Back to Journals » Veterinary Medicine: Research and Reports » Volume 15
Prevalence, Antimicrobial Resistance, and Characterization of Listeria Spp. Isolated from Various Sources in Ethiopia: A Comprehensive Review
Authors Tola EH
Received 26 November 2023
Accepted for publication 31 March 2024
Published 6 April 2024 Volume 2024:15 Pages 109—116
DOI https://doi.org/10.2147/VMRR.S451837
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Young Lyoo
Eyob Hirpa Tola
Department of Microbiology, Immunology and Public Health, College of Veterinary Medicine and Agriculture, Addis Ababa University, Bishoftu, Oromia, Ethiopia
Correspondence: Eyob Hirpa Tola, Email [email protected]; [email protected]
Abstract: Listeriosis is an important foodborne zoonotic disease affecting humans and animals in Ethiopia. This review aims to synthesize the epidemiology, prevalence, distribution, and antimicrobial resistance of Listeria species in the country. The literature reveals a widespread occurrence of Listeria infection in humans, animals, and food products, with an average prevalence of 21.6% for Listeria species and 6.9% for L. monocytogenes. Three sequence types (STs) of L. monocytogenes (2, 145, and 18) and twelve STs of L. innocua (1489, 1619, 603, 537, 1010, 3186, 492, 3007, 1087, 474, 1008, and 637) were reported from milk and dairy products. Contamination rates ranged from 4.1% to 42.9% across livestock, dairy, slaughterhouses, and processing facilities, indicating faults in production practices. Sporadic human listeriosis outbreaks have occurred since 1967, causing meningitis, perinatal infections, and deaths, with recent studies showing L. monocytogenes isolation in up to 10.4% of febrile patients, confirming foodborne transmission. Non-pathogenic Listeria species were also common on farms and in facilities. Ovine listeriosis poses a threat to Ethiopia’s sheep and goat industries, with over 40% seroprevalence in some herds. Comprehensive control measures across the food chain are needed to curb contamination and protect public health. Isolates from various foods show antibiotic resistance to first-line agents but susceptibility to others like gentamicin and cephalosporins. In conclusion, this review synthesizes evidence on Listeria distribution in Ethiopia’s food system and disease burden, highlighting the need for improved food safety policies and awareness.
Keywords: animals, listeriosis, foodborne, pregnancy, zoonotic
Introduction
Listeriosis is a serious foodborne disease that can cause severe illness and death in high-risk groups like pregnant women, newborns, the elderly and immunocompromised individuals.1 The disease is caused by Listeria species, with L. monocytogenes being the main pathogenic species that affects both animals and humans.2–4 L. monocytogenes is a gram-positive bacterium that grows intracellularly and produces virulence factors like listeriolysin O and hemolysin to evade host defenses.2–4
L. monocytogenes is primarily transmitted through contaminated food, including raw dairy and ready-to-eat meals.1 Direct contact with infected livestock like cattle, sheep, and goats can also transfer L. monocytogenes to humans; especially agricultural workers.5 In animals, the bacterium is shed in feces and secretions. In humans, L. monocytogenes typically causes flu-like symptoms but can also lead to severe invasive diseases like septicemia, meningitis, encephalitis and miscarriage or newborn sepsis.5,6
Listeria contamination rates in Ethiopian foods range from 14.3% to 62% in raw meats, dairy products, and vegetables.7 The highest Listeria prevalence was found in raw beef (62%) and ice cream (43%) samples.8 Sporadic human listeriosis cases and outbreaks have been reported from Addis Ababa and central Ethiopian towns.9,10
Antibiotics like ampicillin, penicillin, gentamicin, trimethoprim-sulfamethoxazole, and chloramphenicol can effectively treat listeriosis.11 However, the emergence of multidrug resistant L. monocytogenes strains has raised public health concerns globally.12 In Ethiopia, there is limited data on current antimicrobial resistance trends and molecular subtyping of Listeria strains from humans and animals.
