Back to Journals » Research Reports in Clinical Cardiology » Volume 14
Prevalence of Periprocedural Complications and Associated Factors of Percutaneous Coronary Intervention in Patients with Ischemic Heart Disease at Coronary Care Units of Tikur Anbessa Specialized Hospital and Gesund Cardiac and Medical Center, Addis Ababa, Ethiopia: A Retrospective Cohort Study
Authors Demssis Y, Demissie Z , Alemayheu B, Fekadu C
Received 13 July 2023
Accepted for publication 30 August 2023
Published 4 September 2023 Volume 2023:14 Pages 55—68
DOI https://doi.org/10.2147/RRCC.S419385
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Kones
Yidnekachew Demssis,1 Zekewos Demissie,1 Bekele Alemayheu,2 Chala Fekadu2
1Department of Internal Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 2Department of Internal Medicine, Division of Cardiology, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Yidnekachew Demssis, Email [email protected]
Background: Percutaneous coronary intervention (PCI) is a wire- or catheter-based procedure used to dilate the lumen of stenotic coronary arteries. Even though it is a lifesaving procedure it comes at the cost of rare but detrimental complications. There is no reported study on periprocedural complications of PCI in our setup.
Methodology: We conducted a retrospective cohort study at Tikur Anbessa Specialized Hospital (TASH) and Gesund Cardiac and Medical Center (GCMC) to assess prevalence and associated factors of periprocedural complications of PCI with focus on bleeding, contrast induced nephropathy (CIN) and stroke among patients who underwent PCI from May 1, 2017 to June 30, 2022. A total of 236 participants were included. Data were collected from patient charts, electronic media, and procedure log books. Analysis was done using SPSS 26.0.
Results: The prevalence of CIN and minor bleeding, which is observed blood loss ≥ 3 g/dl to 5 g/dl, were 9% and 9.8%, respectively. On multivariate analysis, the likelihood of having CIN was higher among patients with history of smoking (AOR: 3.374, 95% CI (1.092, 10.421)), taking diuretics (AOR: 3.858, 95% CI (1.357, 10.965)), and patients with chronic kidney disease (AOR: 6.188, 95% CI (1.346, 28.458)).
Conclusion: There is a low prevalence of contrast induced nephropathy and bleeding among cardiac patients who underwent PCI. Smoking, diuretics use and having chronic kidney disease were identified to be strongly associated with periprocedural CIN in this population. There was no stroke or major bleeding in our study and no association was identified between bleeding and the independent factors in this study.
Keywords: contrast induced nephropathy, major and minor bleeding, PCI, periprocedural complication, stroke
Introduction
Cardiovascular disease (CVD) is a major contributor to early mortality and rising medical costs. Within three decades, the overall CVD prevalence nearly doubled.1–3
According to the World Health Organization, over 30% of Ethiopians died from non-communicable diseases in 2014, with cardiovascular disease (CVD) accounting for 9% of these deaths. However, a comprehensive analysis found that the prevalence of CVD in Ethiopia ranged from 7.2 to 24%.4,5
Ischemic heart disease (IHD) is caused by atherosclerotic changes in the coronary arteries.6 Clinically, coronary artery disease (CAD) presents as either an acute or chronic coronary syndrome.7 Growing rates of diabetes, obesity, hypertension, metabolic syndrome, and population aging are all contributing to an increase in the incidence of IHD.8 More than 126 million people worldwide are afflicted by this illness.9
The eighth-leading cause of mortality for both men and women in the area is IHD, which was long thought to be uncommon in sub-Saharan Africa. The incidence of IHD and related morbidity may also be rising due to unfavorable behavioral and lifestyle changes.10 Hospital-based research has established that CAD is a significant health concern, despite the fact that larger studies illustrating the magnitude of the problem in the population are scarce in Ethiopia.11 From May 1, 2007, to December 30, 2011, 300 patients had diagnostic coronary angiography at the Addis Cardiac Centre in Addis Ababa, Ethiopia. 227 (75.7%) individuals with catheterization showed signs of CAD.12
