Back to Journals » Veterinary Medicine: Research and Reports » Volume 14
Prevalence of Bovine Trematodiases and Associated Risk Factors in Nyagatare District, Rwanda
Authors Tumusiime M , Manishimwe JC, Ntampaka P
Received 8 August 2023
Accepted for publication 27 November 2023
Published 11 December 2023 Volume 2023:14 Pages 221—231
DOI https://doi.org/10.2147/VMRR.S430581
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Margaret Tumusiime,* Jean Christian Manishimwe, Pie Ntampaka*
Department of Veterinary Medicine, University of Rwanda, Nyagatare city, Eastern Province, Rwanda
*These authors contributed equally to this work
Correspondence: Pie Ntampaka, Email [email protected]
Introduction: Trematodiases cause significant financial losses to livestock worldwide and some of which are zoonotic, raising public health concerns. In Rwanda, information on the prevalence of bovine trematodiases is scanty, and this hampers efforts to control and prevent them in the country.
Methods: This cross-sectional study aimed to determine the prevalence of bovine trematodiases in Nyagatare district and associated risk factors. One hundred cattle were randomly selected for this study and faecal samples were collected directly from the rectum to identify trematode eggs using a simple sedimentation technique. To analyze the data, frequencies, chi-square test, and binary logistic regression were computed.
Results: Overall, the prevalence of bovine trematodiases was 69%, and Paramphistomum spp. predominated (69%), followed by Dicrocoelium spp. (23%), Fasciola spp. (20%), and Echinostoma spp. (1.0%). The study also recorded mixed paramphistomiasis, fascioliasis and dicrocoeliasis (11.6%), paramphistomiasis and fascioliasis (15.9%) as well as paramphistomiasis and dicrocoeliasis (20.3%). The odds of having trematodiasis (mono or mixed fascioliasis and dicrocoeliasis) for the cow located in Barija cell (AOR = 0.143; 95% C.I. 0.026– 0.793) were 14% lower compared to those of developing such parasitosis for the cow located in Bushoga cell.
Conclusion: Taken together, the study shows that trematodes are a significant contributor to lowering livestock production and productivity and pose a threat to human health. Different approaches should be applied to prevent and control the trematodiases in cows and other livestock (sheep and goats) and reduce the risk of contracting fascioliasis and echinostomiasis in humans in Nyagatare district, Rwanda.
Keywords: prevalence, cattle, paramphistomiasis, fascioliasis, echinostomiasis, dicrocoeliasis, Nyagatare district, Rwanda
Introduction
Agriculture is a vital sector in Rwanda’s journey towards becoming a middle-income economy.1 The sector is critical in achieving long-term economic growth and improving citizen livelihoods. Approximately two-thirds of the working population is employed in agriculture2 which contributes around 30% of the GDP and employs over 70% of the Rwandan population.3 The livestock subsector output is a major component of agriculture and contributes 3% of the overall agriculture GDP.3 Cattle production system in Rwanda is a mixed farming system characterized majorly by keeping Ankole-Friesian crosses that graze on natural communal pastures.4 In Rwanda, cattle contribute to livelihoods as they supply milk, meat, manure, and cash to the communities and at the same time, they are also used in social and cultural necessities. Worldwide, cattle are a major contributor to animal protein, supplying a good percentage of daily meat.5 In Rwanda, the indigenous long-horned Ankole cattle and exotic breeds, particularly the Holstein Friesian, are the most common breed raised – Ankole cattle represent 76% of the national cattle herd.5
On the other hand, Mazimpaka et al, 4 reported that gastrointestinal parasitoses hold back livestock production in the country. Indeed, these parasites cause tremendous economic losses through reduced milk and meat productivity, fertility, work capacity, food intake, weight gain, treatment costs, involuntary culling, and death of heavily infected cattle.6–8 Gastrointestinal trematodes (Fasciola spp, Dicrocoelium spp, Paramphistomum spp) cause serious economic losses globally and are zoonotic (Fasciola spp) and thus of public health concern.9–11 For example, a microscopy-based study carried out in Tanzania reported a prevalence of human fascioliasis accounting for 21%.12 The losses are due