate ectopic parathyroid glands.23 A focus of tissue that appears on imaging and is thought to be parathyroid tissue may be sampled by FNA, and the aspirate’s PTH level can then be determined. Most of the time, FNA of suspect parathyroid adenomas is not required for a conclusive diagnosis, although it can be very helpful after surgery or when the suspected location is not typical for parathyroid disease.21
The presence of overt bone disease, such as osteitis fibrosa cystica, or cortical bone mineral density of more than 2 SD below the adjusted mean for age, and sex, as well as reduced renal function and stone disease (nephrolithiasis or nephrocalcinosis), are among the indicators for surgery in PHPT, according to a set of guidelines by the Health Consensus Development Conference in 1991. A relative indication can also be a younger age (under 50).30 About 50% of PHPT patients will qualify for surgery using these criteria. More than 90% of the time when performed by a skilled parathyroid surgeon, parathyroidectomy is curative and safe.
Invasive or non-invasive preoperative localization studies are both possible. For patients who have never undergone parathyroid surgery before, non-invasive procedures like scintigraphy, computed tomography, magnetic resonance imaging, and ultrasonography are typically not recommended. Technetium sestamibi scintigraphy with single-photon emission computed tomography imaging appears to be the most promising new technology among non-invasive localization studies. Non-invasive procedures frequently fail to accomplish localization, necessitating invasive investigations such as parathyroid arteriography or selective venous sampling for PTH.31
Since FNAC is technically straightforward and affordable, it is usually the primary course of investigation when working up neck nodules. However, due to their similar cytomorphological characteristics, thyroid and parathyroid might be challenging to differentiate on FNAC.32,33 Furthermore, it is almost impossible to differentiate between parathyroid hyperplasia, adenoma, and cancer on FNAC, and it is not required.34,35 Although FNAC was performed on our patient, the authors do not recommend it for patients with parathyroid lesions because the procedure is not an evidence-based practice. In our case, a planned parathyroidectomy was carried out after the lesion was localized using neck ultrasonography, the other investigations are not available in our set up.
The indications for surgery according to the National institutes of health guidelines for parathyroidectomy include, Serum Ca level > 3 mmol/L, unexpected decline of creatinine clearance by 30%, marked calciuria with ca level > 10 mmol/L in 24 hour urine collection, cortical bone mineral density > 2 standard deviations below the mean for age matched control subjects, patient requests surgery, patient unable to be followed up for monitoring, and age less than 50 years.36,37 The only effective treatment for PHPT is surgery, and bilateral neck exploration and minimally invasive parathyroidectomy (MIP) are the two primary surgical techniques. Our patient was managed with the former. Patients who are unsuited for surgery or refuse it, pregnant women in the first or third trimester, patients who are waiting for surgery and patients who have had unsuccessful surgical therapy are all cases when medical management of PHPT is recommended.
Regardless of the type of surgery, there are several complications that can happen after a parathyroidectomy. These complications include persistent or recurring PHPT, hypoparathyroidism/hypocalcemia, hematoma formation resulting in airway obstruction, and recurrent laryngeal nerve injury. The severity of hypocalcaemia can be explained by the so-called hungry bone syndrome, prior parathyroid or thyroid surgery, and residual gland atrophy in the face of a single hyperfunctioning adenoma (particularly if there has been long-term and substantial hypercalcemia).38 Evidence suggests that a reliable predictor of postoperative hypocalcaemia following parathyroidectomy for primary hyperparathyroidism can be the reduction