Percutaneous coronary intervention (PCI) is a minimally invasive, wire- or catheter-based procedure used to dilate the lumen of coronary arteries to treat coronary artery stenosis or obstruction and enhance blood flow to ischemic tissue, which is often accomplished with ballooning the narrow segment or deploying a stent to keep the artery open, these being the most effective.13
Andreas Grüntzig performed the first percutaneous transluminal coronary angioplasty in 1977.14,15 After Dr. Fikru Maru Wordofa, a cardiologist, established the nation’s first catheterization laboratory, Dr. Bekele Alemayehu Shashu, an interventional cardiologist, performed the first coronary angiography and PCI at Addis Cardiac Hospital in 2007.16
PCI is a frequent invasive cardiac procedure with a low likelihood of complications. PCI complications have a large impact on patient healthcare costs, as well as severe morbidity and mortality. Major PCI problems are rare but can have disastrous effects if not effectively controlled.17 PCI-related problems are getting less common compared with the early days of balloon angioplasty.18–20 Adverse effects of PCI can include myocardial injury, hemodynamic or electrical instability, tamponade, peripheral vascular injury with bleeding, stroke, acute renal injury, or fatalities.17,21–23 Acute problems are less common overall as a result of improvements in mechanical devices, pharmacotherapies, and PCI procedures.24–29
As mentioned above, IHD is becoming more and more common. Currently, PCI is among the most commonly performed cardiac interventions and this procedure is well known to be associated with rare but life-threatening complications. There has yet to be a study that evaluated the incidence or prevalence of periprocedural complications and the factors that influence PCI in Ethiopia. With a particular focus on periprocedural bleeding, stroke, and CIN and the associated factors, this study offers information on the frequency of PCI-related complications and associated risk factors.
Research Methodology
A retrospective cohort study was conducted on the prevalence and associated factors of periprocedural complications of percutaneous coronary intervention in patients with IHD at Coronary Care Units of Gesund Cardiac and Medical Center and Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia from May 1, 2017 to June 30, 2022.
The cardiac unit provides treatment and follow up for both inpatient and outpatient clients. The coronary care unit which is under the cardiac unit was established May 2017 and the catheterization laboratory has run since then. Gesund Cardiac and Medical Center is one of the private cardiac facilities in Addis Ababa, Ethiopia.
Study Participants
Inclusion Criteria
We included patients with age 18 years and above who underwent PCI for diagnosis of both ACS and CCS from May 1, 2017 to June 30, 2022 at TASH and GCMC.
Exclusion Criteria
- Patients with estimated GFR less than 30 ml/min/1.73M2 based on CKD-EPI calculator
- Patients on dialysis
- Patients with unilateral kidney
- Patients who have undergone renal transplantation
- Patients who have history of CIN
- Patients who have AKI
- Patients with sepsis
- Patients who survived cardiac arrest
- Patients with incomplete data or lost charts
All patients who underwent percutaneous coronary intervention at Coronary Care Units of Tikur Anbessa Specialized Hospital and Gesund Cardiac and Medical Center from 1 May, 2017 to 30 June, 2022 were included in the study. A total of 236 participants were included in the study.
Data Collection Procedure
A structured questionnaire and checklists were developed after review of relevant literatures. Data on socio-demographics, clinical and behavioral characteristics, co-morbid disease, and drugs relevant to this study was retrieved by chart and electronic record review using a structured questionnaire. Data on periprocedural complications were collected from procedure notes and patient charts.
Operational Definitions
- Adequate post procedural hydration: Patient received Intravenous fluid at a dose of 1 ml/kg/h for at least 6 hours.
- Contrast induced nephropathy: A rise in serum creatinine by ≥0.5 mg/dl or an increment by 25 or more percent from its baseline value within 24–72 hours after administration of contrast media.
- Major bleeding: Patients with intracranial hemorrhage or a ≥5 g/dl decrease in hemoglobin concentration or a ≥15% absolute decrease in hematocrit within 72 hours of procedure.