to reduced productivity and damages done to the animal organ, for instance, the liver parasitized with the trematodes is condemned at slaughter.7,13 Snails are the common intermediate hosts of most trematodes, therefore their occurrence and how definitive hosts are grazed determine the epidemiology and seasonal pattern of trematodiasis.10,14,15 Trematodiases’ incidences occur in immense water-lodged, marshy grazing fields and this supports their propagation and continuation in the snail intermediate hosts and therefore high prevalence of infection.16 Important genera of flukes recorded in ruminants in different places include Fasciola, Dicrocoelium, Paramphistomum, and Schistosoma.9,10,17 Trematodiases that significantly hinder livestock production profitability worldwide, particularly in the humid tropics and sub-tropics, are fascioliasis, paramphistomiasis, and schistosomiasis.15,18 The prevalence of helminthiases and their severity vary significantly depending on the climatic conditions of the area, the type of vegetation, and management practices.6,15,19
In 2019, the prevalence of bovine helminthiases in Rwanda accounted for 24.5%.2 In a study that investigated bovine hepatic lesions at an abattoir in the country, fascioliasis topped the condemnations with 78.7%.20 Again, a copromicroscopic study conducted on Ankole cattle in Nyagatare district in Rwanda reported the prevalence of fascioliasis totaling 19.9% and 49.3% at individual cow level and herd level, respectively 21 Copromicroscopic studies conducted on cattle in other countries reported the prevalence of trematodiases that varied between 50% and 68.9%.7,10,17,18 Therefore, the study investigated bovine trematodiasis prevalence in Nyagatare district, Rwanda to generate data that could assist in designing effective control measures.
Materials and Methods
Study Area
The study area was Nyagatare district, which is among the 7 districts of Eastern Province, Northeastern extremity of Rwanda. The district comprises 14 Sectors and is further subdivided into 106 administrative cells. Nyagatare district is bordered in the East by Tanzania, the north by Uganda, the west by Gicumbi District, and Gatsibo District in the south. The total surface area of the district is 1919 Km² and it is considered the largest district in Rwanda.
In 2022, the cattle population in Rwanda was 1,424,180 and the Eastern province was the populous region with 390,826 cattle. Again, Nyagatare district was home to 8.6% of the national cattle population, i.e., the populous district with 122,638 cattle.22 Thus, Nyagatare district was purposively selected in the first instance, as the study district. Nyagatare district is in a region with low altitude and an average rainfall ranging from 1250 to 1500 mm. Its rainfall is less varied with an annual average temperature varying between 25.3°C and 27.70°C. In the second instance, Nyagatare sector was selected. In 2022, Nyagatare district record showed that families that reared cows were 13,355 including 8.7% who lived in Nyagatare sector. The latter is generally made by savannah vegetation and some forest gallery, and it is subdivided in 9 administrative cells. The study sector (Nyagatare) and cells are shown (Figure 1).23
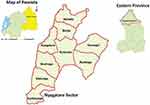 |
Figure 1 A map of the study area – shows the study sector (bounded by red limits) and its nine cells. Only study cows situated in Nyagatare, Barija, Bushoga, Rutaraka, and Nsheke cells were sampled. |
Study Design and Sample Size
This cross-sectional study recruited apparently healthy cows for fecal sampling. Cows were randomly selected and those aged below 18 months were considered young while those aged above 18 months were considered old. Both male and female cows were sampled, and cattle were of local, exotic, and crossbreeds. The sample size was computed based on the cattle population in Nyagatare sector in 2022 (19,734 cows – as per records of the sector administration) and previously reported copromicroscopic prevalence (49.3%).21 Cochran’s formula for calculating sample size for proportions24 was used to compute the sample size of study cows as follows:
Where N is the population; e is the precision level and p is the previously reported prevalence. To compensate for possible non-response, we increased the sample size by 10%,25 i.e., our target sample was 105 cows of which 100 were sampled successfully.