of intraoperative parathyroid hormone by >85% after parathyroidectomy.39 We could not determine the intra-operative serum PTH level in our patient, because the test was not readily available. There are no much data on post-operative hypotension in patients who undergo parathyroidectomy for PHPT; however, a prospective cohort study conducted in hemodialysis patients revealed that parathyroidectomy lowers blood pressure regardless of the patients’ preintervention values. Variations in blood pressure are clinically significant and may be linked to calcium efflux from the walls of arteries following parathyroidectomy.40
Prolonged (longer than 4th day post-operatively) profound hypocalcemia (serum calcium <2.1 mmol/l) that occurs after parathyroidectomy for severe hyperparathyroidism is known as “hungry bone syndrome” (HBS). Osteitis fibrosa cystica, “brown tumors”, and/or significant preoperative markers of bone turnover are typically linked to skeletal symptoms. Although there is not enough evidence to support this theory, it is thought that the severe hypocalcaemia is caused by the significantly increased calcium used by the skeleton. This is thought to happen when the effects of high circulating PTH levels on the bones are removed, leading to an immediate arrest of bone resorption in the face of ongoing and enhanced bone formation.41 Patients present with symptoms of severe hypocalcemia, associated with neuromuscular irritability clinically manifested by carpopedal spasms, perioral paresthesia, tingling extremities, Chvostek sign and Trousseau sign. They can also develop generalized seizures, and if it remains untreated, coma and death.41,42 In patients with HBS, serum calcium levels fall to less than 2.1 mmol/l during the first 3–5 days following surgery, and they continue to decline after the fourth post-operative day. They might also develop hypophosphatemia and hypomagnesemia. Additionally, serum alkaline phosphatase levels sharply rise. On radiological images, their bone mineral density will increase quickly.42,43 In the short term, the main goal of treating HBS is to restore the skeletal calcium reserves that have been depleted while also using appropriate dosages of vitamin D’s active metabolites. Additionally, magnesium has to be replenished as needed.41,44
Our patient had two post-operative complications: hypotension severe enough to require vasopressors and post-operative severe hypocalcemia manifesting as tetany. Hypoparathyroidism or hungry bone syndrome, two possible complications of parathyroidectomy for PHPT, can account for the hypocalcemia. We were unable to distinguish between the two potential underlying problems because we were unable to measure the post-operative serum PTH level. He was given low-dose adrenaline infusion, intravenous calcium gluconate, vitamin D, and fluid resuscitation. Eventually, the blood pressure returned to normal, but sadly, the patient left the facility without completing his treatment for the severe hypocalcemia, which led to his demise at home.
Conclusion
Although it is uncommon in industrialized countries, the traditional PHPT presentation with “Bones, Stones, Abdominal groans, and psychic moans” is nevertheless common in impoverished nations like Ethiopia. Surgery is the mainstay of care, particularly for individuals with renal impairment and metabolic bone disease. Most patients see long-term improvements in their symptoms and quality of life after parathyroidectomies. However, post-operative complications including severe hypocalcemia might raise the morbidity of parathyroid surgery. Due to the increased risk of hungry bone syndrome, this is particularly true for patients who have long-term, severe hypercalcemia and generalized metabolic bone disease.
Ethical Approval and Informed Consent
Ethical approval is exempt by Jimma University institutional review board as long as written informed consent is obtained from the patient or the patient’s next of kin in the event that the patient has passed away.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Erickson LA, Mete O, Juhlin CC, Perren A, Gill AJ. Overview of the 2022 WHO classification of parathyroid tumors. Endocr Pathol. 2022;33(1):64–89. doi:10.1007/s12022-022-09709-1