- Minor bleeding: Observed blood loss, ≥3 g/dl decrease in hemoglobin concentration or ≥10% decrease in hematocrit within 72 hours of procedure.
- Percutaneous coronary intervention: A procedure involving either balloon angioplasty or stent deployment of the coronary arteries.
Data Analysis
After checking the data for consistency, completeness, clarity, and accuracy, they were coded manually, then fed into a spreadsheet. Data were analyzed using Statistical Package for Social Sciences (SPSS) version 26.0. Socio-demographic data were described by descriptive analysis. Mean and standard deviation were used to describe continuous variables and percentage and frequency were used to express categorical variables. The prevalence of periprocedural bleeding, stroke, and CIN were expressed using percentages. After assessing multicollinearity test, univariate and multivariate logistic regression analyses were performed to determine the association of independent variables with periprocedural bleeding and CIN. P-value less than 0.05 were considered to determine the statistical significance of the associations.
Ethical Clearance
The Institutional review board of AAU gave ethical clearance to conduct this study and permission was requested from the cardiology unit at TASH and GCMC prior to data collection. Since the data were collected retrospectively, no consent was required from study participants. Participants’ identity remained confidential. Individuals’ anonymity is protected. Ethical principles of the Declaration of Helsinki were respected during all processes of the study.
Results
Socio-Demographic Characteristics of the Study Participants
A total of 236 patients who underwent PCI were included in the study out of 317 retrieved charts (response rate 74.5%). The remaining patient charts were excluded due to: 46 patients with lost charts, 11 patients had acute kidney injury prior to PCI, 2 patients were post cardiac arrest, 7 patients had CKD with estimated GFR<30 ml/min/1.73m2 and the remaining patients had sepsis.
The majority, 198 (83.9%), of the participants were males. The mean (SD) age of the study participants was 58.58±11.13 years with range between 29–89. One hundred and thirty-four (56.8%) of the participants were between 55–74 years of age. Only 35 (14.8%) of the study participants had a history of smoking with median (IQR) pack years of 12 (IQR = 7–20). More than half (20) of those with smoking history were active smokers.
The socio-demographic characteristics are shown in Table 1 below.
Clinical Characteristics of the Study Participants
Clinical Characteristics
The median systolic and diastolic blood pressure of the study participants during admission were 130 (IQR = 110, 140) and 80 (IQR = 70, 80), respectively, with median mean arterial pressure of 80 (IQR = 70, 80). Out of the 43 patients with documented BMI, 19 (44.2%) had normal BMI, 20 (46.5%) were overweight, and the remaining 4 (9.3%) of the patients were obese.
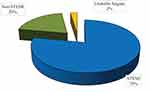
Acute coronary syndrome (ACS) was the most frequent clinical diagnosis in the participants, reported in two-thirds of the patients who underwent PCI. Among patients with ACS, STEMI was the most frequent diagnosis which was found in 123 (77.9%) of patients. The rest of the patients with ACS had NSTEMI, 31 (19.6%), and only 4 (2.5%) patients had unstable angina. Chronic coronary syndrome was found in 78 (33.1%) of the patients.
Among patients with ACS, 98 (63.6%), 47 (30.5%), 8 (5.3%), and only one (0.6%) of the patients had Killip class I, II, III, and IV ACS, respectively. All patients underwent echocardiographic assessment and determination of left ventricular systolic function before PCI. 121 (51.3%), 45 (19.1), and 70 (29.7%) of the patients had ejection fraction of ≥50%, 41–49%, and ≤40%, respectively.
Acute coronary syndrome presentation of patients with ischemic heart disease who underwent PCI is shown in Figure 1.
Co-morbidities
Half (118) of the study participants had hypertension and 89 (75.4%) of them were on treatment. The prevalence of diabetes mellitus and dyslipidemia among the study population were 121 (51.3%) and 51 (21.6%), respectively. Nearly 90% of those with diabetes mellitus and 47.1% of those with dyslipidemia were on medication. Furthermore, chronic kidney disease was reported in 11 (4.7%) of the patients; 6 (54.5%) of them had an estimated GFR of 30 ml/min/1.73M2 to 44 ml/min/1.73M2, while the remaining 5 had an estimated GFR of 45 ml/min/1.73M2 to 59 ml/min/1.73M2.