Collection of Fecal Samples and Analysis
Each fecal sample was directly collected from the rectum using a rectal palpation glove that was turned inside out to serve as a fecal container. The sample was labeled with relevant information (study location, cow’s age, sex, and breed). In the field, the samples were kept in a cool box and transported to the parasitology laboratory of the University of Rwanda, School of Veterinary Medicine for coprological analysis using a simple fecal egg sedimentation technique.17,26
For preparing a fecal suspension, 3 grams of feces were suspended in 50 mL of tap water in a beaker and mixed thoroughly.17 The mixture was then filtered into another beaker using a tea strainer and left undisturbed for 10 minutes. After discarding the supernatant, the sediment was re-suspended three times in 50 mL of water – each time the sediment was left undisturbed for 10 minutes. Finally, the supernatant was discarded carefully and the sediment was poured on a petri dish where a 1% methylene blue drop was added. The dye stained the fecal particles a deep blue leaving the trematode eggs unstained. Finally, the eggs were identified using a light microscope at 10x magnification and based on their morphological characteristics.
Data Analysis
The data was entered into Excel Microsoft 365 and transferred to an IBM Statistical Package for Social Sciences (SPSS) version 23 for analysis. To calculate the prevalence, the number of study cows infected with the trematode(s) was divided by the total number of study cows and then multiplied by a hundred. Pearson chi-square test of independence27 and binary logistic regression were also computed to determine the effect of the cow’s location (study administrative cell), age, breed, and sex on the prevalence of trematodiases. The statistical tests were performed at a precision level of 5%.
Results
In total, 100 fecal samples were collected – cows were in various administrative cells and were also of different age, sex, and breeds. Information on the overall prevalence of bovine trematodiases in Nyagatare district is detailed (Table 1).
 |
Table 1 Overall Prevalence of Bovine Trematodiases in Nyagatare District, Rwanda (n = 100) |
Bovine trematodiasis prevalence in relation to the study location is detailed (Table 2).
 |
Table 2 Prevalence of Bovine Trematodiases in Nyagatare District According to the Study Location |
The prevalence of bovine trematodiases in Nyagatare district in relation to the cow’s sex is shown (Table 3).
 |
Table 3 Prevalence of Bovine Trematodiases in Nyagatare District in Relation to the Cow’s Sex |
The prevalence of bovine trematodiases in Nyagatare district in relation to the cow’s age is shown (Table 4).
 |
Table 4 Prevalence of Bovine Trematodiases in Nyagatare District in Relation to the Cow’s Age |
The prevalence of bovine trematodiases in Nyagatare district in relation to the cow’s breed is detailed in Table 5.
 |
Table 5 Prevalence of Bovine Trematodiases in Nyagatare District in Relation to the Cow’s Breed |
The occurrence of single and mixed bovine trematodiasis in Nyagatare district is itemized in Table 6.
 |
Table 6 Occurrence of Single and Mixed Bovine Trematodiasis in Nyagatare District (N = 69) |
The results of binary logistic regression are presented in Table 7.
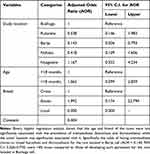 |
Table 7 Effect of the Cow’s Study Location, Age, and Breed on the Occurrence of Mono or Mixed Fascioliasis and Dicrocoeliasis in Nyagatare District |
Discussion
The overall bovine prevalence of trematodiases in Nyagatare district, Rwanda was 69.0% and the cow’s location (study cell) influenced the prevalence of fascioliasis and dicrocoeliasis. This study’s overall prevalence was higher than 57%, 50%, 61%, and 52.6% reported in Tanzania, Indonesia, and Ethiopia, respectively.7,10,17,19 It was, however, comparable to 68.9% reported in Bangladesh18 and lower than 78.1% recorded in Nigeria.9 The dissimilarities in prevalence in the different studies might be due to variations in ecological and climatic conditions. The infection rate of pasture is the major epidemiological variable influencing the trematode burden of animals.16,28,29 Indeed, trematodiasis occurrence depends on the presence of the snails’ intermediate host.