Listeria is widespread in the environment.13 Most infections are acquired by ingestion, but Listeria can also be transmitted through inhalation or direct contact. In sheep, listeriosis often occurs after eating contaminated silage.14 Contaminated foods that can infect humans include raw meat, seafood, unpasteurized dairy, and uncooked vegetables.14 In newborn infants and ruminants, vertical transmission from mother to baby during pregnancy or birth is the most common source of infection.15 Humans can also become infected from direct contact with sick animals during birthing or necropsies.15 Sheep and goat feces, human waste, farm slurries, sewage, water troughs, surface water, plants, animal feed, and barn walls are primary sources.16 While L. monocytogenes is ubiquitous on farms, its growth is limited during food preparation.
In their 2024 study, Wei et al17 investigated Listeria isolates from Ethiopia. They identified three sequence types (STs) of Listeria monocytogenes (2, 145, and 18) and twelve STs of Listeria innocua (1489, 1619, 603, 537, 1010, 3186, 492, 3007, 1087, 474, 1008, and 637). Some of these STs exhibited region-specific occurrence, while others were widely distributed across regions. Through high-quality single nucleotide polymorphism (SNP) analysis, they found that among the 13 L. monocytogenes isolates of ST2, 11 were highly similar, differing by only 1 to 10 SNPs, suggesting potential selection in the dairy food supply chain. The L. innocua isolates also exhibited low intra-ST genetic variation (0–10 SNP differences), except for ST1619, which displayed greater diversity.
Overall, listeriosis remains an underdiagnosed and underreported disease in Ethiopia due to lack of routine surveillance, reporting, and laboratory infrastructure in healthcare and veterinary sectors. Enhanced surveillance, diagnostics, antimicrobial susceptibility testing, and molecular characterization of Listeria strains circulating across Ethiopia are warranted to determine the public health impact of foodborne listeriosis. Findings can inform evidence-based control strategies to prevent listeriosis in high-risk groups and livestock production. The objective of this paper is to comprehensively review the epidemiology, prevalence, distribution, and antimicrobial resistance patterns of Listeria monocytogenes and other Listeria spp in Animals and Food products in Ethiopia.
Materials and Methods
A comprehensive search for relevant studies was performed using several major scientific databases, including PubMed, Web of Science, EMBASE, Google Scholar and the Cochrane Library. The search focused on identifying published observational studies that reported on the prevalence of Listeria species isolated from various sources in Ethiopia, including animal- and plant-based food products as well as human clinical samples (Figure 1). Strict inclusion and exclusion criteria were developed and applied to select the most pertinent articles. After removing duplicate reports, 37 studies met the predetermined inclusion criteria and were selected for review. This comprehensive search and screening process aimed to identify the most relevant published studies on Listeria spp, Listeria monocytogenes, prevalence, distributions in animal, human, food and feed reported in Ethiopia (Figure 2).