Co-morbidities among patients with ischemic heart disease who underwent PCI are shown in Figure 2.
Medications
Drug intakes of the study participants are shown in Figure 3. For diabetes mellitus, 90 (38.1%), 32 (13.6%), 17 (7.2%), 3 (1.3%), and 5 (2.1%) of the patients were taking metformin, insulin, sulfonylurea, SGLT2 inhibitors, and DPP4 inhibitors, respectively. The most commonly used medication for hypertensive patients were RAS inhibitors 50 (56.2%), beta blockers 41 (46.1%) and Diuretics 35 (39.3%). Beta blockers (17.8%), RAS inhibitors 25 (10.6%), MRA 15 (6.4%) and diuretics 43 (18.2%) were also given for patients with diagnosis other than hypertension after being diagnosed with IHD or heart failure. 88 (37.3%) of the patients were on statins. Double antiplatelet agents were used in 168 (71.2%) patients. All patients with ACS were taking dual antiplatelet agents (aspirin and clopidogrel) and anticoagulant, unfractionated heparin in 152 (64.4%) and enoxaparin in 6 (2.5%). Four patients received warfarin for concomitant left ventricular thrombus.
Medications among patients with ischemic heart disease who underwent PCI are shown in Figure 3.
Clinical and treatment-related characteristics of the study participants are shown in Table 2.
Prevalence of Periprocedural Complications of PCI
Contrast Induced Nephropathy
During the study period, out of the 236 patients who underwent PCI, 201 of them had documented pre- and post-procedure creatinine determination. 201 participants were included for assessment of development of CIN. The mean baseline and post-procedural serum creatinine were 1.02±0.26 and 1.09±0.34, respectively. Out of the 201 patients, 18 (9%) developed CIN. Hydration with intravenous fluids was reported in 187 (79.2%) of the patients.
Among patients who developed CIN the mean pre- and post-PCI creatinine were 1.15±0.37 and 1.72±0.44, respectively. For pre- and post-procedure, the mean serum creatinine difference was −0.58±0.14, which was significantly higher post-PCI (P <0.001).
The mean serum creatinine differences of pre- and post-procedure are shown in Figure 4.
 |
Figure 4 The mean serum creatinine difference of pre- and post-procedure which was significantly higher post-PCI (Left) and no mean difference in the group with no CIN (Right). |
Bleeding
The study sample consisted of 217 (91.9%) patients who underwent PCI over the period under study who had documentation on the presence or absence of post-procedural bleeding as documented by the treating physician and 163 (69.1%) patients with both pre- and post-procedure hemoglobin level determination.
Out of the 163 patients with both pre- and post-procedure hemoglobin, hemoglobin drop was reported in 120 (73.6%). One hundred and four (63.8%) of the patients had hemoglobin drop <3 g/dl which does not fulfill the criteria for minor bleeding.
The prevalence of minor bleeding which was defined by hemoglobin drop of ≥3 g/dl to 5 g/dl, was 16 (9.8%) out of 163 patients with both pre- and post-procedure hemoglobin results. The mean pre- and post-procedural hemoglobin score among patients with hemoglobin drop between ≥3 g/dl to 5 g/dl were 16.04±2.36 g/dl and 12.25±2.46 g/dl, respectively. Among those with documented bleeding or minor bleeding as defined by hemoglobin drop of ≥3 g/dl to 5 g/dl, only two required transfusion. There was a statistically significant mean difference of 3.79±0.72 g/dl between pre- and post-PCI hemoglobin with the lower documented post-procedure (P <0.001).