2. Ahmad R, Hammond JM. Primary, secondary, and tertiary hyperparathyroidism. Otolaryngol Clin North Am. 2004;37(4):701–713. doi:10.1016/j.otc.2004.02.004
3. Heath H III, Hodgson SF, Kennedy MA. Primary hyperparathyroidism: incidence, morbidity, and potential economic impact in a community. N Engl J Med. 1980;302(4):189–193. doi:10.1056/NEJM198001243020402
4. Pradeep PV, Jayashree B, Mishra A, Mishra SK. Systematic review of primary hyperparathyroidism in India: the past, present, and the future trends. Int J Endocrinol. 2011;2011. doi:10.1155/2011/921814
5. Yeh MW, Ituarte PH, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab. 2013;98(3):1122–1129. doi:10.1210/jc.2012-4022
6. Silva BC, Bilezikian JP. Skeletal abnormalities in hypoparathyroidism and in primary hyperparathyroidism. Rev Endocr Metab Disord. 2021;22(4):789–802. doi:10.1007/s11154-020-09614-0
7. Sharma S, Rastogi A, Bhadada SK, et al. Prevalence and predictors of primary hyperparathyroidism among patients with urolithiasis. Endocr Pract. 2017;23(11):1311–1315. doi:10.4158/EP171759.OR
8. Ganesan C, Weia B, Thomas IC, et al. Analysis of primary hyperparathyroidism screening among US veterans with kidney stones. JAMA Surg. 2020;155(9):861–868. doi:10.1001/jamasurg.2020.2423
9. Joborn C, Hetta J, Johansson H, et al. Psychiatric morbidity in primary hyperparathyroidism. World J Surg. 1988;12:476–480. doi:10.1007/BF01655425
10. Bauman BD, Evasovich M, Louiselle A, et al. An occult ectopic parathyroid adenoma in a pediatric patient: a case report and management algorithm. J Pediatr Endocrinol Metab. 2017;30(9):995–999. doi:10.1515/jpem-2017-0077
11. Bhadada SK, Arya AK, Mukhopadhyay S, et al. Primary hyperparathyroidism: insights from the Indian PHPT registry. J Bone Miner Metab. 2018;36:238–245. doi:10.1007/s00774-017-0833-8
12. Agha RA, Franchi T, Sohrabi C, Mathew G. Ligne directrice SCARE 2020: mise à jour des lignes directrices du rapport sur les cas chirurgicaux de consensus (SCARE). Int J Surg. 2020;84:226–230. doi:10.1016/j.ijsu.2020.10.034
13. Carneiro-Pla DM, Irvin GL III, Chen H. Consequences of parathyroidectomy in patients with “mild” sporadic primary hyperparathyroidism. Surgery. 2007;142(6):795–799. doi:10.1016/j.surg.2007.07.023
14. Gopinath P, Mihai R. Hyperparathyroidism. Surgery (Oxford). 2011;29(9):451–458. doi:10.1016/j.mpsur.2011.06.015
15. Cordellat IM. Hyperparathyroidism: primary or secondary disease?. Reumatología Clínica (English Edition). 2012;8(5):287–291. doi:10.1016/j.reuma.2011.06.001
16. Conrad DN, Olson JE, Hartwig HM, Mack E, Chen H. A prospective evaluation of novel methods to intraoperatively distinguish parathyroid tissue utilizing a parathyroid hormone assay. J Surg Res. 2006;133(1):38–41. doi:10.1016/j.jss.2006.02.029
17. Kebede T, Hagos E. Primary hyperparathyroidism presenting with musculoskeletal manifestations in a young patient: a case report. Ethiopian Med J. 2004;42(4):299–301.
18. Tinsae Alemayehu MD, Workeabeba Abebe MD. Case report parathyroid adenoma in an Ethiopian adolescent living with human immunodeficiency virus: a rare association. Ethiop Med J. 2019;57(2):283–285.
19. Wondimu S, Nega B. The Surgical Management of Primary Hyperparathyroidism: the Experience in Tikur Anbessa Specialized Tertiary Referral and Teaching Hospital, Addis Ababa, Ethiopia. East Cent Afr J Surg. 2016;21(3):56–62. doi:10.4314/ecajs.v21i3.10
20. Lundgren E, Rastad J. Diagnosis, natural history and intervention in sporadic primary hyperparathyroidism. Surg Endocrinol. 2001:137–162.
21. Madkhali T, Alhefdhi A, Chen H, Elfenbein D. Primary hyperparathyroidism. Turk J Surg. 2016;32(1):58.
22. Silverberg SJ, Lewiecki EM, Mosekilde L, Peacock M, Rubin MR. Presentation of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J Clin Endocrinol Metab. 2009;94(2):351–365. doi:10.1210/jc.2008-1760
23. Silverberg S, Bilezikian JP. Primary hyperparathyroidism. In: Primer of the Metabolic Bone Diseases and Disorders of Mineral Metabolism; 2008:302–306.