This current study recorded Paramphistomum spp as the prevalent trematode followed by Fasciola spp and Dicrocoelium spp, while Echinostoma spp was the least prevalent. Similar patterns of occurrence of Paramphistomum spp, Fasciola spp, and Dicrocoelium spp were also reported in Nigeria.9,16 Similarly, studies done in Tanzania and Ethiopia10,17 found Paramphistomum spp to be predominant followed by Fasciola spp. We found that the most prevalent trematode was Paramphistomum spp (69.0%) and this was higher than 62.8%, 50.5%, and 47% reported in Tanzania, Bangladesh, and Indonesia, respectively.7,18,30 It was, however, lower than 76% reported in Zambia.13 The high prevalence in the current study may be due to the abundance of intermediate hosts in the environment, grazing on infected pastures, contaminated water sources and the lack of efficient helminth management systems in the study area.14 Furthermore, it may be related to parasite biology, its ability to survive for a long time in the host, and also its capacity to produce many eggs, consequently massive development in the affected snails.17,29 Additionally, it is reported that Paramphistomum spp occur worldwide, can adapt to a wide range of snails19 and are also common in the tropical and sub-tropical regions.14,28,29 Furthermore, bovine paramphistomiasis has been reported as an endemic disease in East African countries.19,31 A study conducted in Zambia32 reported a higher prevalence of Fasciola spp (68.8%) compared to Paramphistomum spp (50.0%) and this might be due to the abundance of Fasciola intermediate hosts in Zambia compared to Rwanda (Nyagatare district). Astudy done in Nigeria also reported a higher prevalence of Fasciola spp (74.9%) followed by Paramphistomum spp (16.1%), Dicrocoelium spp (7.3%), and Schistosoma spp (1.2%)9 and this might be due to high adaptability of Fasciola intermediate hosts in the environment of North-central Nigeria.
Contrarily, the low Fasciola prevalence recorded in this study was comparable to 20.1% and 22.2% reported in Ethiopia.17,19 It was however lower than 18% and 19% that were previously reported in the Democratic Republic of Congo and Rwanda, respectively.21,33 On the other hand, a study done on slaughtered cattle at Nyabugogo abattoir in Rwanda reported a higher prevalence of fasciolosis (78.7%) and cattle from Nyagatare district had a prevalence of 30.2%.20 Other studies have also recorded a higher prevalence of fascioliasis, 67% and 48% in Malaysia and Indonesia, respectively.7,34 It is reported that the risk of fasciolosis happens in areas with higher annual rainfall and with the risk-reducing in areas with shorter wet seasons and/or lower temperatures, since in hot conditions metacercariae survival is reduced16,35 like what is experienced in the current study area.
Differently, the prevalences of Dicrocoelium spp reported in other studies were 7.3%,9 5.68%,36 and 0.52%37 which were lower than that reported in this study (23.0%), and this might be due to the presence of ants as second intermediate hosts for the parasite in Nyagatare district and this factor could contribute to the higher prevalence compared to other regions. However, a higher prevalence of Dicrocoelium spp has been reported elsewhere, i.e., 37.6% in Spain38 and 39% in Nigeria.39 In addition, the survivability and distribution of Dicrocoelium spp is reported to be sustained by environmental factors, intermediate host, and definitive host factors.16,40
This study shows significant associations between the location of the cows with fascioliasis and dicrocoeliasis. It has been reported that differences in parasite prevalence in cattle might be influenced by different geographical areas (ecology) and this is also associated with snail habitats in grazing areas of animals.14,16,19,41,42 Fascioliasis has been particularly linked to climatic conditions of the area and a grazing history of animals.35
The study also recorded mixed trematodiases. Similarly, mixed paramphistomiasis and fascioliasis have been reported elsewhere: in Ethiopia,19 in Tanzania,17,30 in Zambia,13 in Nigeria,9 and in Malaysia.34 The co-infection of Fasciola with Dicrocoelium was also reported in Nigeria.39 Co-infections might be due to different factors such as simultaneous exposure of cattle to different trematodes, probability of the different trematodes surviving in the same intermediate hosts, and similarities of some of the trematode life cycles.9 Some metacercariae of different trematode species might be ingested together by cattle and cause co-infections. The mixed infections recorded in this study may suppress the host immune system predisposing it to other diseases and reduce livestock production. The co-infection could also lead to a high worm burden which would result in high egg contamination of the environment - this would increase the risk of contracting the zoonotic trematodes (Fasciola spp, Echinostoma spp) in humans. Indeed, both Fasciola hepatica and F. gigantica can cause zoonotic infections.43 Although there is no data on human fascioliasis in Rwanda, human cases have been reported in a neighboring country (Tanzania).