Factors Associated with Complications Among Patients Who Underwent PCI
Contrast Induced Nephropathy
Independent variables were checked for the presence of association with CIN in bivariate logistic regression analysis. Smoking history, diuretics use, and CKD were found to be associated with CIN in bivariate analysis (P <0.05) and were entered for multi-variable logistic regression.
We carried out a multivariable logistic regression in which these factors were modeled against a dependent variable of presence of CIN. In this analysis (Table 2), the model explained only 7–16% of the variation (Cox and Snell R2 = 0.073, Nagelkerke R2 = 0.16) in outcome. All the three factors were found to have significant association with the occurrence of CIN.
On multivariate analysis, the likelihood of having CIN was 3.4 times higher among patients with smoking history as compared with those who do not have smoking history (AOR: 3.374, 95% CI (1.092,10.421)). Diuretic use increased by 3.9 times the chance of having CIN compared with patients who did not take diuretics (AOR: 3.858, 95% CI (1.357, 10.965)). Furthermore, the odds of having CIN are 6.2 times higher among patients with CKD than those without (AOR: 6.188, 95% CI (1.346, 28.458)).
Multivariable logistic regression to assess factors associated with CIN complications are shown in Table 3.
Bleeding
Age, sex, smoking history, CKD, type of IHD, ACS type, Killip class and ejection fraction were the independent variables checked for the presence of association with post PCI bleeding in bivariate logistic regression analysis. No significant association was found with any of the independent variables and post-PCI bleeding in bivariate analysis.
Univariate logistic regression to assess factors associated with bleeding complications are shown in Table 4.
 |
Table 4 Univariate Logistic Regression to Assess Factors Associated with Bleeding Complication on Patients with Ischemic Heart Disease That Underwent PCI in Addis Ababa, Ethiopia, 2022 |
Discussion
This study aimed to assess the prevalence and predictors of periprocedural complications of PCI in patients with ischemic heart disease at Coronary Care Units of Tikur Anbessa Specialized Hospital and Gesund Cardiac and Medical Center, Addis Ababa, Ethiopia.
The most common co-morbid condition found in the study was hypertension, while diabetes and dyslipidemia were the second and the third, respectively. The findings were higher than a study in Egypt and lower than a study in India.30,31 This might be due to the differences in the study setting, patient population, and smaller sample size in ours compared with the Indian study. The reason behind these co-morbidities being most prevalent might be the fact that these illnesses are the most common causes for vascular injury and IHD.
The study revealed that the prevalence of CIN was found to be 9%. Our study showed a lower prevalence of CIN compared with studies from Egypt, China, and India.30–32 The prevalence of CIN in these studies was 10.6% in the Egyptian study, 15.3% in a study in China, and 29% in the Indian study. This may be due to the retrospective nature of our study and high non-response rate due to data loss.
Patients with pre-existent CKD have a higher risk of developing CIN. This is in alignment with studies.31,33 This can be explained by the fact that an already compromised kidney function can get worse with administration of contrast.
As was found in our study, a study conducted in India identified diuretic use as strong risk factor for CIN development.31 Diuretics put patients undergoing PCI at risk of dehydration from over-diuresis which subsequently results in increased concentration of the contrast media in the renal tubules. This subsequently results in decreased renal blood flow and medullary hypoxia. Central venous pressure measurement is advocated by some studies in patients with heart failure to guide diuretic therapy.34
Many studies and meta-analysis showed a significant association between occurrence of CIN with diabetes and hypertension.31,33 In contrast, despite a high prevalence of hypertension and diabetes mellitus found in our study, these co-morbidities were not found to be associated with the occurrence of CIN.35 Furthermore, the meta-analysis has found age, Killip class ≥2 and having decreased left ejection fraction as independent determinants for the occurrence of CIN. These factors were not found to be associated with CIN in our study. The reason for not showing significant association might be due to the small sample size in our study.
The prevalence of minor bleeding was found to be 9.8% in our study and there was no reporting of major bleeding incidents following PCI. Contrary to this finding, major bleeding incidence was reported to be 3.1%, 3.9%, and 3% from Japan, Grace Data from USA and France studies.33,36,37 This discrepancy might be due to information bias in our study that occurred because we conducted a retrospective study design by assessing secondary data. Also, the small sample size, population and study setting differences might be reasons for this finding.