 |
Figure 1 Map of Listeria spp reported area. |
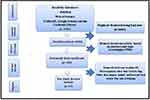 |
Figure 2 Strategy of Searching and Selection of Published Article. |
Results
Multiple studies in Ethiopia have found Listeria contamination in animal feed and human foods, though comprehensive nationwide data is lacking (Figure 1). A wide range of sample types were tested, including bovine, poultry, pork, fish, dairy products, eggs, farm environments and human clinical samples for detection of Listeria monocytogenes and other Listeria spp. The total number of samples analysed was 5144 across all the studies. The overall prevalence of any Listeria species ranged from 4.1% to 42.9%, with an average prevalence of 21.6% across the studies. This indicates widespread Listeria contamination of livestock, animal products, produce and the farm environment in the country. Bovine sources and raw dairy products showed some of the highest rates of contamination. The prevalence specifically for the pathogenic species L. monocytogenes averaged 6.9% across the studies, ranging from 1.2% to 32.6% based on the sample type. Key food sources showing L. monocytogenes contamination included raw milk, meat, eggs and fish. Human clinical samples also tested positive, confirming human listeriosis cases. Several non-pathogenic Listeria species were isolated from the various sources, including L. innocua, L. ivanovii, L. seeligeri, L. welshimeri, L. grayi and L. murrayi. These species are generally saprophytes but can cause opportunistic infections. Their presence also indicates faults in food handling and processing (Table 1)
 |
Table 1 Listeria Spp Isolated and Identified from Various Source in Ethiopia |
In Addis Ababa, Listeria was isolated from 26.1% of 391 samples including raw milk, beef, cheese, and eggs. L. monocytogenes, L. innocua, L. ivanovii, L. seeligeri, L. welshimeri, L. grayi, and L. murrayi were identified.18 In Debre-Birhan, 20.88% of 407 dairy milk samples tested positive for L. monocytogenes, L. innocua, L. seeligeri, L. welshimeri, L. grayi, and L. murrayi.27 In Gondar town, 27% of 711 raw milk, beef, pork, and egg samples were positive, with isolation of L. monocytogenes and other species.19 Another Gondar study found 25% of 384 raw milk and beef samples positive, with isolation of multiple Listeria species.31 In Ambo and Holeta, 28.4% of 450 beef samples were positive, with isolation of L. monocytogenes, L. ivanovii, L. seeligeri, L. welshimeri, L. innocua, and L. grayi.28
Listeria species were isolated from 27.4% of food samples in Addis Ababa, with L. monocytogenes specifically in 5.4%.6 Another Addis Ababa study isolated L. monocytogenes and other Listeria from various raw and ready-to-eat foods at 5.1% frequency.32 Identified L. monocytogenes serotypes belonged to serogroups 1/2b, 4b and 4e.18 Listeria was found in 20% of samples from silage, water, cow barns, cows, and milking at Haramaya University.20 L. monocytogenes was isolated from 20.88% of raw bovine milk in Debre-Birhan,24 High contamination was reported in foods of animal origin from Bishoftu and Dukem at 30%.21,23 In Arsi, East Shewa, and the central highlands, prevalence was 42.9% and 28.4% in raw milk and dairy, respectively.22 Other studies found Listeria rates of 25% in animal foods in Gondar,31 24.2% in milk and dairy in North Shewa33 14% in meats and dairy in Jimma19 and 4.1% in sheep meat from Addis Ababa.25 Contamination ranging from 3.8% to 42.9% indicates Listeria is common in animal-derived foods across Ethiopia.
Sporadic cases and outbreaks of human listeriosis have also been reported in Ethiopia. The first documented case was in 1967 in a diabetic patient.34 L. monocytogenes was isolated from 16 cases of meningitis from 1983 to 1984.35 An outbreak occurred in 1988 with 16 perinatal infections and 2 maternal deaths linked to L. monocytogenes contamination in a hospital.36 More recent studies have reported L. monocytogenes isolation rates of 10.4% from febrile patients, 31.7% from spinal fluid cultures, and 5.6% from cases of abortion in pregnant women.29,34 Overall, human listeriosis appears to be an endemic yet underdiagnosed condition in Ethiopia, warranting improved food safety measures and education on prevention.
The highest prevalence has been reported from the central highlands of Ethiopia. A study by25 conducted on 4 farms near Addis Ababa found L. monocytogenes in 7.6% of sheep and 4.8% of goats sampled. This indicates ovine listeriosis may be more common in certain agro-ecological zones of Ethiopia. The variable prevalence rates across different regions highlight the need for further epidemiological studies to determine the distribution and associated risk factors for ovine listeriosis in Ethiopia. Overall, the available studies indicate ovine listeriosis is an endemic problem in the Ethiopian small ruminant population. Prevalence of Listeria in animal feed and human food in Ethiopia is indicated in Table 1.