The management and diagnosis of mixed trematodiases are challenging: a host suffering from acute F. hepatica infection may not shed eggs in its faeces, and acute fascioliasis can be diagnosed with ELISA rather than fecal egg sedimentation. Major damages to the host suffering from acute fascioliasis is due to the migration of juvenile flukes from the intestine to the liver.44 The fact that this study’s cows were apparently healthy may indicate that the cows that were infected had chronic or asymptomatic trematodiasis: in ruminants, fascioliasis is predominantly chronic.45 Again, adult Paramphistomum worms are non-pathogenic, but their migrating larvae may impact the host production and even cause death. Further, bovine dicrocoeliasis is also asymptomatic.44
Given that a sedimentation technique was used to differentiate trematodes based on their egg morphology; this might have resulted in misclassification of the trematodes. Although Paramphistomum spp eggs can be differentiated from those of F. hepatica,44 eggs of F. hepatica can be mistaken for those of Fasciolopsis buski and Echinostoma spp.45 According to Verocai et al,44 eggs of Dicrocoelium dendriticum cannot be confused with those of other flukes because they are too small. However, given such eggs are brown, when they are detected using a sedimentation technique, the test is difficult to read due to excessive debris.44
Fascioliasis is refractory to praziquantel, while it is responsive to triclabendazole. Echinostomiasis can also be treated with praziquantel.46 Nitroxinil is one of anthelminthics that are effective against F. hepatica and Paramphistomum spp.47
This study’s major limitation was that the trematodes were identified at the genus level based on morphology of the eggs detected using a simple sedimentation technique. This diagnostic technique could miss some small eggs of Dicrocoelium spp. Molecular-based diagnostics would help to avoid misclassifying the trematodes.
Conclusion
The overall prevalence of bovine trematodiases in Nyagatare district, Rwanda was 69.0%. Different trematodes were identified including Paramphistomum spp, Fasciola spp, Dicrocoelium spp, and Echinostoma spp. The location of the cow was associated with the occurrence of bovine trematodiasis (mono or mixed fascioliasis and/or dicrocoeliasis). The high overall prevalence of trematodes in cattle in this study is adequate to limit and constrain cattle production leading to significant economic losses to small-scale farmers due to decreased productivity, treatment costs, and potential condemnation of affected organs during slaughter. Trematodiases constitute a major hindrance to livestock production and human health : for example, Fasciola spp and Echinostoma spp are zoonotic. Therefore, different approaches should be applied to prevent and control the trematodiases in cows and other livestock (sheep and goats) and reduce the risk of contracting fascioliasis and echinostomiasis in humans in Nyagatare district in Rwanda.
Ethics Approval and Informed Consent
This study was concerned with live cows, not humans; therefore, study approval was given by the academic council of the School of Veterinary Medicine, University of Rwanda. Before collecting the data, the cattle farmers were briefed about the study, and only those who consented to admit their cows for faecal sampling were selected. Further, the cows were also treated with the best practice of veterinary care.