24. Jacobs TP, Bilezikian JP. Rare causes of hypercalcemia. J Clin Endocrinol Metab. 2005;90(11):6316–6322. doi:10.1210/jc.2005-0675
25. Marcocci C, Cetani F. Primary hyperparathyroidism. N Engl J Med. 2011;365(25):2389–2397. doi:10.1056/NEJMcp1106636
26. Silverberg SJ. Vitamin D deficiency and primary hyperparathyroidism. J Bone Miner Res. 2007;22(S2):V100–4. doi:10.1359/jbmr.07s202
27. Marx SJ, Attie MF, Levine MA, Spiegel AM, Downs RW JR, Lasker RD. The hypocalciuric or benign variant of familial hypercalcemia: clinical and biochemical features in fifteen kindreds. Medicine. 1981;60(6):397–412. doi:10.1097/00005792-198111000-00002
28. Eastell R, Arnold A, Brandi ML, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J Clin Endocrinol Metab. 2009;94(2):340–350. doi:10.1210/jc.2008-1758
29. Silverberg SJ, Shane E, de la Cruz L, et al. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res. 1989;4(3):283–291. doi:10.1002/jbmr.5650040302
30. Consensus Development Conference on Diagnosis and Management of Asymptomatic Primary Hyperparathyroidism (1990, Bethesda, Md.), Potts JT.
31. Al Zahrani A, Levine MA. Primary hyperparathyroidism. Lancet. 1997;349(9060):1233–1238. doi:10.1016/S0140-6736(96)06176-4
32. Abati A, Skarulis MC, Shawker T, Solomon D. Ultrasound-guided fine-needle aspiration of parathyroid lesions: a morphological and immunocytochemical approach. Human Pathol. 1995;26(3):338–343. doi:10.1016/0046-8177(95)90068-3
33. Absher KJ, Truong LD, Khurana KK, Ramzy I. Parathyroid cytology: avoiding diagnostic pitfalls. Head Neck. 2002;24(2):157–164. doi:10.1002/hed.10003
34. Tseng FY, Hsiao YL, Chang TC. Ultrasound-guided fine needle aspiration cytology of parathyroid lesions. Acta Cytol. 2002;46(6):1029–1036. doi:10.1159/000327103
35. Kumari N, Mishra D, Pradhan R, Agarwal A, Krishnani N. Utility of fine-needle aspiration cytology in the identification of parathyroid lesions. J Cytol/Ind Acad Cytol. 2016;33(1):17. doi:10.4103/0970-9371.175490
36. Khan A, Bilezikian J. Primary hyperparathyroidism: pathophysiology and impact on bone. Cmaj. 2000;163(2):184–187.
37. Consensus development conference statement. J Bone Miner Res. 1991;6(Suppl 2):S9–13. PMID: 1763674. doi:10.1002/jbmr.5650061406
38. Wong WK, Wong NA, Farndon JR. Early postoperative plasma calcium concentration as a predictor of the need for calcium supplement after parathyroidectomy. Br J Surg. 1996;83(4):532–534. doi:10.1002/bjs.1800830433
39. Crea N, Pata G, Casella C, Cappelli C, Salerni B. Predictive factors for postoperative severe hypocalcaemia after parathyroidectomy for primary hyperparathyroidism. Am Surg. 2012;78(3):352–358. doi:10.1177/000313481207800347
40. Pizzarelli F, Fabrizi F, Postorino M, Curatola G, Zoccali C, Maggiore Q. Parathyroidectomy and blood pressure in hemodialysis patients. Nephron. 1993;63(4):384–389. doi:10.1159/000187239
41. Witteveen JE, Van Thiel S, Romijn JA, Hamdy NA. Hungry bone syndrome: still a challenge in the post-operative management of primary hyperparathyroidism: a systematic review of the literature. Eur J Endocrinol. 2013;168(3):R45–53. doi:10.1530/EJE-12-0528
42. Rathi MS, Ajjan R, Orme SM. A case of parathyroid carcinoma with severe hungry bone syndrome and review of literature. Exp Clin Endocrinol Diab. 2007;20:487–490. doi:10.1055/s-2007-992155
43. Ahuja MM, Chopra IJ. Coexistent hyperthyroidism and hyperparathyroidism. Metabolism. 1968;17(10):854–866. doi:10.1016/0026-0495(68)90150-9
44. Corsello SM, Paragliola RM, Locantore P, et al. Post-surgery severe hypocalcemia in primary hyperparathyroidism preoperatively treated with zoledronic acid. Hormones. 2010;9:338–342. doi:10.14310/horm.2002.1286
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.