Studies across various regions of Ethiopia have reported a significant prevalence of Listeria monocytogenes contamination in food sources of animal origin as well as human clinical samples. The prevalence ranges from 1.2% in bovine bulk milk samples from Arsi and East Shewa zones22 to 8.8% in dairy milk from Debre-Birhan.27 In meat and milk products, a 4% prevalence was found in bovine milk, cheese, ice cream and yoghurt samples in Jimma Town,23 while a 6.25% prevalence was detected in beef meat and milk samples in Gondar.24 Clinical samples also showed contamination, with 8.5% prevalence in human blood samples from Mekele26 and 5.56% in human blood samples from Jimma.29 The pooled average prevalence across the various studies was 6.9%, highlighting the need for improved food safety practices to reduce L. monocytogenes contamination.
In raw cow milk samples, L. monocytogenes exhibited complete resistance (100%) to nalidixic acid, while also showing high resistance to erythromycin (88%), ampicillin (23.5%), chloramphenicol (17.65%), streptomycin (11.76%), and cefotaxime (5.88%).30 Isolates from milk and milk products displayed 100% resistance to oxacillin, followed by 90.91% resistance to amoxicillin and 54.5% resistance to nalidixic acid.37 In sheep meat samples, high resistance levels were observed against ampicillin (88.9%), chloramphenicol (88.9%), and penicillin (66.7%), while lower resistance was seen against sulfamethoxazole-trimethoprim (66.7%) and tetracycline (22.2%).25 Listeria from raw milk exhibited 30.5% resistance to nalidixic acid, 25% to tetracycline, 22.2% to chloramphenicol, and 11.1% to streptomycin.27 Clinical isolates from pregnant women’s blood showed the highest resistance to penicillin G (66.7%), clindamycin (66.7%), amoxicillin (50%), and vancomycin (50%).26
Research showed L. monocytogenes isolates from raw cow milk samples had higher susceptibility to antibiotics like vancomycin, gentamicin, and sulfamethoxazole. Studies on milk products demonstrated retained sensitivity to gentamycin, norfloxacin, chloramphenicol, nitrofurantoin, and tetracycline. Analysis of L. monocytogenes from sheep meat samples found higher susceptibility rates to vancomycin, co-trimethazole, amoxyclav, gentamycin, and streptomycin. Investigations of isolates in raw milk indicated continued efficacy of cephalothin, kanamycin, vancomycin, ampicillin, and gentamicin against L. monocytogenes.(Table 2)
 |
Table 2 Antimicrobial Resistance Profiles of Listeria Monocytogenes Isolated from Various Sample |
Conclusion
In conclusion, this review highlights that listeriosis remains an underdiagnosed and underreported disease in Ethiopia, warranting strengthened nationwide surveillance, diagnostics, antimicrobial susceptibility testing, and molecular characterization of Listeria strains circulating in livestock, foods, and humans across diverse agro-ecological zones. Reported contamination rates of Listeria species in Ethiopian foods ranging from 3.8% to 42.9% indicate it is a common foodborne hazard needing improved farm biosafety and food hygiene practices for control. Sporadic human cases and outbreaks also showcase listeriosis as an endemic yet overlooked public health issue in Ethiopia. However, its true distribution and burden across high-risk groups like pregnant women and neonates are unknown due to limited systematic monitoring, reporting, and laboratory infrastructure. Thus, enhanced surveillance and epidemiological studies are recommended to determine the prevalence, incidence, and impacts of listeriosis in vulnerable populations nationwide. Routine screening, outbreak reporting, advanced diagnostic testing, antimicrobial resistance profiling, and molecular subtyping of Listeria strains in veterinary and public health laboratories would help elucidate the public health risks. Evidence-based control strategies should be implemented, including education programmes on food hygiene and safety targeted at farmers, processors, and consumers. Livestock vaccination if available, sanitation protocols in food production facilities, and restrictions on the distribution of high-risk products are also advised. Further research can identify predominant Listeria strains, transmission routes, and risk factors in diverse regions to support tailored prevention approaches to control listeriosis across Ethiopia. Overall, this review summarizes current knowledge on ovine and human listeriosis in Ethiopia while underscoring critical needs for improved monitoring, diagnostics, molecular characterization, risk factor research, and the implementation of food safety practices and policies to combat this major zoonotic disease.