Acknowledgments
The authors are thankful to the University of Rwanda, Nyagatare Campus, for allowing them to use the laboratory facilities. They also thank the leadership of Nyagatare sector for allowing them to collect data and are also grateful to the farmers who allowed the collection of fecal samples from their cows.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Habiyaremye N, Ouma EA, Mtimet N, Obare GA. A review of the evolution of dairy policies and regulations in Rwanda and its implications on inputs and services delivery. Front Vet Sci. 2021;8:611298. doi:10.3389/fvets.2021.611298
2. MINAGRI. Ministry of agriculture and animal resources (MINAGRI), Annual report 2018–2019, Republic of Rwanda; 2019. Available from: https://www.minagri.gov.rw/.
3. NISR. Rwanda Statistical Yearbook 2018. NISR; 2018.
4. Mazimpaka E, Mbuza F, Michael T, Gatari EN, Bukenya EM, James OA. Current status of cattle production system in Nyagatare District-Rwanda. Trop Anim Health Prod. 2017;49(8):1645–1656. doi:10.1007/s11250-017-1372-y
5. Hirwa DC, Donald RK, Tiba M, et al. Management and phenotypic features of indigenous cattle in Rwanda. Int J Livest Prod. 2017;8(7):95–112. doi:10.5897/IJLP2017.0362
6. Degefu H, Abera C, Yohannes M, Tolosa T. Gastrointestinal helminth infections in small-scale dairy cattle farms of Jimma town, Ethiopia. Ethiop J Appl Sci Technol. 2011;2(1):31–37.
7. Rinca KF, Prastowo J, Widodo DP, Nugraheni YR. Trematodiasis occurrence in cattle along the Progo River, Yogyakarta, Indonesia. Vet World. 2019;12(4):593–597. doi:10.14202/vetworld.2019.593-597
8. Yuguda AU, Samaila AB, Panda SM. Gastrointestinal helminths of slaughtered cattle in Bauchi Central Abattoir, Bauchi State, Nigeria. GSC Biol Pharm Sci. 2018;4(2):058–065. doi:10.30574/gscbps.2018.4.2.0036
9. Elelu N, Ambali A, Coles GC, Eisler MC. Cross-sectional study of Fasciola gigantica and other trematode infections of cattle in Edu Local Government Area, Kwara State, north-central Nigeria. Parasit Vectors. 2016;9(1):470. doi:10.1186/s13071-016-1737-5
10. Nzalawahe J, Kassuku AA, Stothard JR, Coles GC, Eisler MC. Trematode infections in cattle in Arumeru District, Tanzania are associated with irrigation. Parasit Vectors. 2014;7(1):107. doi:10.1186/1756-3305-7-107
11. de Waal T, Mehmood K. Editorial: trematode infection in ruminants. Front Vet Sci. 2021;8:719577. doi:10.3389/fvets.2021.719577
12. Lukambagire AHS, Mchaile DN, Nyindo M. Diagnosis of human fascioliasis in Arusha region, northern Tanzania by microscopy and clinical manifestations in patients. BMC Infect Dis. 2015;15(1):578. doi:10.1186/s12879-015-1326-9
13. Yabe J, Phiri IK, Phiri AM, Chembensofu M, Dorny P, Vercruysse J. Concurrent infections of Fasciola, Schistosoma and Amphistomum spp. in cattle from Kafue and Zambezi river basins of Zambia. J Helminthol. 2008;82(4):373–376. doi:10.1017/S0022149X08054904
14. Pfukenyi DM, Mukaratirwa S. Amphistome infections in domestic and wild ruminants in East and Southern Africa: a review. Onderstepoort J Vet Res. 2018;85(1). doi:10.4102/ojvr.v85i1.1584
15. Sanchís J, Sánchez-Andrade R, Macchi MI, et al. Infection by Paramphistomidae trematodes in cattle from two agricultural regions in NW Uruguay and NW Spain. Vet Parasitol. 2013;191(1–2):165–171. doi:10.1016/j.vetpar.2012.07.028
16. Chongmobmi M, Panda S. Bovine Gastrointestinal Trematodosis In Nigeria: a Review. IOSR J Agric Vet Sci. 2018;11(11):08–19.