In contrast to other studies,35,36 age, sex, diabetes mellitus, history of smoking, and medications were not associated with post-PCI procedure in our study. The reason for not showing significant difference might be due to the smaller proportion of these subjects and smaller sample size of our study.
Strengths and Limitations of the Study
The study tried to look at the different variables related to PCI complications and determinants of complications in two centers with different setups. This study also tried to involve all patients who underwent PCI procedure over the study duration which will increase representativeness and decrease information loss. Additionally this is the first study in our setup that assessed the prevalence and predictors of periprocedural complications of PCI, which may lay a ground for further prospective study.
Limitations of this study include its retrospective nature and use of medical record reports which are secondary data sources with missing information and absence of important investigation results in a proportion of patients. Results from different laboratories may also result in measurement bias and affect the outcome of the study. The sample size is also small which possibly affects the results in both negative and positive directions.
Conclusion
Our study found, a low prevalence of contrast induced nephropathy and bleeding among cardiac patients who underwent PCI. Smoking, diuretics use and having chronic kidney disease were found to have a significant association with periprocedural CIN in this population. There is no case of stroke among all the study population and no association was identified between bleeding and the independent factors in this study.
Recommendations
Based on the findings of this study we have made the following recommendations.
For Care Providers
- As the study demonstrated there is an association between diuretic use and potential dehydration of patients that worsens the occurrence of CIN which mandates that care providers should assess fluid status of patients closely to balance between dehydration and fluid overload based on the proposed guidelines with particular attention in patients with underlying CKD.
For Health Institutions
- The findings from this study may be used by health institutions to enforce protocols and guidelines in a standard management of post-PCI CIN and bleeding patients.
- Advocate renal protection strategies for high-risk patients.
For Future Researchers
- Since this is a cross-sectional study with difficult retrieval of medical records and outcomes, it will be best to conduct a prospective cohort study with better determination cause and effect relationship and further follow up for adverse events.
Abbreviations
AAU, Addis Ababa University; ACS, Acute Coronary Syndrome; ACE-Is, Angiotensin Converting Enzyme Inhibitors; ARBs, Angiotensin Receptor Blockers; ARNI, Angiotensin Receptor Neprilysin Inhibitor; BB, Beta Blockers; CAD, Coronary Artery Disease; CCB, Calcium Channel Blockers; CCS, Chronic Coronary Syndrome; CHS, College of Health Science; CIN, Contrast Induced Nephropathy; CKD, Chronic Kidney Disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CVA, Cerebrovascular Accident; CVD, Cardiovascular Disease; GC, Gregorian Calendar; GCMC, Gesund Cardiac and Medical Center; GFR, Glomerular Filtration Rate; GRACE, Global Registry of Acute Coronary Events; IHD, Ischemic Heart Disease; MI, Myocardial Infarction; MRA, Mineralocorticoid Receptor Antagonists; NSAIDs, Non-Steroidal Anti-Inflammatory Drugs; NSTEMI, Non-ST Segment Elevation Myocardial Infarction; PCI, Percutaneous Coronary Intervention; SPSS, Statistical Package for Social Sciences; STEMI, ST Segment Elevation Myocardial Infarction; TASH, Tikur Anbessa Specialized Hospital; TIMI, Thrombolysis in Myocardial Infarction; TRI, Trans-Radial Intervention; US, United States.
Acknowledgment
We would like to thank the Department of Internal Medicine for this educational opportunity to conduct this research. We also would like to express our heartfelt gratitude to Gesund Cardiac and Medical Center for allowing us to conduct this study at their center. We wish to express our sincere thanks to my advisors Dr. Bekele Alemayehu and Dr. Chala Fekadu for their continued guidance, teaching and constructive comments since the conception of the thesis proposal to completion of the research undertaking.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi:10.1016/j.jacc.2020.11.010
2. Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222.
3. Mensah GA, Roth GA, Fuster V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. Journal of the American College of Cardiology ; 2022. Available from: https://www.jacc.org/doi/full/10.1016/j.jacc.2019.10.009.
4. Misganaw A, Mariam DH, Ali A, Araya T. Epidemiology of major non-communicable diseases in Ethiopia: a systematic review. J Health Popul Nutr. 2014;32(1):1.
5. Angaw DA, Ali R, Tadele A, Shumet S. The prevalence of cardiovascular disease in Ethiopia: a systematic review and meta-analysis of institutional and community-based studies. BMC Cardiovasc Disord. 2021;21(1):1–9. doi:10.1186/s12872-020-01828-z
6. Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25. doi:10.1016/j.jacc.2017.04.052
7. Prabhakaran D, Jeemon P, Sharma M, et al. The changing patterns of cardiovascular diseases and their risk factors in the states of India: the Global Burden of Disease Study 1990–2016. Lancet Glob Health. 2018;6(12):e1339–e1351. doi:10.1016/S2214-109X(18)30407-8
8. Moran AE, Forouzanfar MH, Roth GA, et al. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129(14):1483–1492. doi:10.1161/CIRCULATIONAHA.113.004042
9. Khan MA, Hashim MJ, Mustafa H, et al. Global epidemiology of ischemic heart disease: results from the global burden of disease study. Cureus. 2020;12(7). doi:10.7759/cureus.9349
10. Mensah G. Ischaemic heart disease in Africa. Heart. 2008;94(7):836–843. doi:10.1136/hrt.2007.136523
11. Maru M. The changing pattern of cardiovascular diseases in Ethiopia. East Afr Med J. 1993;70(12):772–776.
12. Shashu BA, Ayele MA. The pattern of coronary artery diseases as diagnosed by coronary angiography and the outcome of Percutaneous Coronary Intervention (PCI) in Ethiopia. Ethiop J Health Dev. 2014;28(1):1.
13. Khan SQ, Ludman PF. Percutaneous coronary intervention. Medicine. 2022;50(7):437–444. doi:10.1016/j.mpmed.2022.04.008
14. Canfield J, Totary-Jain H. 40 years of percutaneous coronary intervention: history and future directions. J Pers Med. 2018;8(4):33. doi:10.3390/jpm8040033
15. Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and re-stenosis after transluminal angioplasty. N Engl J Med. 1987;316(12):701–706. doi:10.1056/NEJM198703193161201
16. The management of coronary artery disease in Ethiopia: emphasis on revascularization. Ethiopian Journal of Health Sciences; 2022 Available from: https://www.ajol.info/index.php/ejhs/article/view/207206.
17. Doll JA, Hira RS, Kearney KE, et al. Management of percutaneous coronary intervention complications. Circ Cardiovasc Interv. 2020;13(6):e008962. doi:10.1161/CIRCINTERVENTIONS.120.008962
18. Bauer T, Boeder N, Nef HM, et al. Fate of patients with coronary perforation complicating percutaneous coronary intervention (from the euro heart survey percutaneous coronary intervention registry). Am J Cardiol. 2015;116(9):1363–1367. doi:10.1016/j.amjcard.2015.07.056
19. Prevalence, predictors, and health status implications of periprocedural complications during coronary chronic total occlusion angioplasty – PubMed; 2022. Available from: https://pubmed.ncbi.nlm.nih.gov/29808821/.
20. Fanaroff AC, Zakroysky P, Dai D, et al. Outcomes of PCI in relation to procedural characteristics and operator volumes in the United States. J Am Coll Cardiol. 2017;69(24):2913–2924. doi:10.1016/j.jacc.2017.04.032
21. George S, Butler R, Nolan J, Mamas MA. Percutaneous coronary intervention and bleeding complications. Eur Med J. 2016;4(1):100–109.
22. Ischemic stroke after percutaneous coronary intervention: rare, but devastating*. JACC: Cardiovascular Interventions; 2022. Available from: https://www.jacc.org/doi/full/10.1016/j.jcin.2019.05.013.