17. Aragaw K, Tilahun H. Coprological study of trematode infections and associated host risk factors in cattle during the dry season in and around Bahir Dar, northwest Ethiopia. Vet Anim Sci. 2019;7:1–7. doi:10.1016/j.vas.2018.11.002
18. Yasin MG, Alim MA, Ahasan SA, et al. Trematode infections in farm animals and their vector snails in Saint Martin’s Island, the southeastern offshore area of Bangladesh in the Bay of Bengal. J Vet Med Sci. 2018;80(4):684–688. doi:10.1292/jvms.17-0308
19. Fromsa A, Meharenet B, Mekibib B. Major trematode infections of cattle slaughtered at Jimma municipality abattoir and the occurrence of the intermediate hosts in selected water bodies of the zone. J Anim Vet Adv. 2011;10(12):1592–1597. doi:10.3923/javaa.2011.1592.1597
20. Habarugira G, Mbasinga G, Mushonga B, Chitura T, Kandiwa E, Ojok L. Pathological findings of condemned bovine liver specimens and associated economic loss at Nyabugogo abattoir, Kigali, Rwanda. Acta Trop. 2016;164:27–32. doi:10.1016/j.actatropica.2016.07.020
21. Sun P, Wronski T, Apio A, Edwards L. A holistic model to assess risk factors of fasciolosis in Ankole cattle. Vet Parasitol Reg Stud Rep. 2020;22:100488. doi:10.1016/j.vprsr.2020.100488
22. NISR. National Institute of Statistics of Rwanda (NISR); the fifth Rwanda population and housing census, Rwanda; 2023. Available from: https://www.statistics.gov.rw/publication/key-figures-5th-rwanda-population-and-housing-census-phc.
23. Ntampaka P, Niragire F, Nkurunziza V, Uwizeyimana G, Shyaka A. Perceptions, attitudes and practices regarding canine zoonotic helminthiases among dog owners in Nyagatare district, Rwanda. Vet Med Sci. 2022;8(4):1378–1389. doi:10.1002/vms3.787
24. Cochran WG. Sampling Techniques.
25. Al-Subaihi AA. Sample size determination. Neurosciences. 2003;8(2):79–86.
26. Hansen J, Perry B. The epidemiology, diagnosis and control of helminth parasites of ruminants. Prev Vet Med. 1994;31(1–2):161–162. doi:10.1016/S0167-5877(97)83404-6
27. McHugh ML. The Chi-square test of Independence. Biochem Med. 2013;23(2):143–149. doi:10.11613/BM.2013.018
28. Hajipour N, Mirshekar F, Hajibemani A, Ghorani M. Prevalence and risk factors associated with amphistome parasites in cattle in Iran. Vet Med Sci. 2021;7(1):105–111. doi:10.1002/vms3.330
29. Hotessa AS, Kanko DK. Review on Paramphistomosis. Adv Biol Res. 2020;14(4):184–192. doi:10.5829/idosi.abr.202.184.192
30. Nzalawahe J, Kassuku AA, Stothard JR, Coles GC, Eisler MC. Associations between trematode infections in cattle and freshwater snails in highland and lowland areas of Iringa Rural District, Tanzania. Parasitology. 2015;142(11):1430–1439. doi:10.1017/S0031182015000827
31. Keyyu JD, Kassuku AA, Msalilwa LP, Monrad J, Kyvsgaard NC. Cross-sectional prevalence of helminth infections in cattle on traditional, small-scale and large-scale dairy farms in Iringa District, Tanzania. Vet Res Commun. 2006;30(1):45–55. doi:10.1007/s11259-005-3176-1
32. Phiri AM, Phiri IK, Chota A, Monrad J. Trematode infections in freshwater snails and cattle from the Kafue wetlands of Zambia during a period of highest cattle–water contact. J Helminthol. 2007;81(1):85–92. doi:10.1017/S0022149X07387786
33. Bisimwa NP, Lugano RM, Wasso SD, Kinimi E, Banswe G, Bajope EA. Prevalence of gastro-intestinal helminths in slaughtered cattle in walungu territory, South Kivu Province, Eastern Democratic Republic of Congo. Austin J Vet Sci Anim Husbandary. 2018;5(1):1–7.