23. Shoukat S, Gowani SA, Jafferani A, Dhakam SH. Contrast-induced nephropathy in patients undergoing percutaneous coronary intervention. Cardiol Res Pract. 2010;2010:e649164. doi:10.4061/2010/649164
24. Stathopoulos I, Jimenez M, Panagopoulos G, Kwak E. The decline in PCI complication rate: 2003–2006 versus 1999–2002. Hellenic J Cardiol. 2009;50(5):379–387.
25. Cowley MJ, Dorros G, Kelsey SF, Van Raden M, Detre KM. Acute coronary events associated with percutaneous transluminal coronary angioplasty. Am J Cardiol. 1984;53(12):C12–C16. doi:10.1016/0002-9149(84)90738-0
26. Detre K, Holubkov R, Kelsey S, et al. Percutaneous transluminal coronary angioplasty in 1985–1986 and 1977–1981. N Engl J Med. 1988;318(5):265–270. doi:10.1056/NEJM198802043180501
27. Agrawal SK, Ho DSW, Liu MW, et al. Predictors of thrombotic complications after placement of the flexible coil stent. Am J Cardiol. 1994;73(16):1216–1219. doi:10.1016/0002-9149(94)90186-4
28. Ellis SG, Roubin GS, King SB, et al. In-hospital cardiac mortality after acute closure after coronary angioplasty: analysis of risk factors from 8207 procedures. J Am Coll Cardiol. 1988;11(2):211–216. doi:10.1016/0735-1097(88)90082-4
29. Kimmel SE, Berlin JA, Strom BL, Laskey WK. Development and validation of a simplified predictive index for major complications in contemporary percutaneous transluminal coronary angioplasty practice. J Am Coll Cardiol. 1995;26(4):931–938. doi:10.1016/0735-1097(95)00294-4
30. Khalfallah M, Allaithy A, Maria DA. Incidence, predictors and outcomes of contrast induced nephropathy in patients with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention. Glob Heart. 2021;16(1). doi:10.5334/gh.1071
31. Valappil SP, Kunjukrishnapillai S, Iype M, et al. Predictors of contrast induced nephropathy and the applicability of the Mehran risk score in high risk patients undergoing coronary angioplasty—a study from a tertiary care center in South India. Indian Heart J. 2018;70(3):399–404. doi:10.1016/j.ihj.2017.08.018
32. Wang J, Zhang C, Liu Z, Bai Y. Risk factors of contrast-induced nephropathy after percutaneous coronary intervention: a retrospective analysis. J Int Med Res. 2021;49(4):03000605211005972. doi:10.1177/03000605211005972
33. Numasawa Y, Kohsaka S, Ueda I, et al. Incidence and predictors of bleeding complications after percutaneous coronary intervention. J Cardiol. 2017;69(1):272–279. doi:10.1016/j.jjcc.2016.05.003
34. Qian G, Fu Z, Guo J, Cao F, Chen Y. Prevention of contrast-induced nephropathy by central venous pressure–guided fluid administration in chronic kidney disease and congestive heart failure patients. JACC Cardiovasc Interv. 2016;9(1):89–96. doi:10.1016/j.jcin.2015.09.026
35. Bundhun PK, Huang F. Post percutaneous coronary interventional adverse cardiovascular outcomes and bleeding events observed with prasugrel versus clopidogrel: direct comparison through a meta-analysis. BMC Cardiovasc Disord. 2018;18(1):1–12. doi:10.1186/s12872-018-0820-6
36. Moscucci M, Fox KA, Cannon CP, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J. 2003;24(20):1815–1823. doi:10.1016/S0195-668X(03)00485-8
37. Kent KC, Moscucci M, Mansour KA, et al. Retroperitoneal hematoma after cardiac catheterization: prevalence, risk factors, and optimal management. J Vasc Surg. 1994;20(6):905–913. doi:10.1016/0741-5214(94)90227-5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.