34. Khadijah S, Ariff Z, Nurlaili MR, Sakiinah A, Izzudin AH, Mursyidah AK. Fasciola and Paramphistomum infection in large Ruminants. Int J Agron Agric Res. 2017;10(6):1–8.
35. Biruk A. Bovine Fasciolosis in Ethiopia-A review. Vet Drug Anim Feed Adm Control Auth Hawassa Ethiop. 2019;2(2):1–13.
36. Shamsi L, Samaeinasab S, Samani ST. Prevalence of hydatid cyst, Fasciola spp. and Dicrocoelium dendriticum in cattle and sheep slaughtered in Sabzevar abattoir, Iran. Ann Parasitol. 2020;66(2):211–216. doi:10.17420/ap6602.256
37. Chougar L, Harhoura K, Aissi M. First isolation of Dicrocoelium dendriticum among cattle in some Northern Algerian slaughterhouses. Vet World. 2019;12(7):1039–1045. doi:10.14202/vetworld.2019.1039-1045
38. Manga-González MY, González-Lanza C. Field and experimental studies on Dicrocoelium dendriticum and dicrocoeliasis in northern Spain. J Helminthol. 2005;79(4):291–302. doi:10.1079/JOH2005323
39. Iyaji FO, Yaro CA, Peter MF, Abutu AEO. Fasciola hepatica and associated parasite, dicrocoelium dendriticum in slaughter houses in Anyigba, Kogi State, Nigeria. Adv Infect Dis. 2018;08(01):1–9. doi:10.4236/aid.2018.81001
40. Otranto D, Traversa D. Dicrocoeliosis of ruminants: a little known fluke disease. Trends Parasitol. 2003;19(1):12–15. doi:10.1016/S1471-4922(02)00009-0
41. Pfukenyi DM, Mukaratirwa S, Willingham AL, Monrad J. Epidemiological studies of amphistome infections in cattle in the highveld and lowveld communal grazing areas of Zimbabwe. Onderstepoort J Vet Res. 2005;72(1):67–86. doi:10.4102/ojvr.v72i1.224
42. Seid U, Melese M. Review on prevalence, distrbution and economic significance of liver Fluke in Ethiopia. ARC J Anim Vet Sci. 2018;4(2):38–48. doi:10.20431/2455-2518.0402006
43. Despommier D, Griffin D, Gwadz R, Hotez P, Knirsch C. Parasitic Diseases, Parasites Without Borders, Inc. NY, New York; 2019.
44. Verocai GG, Chaudhry UN, Lejeune M. Diagnostic methods for detecting internal parasites of livestock. Vet Clin North Am Food Anim Pract. 2020;36(1):125–143. doi:10.1016/j.cvfa.2019.12.003
45. Mahanty S, Maclean† JD, Cross JH. Liver Lung, and Intestinal Fluke Infections. In: Tropical Infectious Diseases: Principles, Pathogens and Practice. Elsevier; 2011:854–867. doi:10.1016/B978-0-7020-3935-5.00123-3
46. Chai JY. Praziquantel treatment in trematode and cestode infections: an update. Infect Chemother. 2013;45(1):32. doi:10.3947/ic.2013.45.1.32
47. Ico-Gómez R, González-Garduño R, Ortiz-Pérez D, et al. Assessment of anthelmintic effectiveness to control Fasciola hepatica and paramphistome mixed infection in cattle in the humid tropics of Mexico. Parasitology. 2021;148(12):1458–1466. doi:10.1017/S0031182021001153
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.