Disclosure
The author reports no conflicts of interest in this work.
References
1. World Health Organization. Listeriosis; 2018. Availble from: https://www.who.int/news-room/fact-sheets/detail/listeriosis.
2. Cheng C, Sun J, Yu H, et al. Listeriolysin O Pore-Forming Activity Is Required for ERK1/2 Phosphorylation During Listeria monocytogenes Infection. Front Immunol. 2020;11:1146. doi:10.3389/fimmu.2020.01146
3. Barbuddhe SB, Malik SVS, Bhilegaonkar KN, Prahlad K, Gupta LK. Isolation of Listeria monocytogenes and anti-listeriolysin O detection in sheep and goats. Small Ruminant Res. 2000;38(2):151–155. doi:10.1016/S0921-4488(00)00155-3
4. Chlebicz A, Sli ́zewska K. Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: a review. Int J Environ Res Public Health. 2018;15(5):1–28. doi:10.3390/ijerph15050863
5. Pagliano P, Ascione T, Boccia G, Decaro F, Esposito S. Listeria monocytogenes meningitis in the elderly epidemiological clinical and therapeutic findings. Infez Med. 2017;24:105–111.
6. Low JC, Donachie W. A review of Listeria monocytogenes and listeriosis. Vet j. 1997;153(1):9–29. doi:10.1016/s1090-0233(97)80005-6
7. Molla B, Yilma R, Alemayehu D. Listeria monocytogenes and other Listeria species in retail meat and milk products in Addis Ababa Ethiopia. J Health Develop. 2004;18:131–212.
8. Sagni D, Habtamu T. Systematic review and meta-analysis of animal listeriosis in Ethiopia. Int J Veter Sci Res. 2021;7(1):029–032. doi:10.17352/ijvsr.000077
9. Diriba K, Awulachew E, Diribsa K. The prevalence of Listeria species in different food items of animal and plant origin in Ethiopia a systematic review and meta-analysis. Eur J Med Res. 2021;26(1):60. doi:10.1186/s40001-021-00532-8
10. Yilma Z. Quality factors that affect Ethiopian formal milk business experience from selected dairy potential areas. Netherlands Development Organization SNV; 2010.
11. Parihar VS, Barbuddhe SB, Danielsson-Tham ML, Tham W. Isolation and characterization of Listeria species from tropical seafood. Food Control. 2008;19(6):566–569. doi:10.1016/j.foodcont.2007.06.009
12. Wang J, King JE, Goldrick M, Lowe M, Gertler FB, Roberts IS. Lamellipodin Is Important for Cell-to-Cell Spread and Actin-Based Motility in Listeria monocytogenes. Infect Immun. 2015;83(9):3740–3748. doi:10.1128/IAI.00193-15
13. Radostits OM, Gay CC, Hinchcliff WK, Constable DP. Veterinary Medicine: A Text Book of the Diseases of Cattle, Sheep, Pigs, Goats and Horses.
14. Enkeshe L, Zenebech K. A review on listeria monocytogenes in food of animal origin. Int J Adv Res Biol Sci. 2019;6:10.
15. Tchatchouang CK, Fourie J, Mazel DS, et al. Listeriosis outbreak in South Africa. Microorganisms. 2020;8(1):135. doi:10.3390/microorganisms8010135
16. Sagni D. Epidemiology of Listerosis in Animal and Human in Ethiopia. Int J Veter Sci Res. 2020;6(2):154–158. doi:10.17352/ijvsr.000067
17. Wei X, Hassen A, McWilliams K, et al. Genomic characterization of Listeria monocytogenes and Listeria innocua isolated from milk and dairy samples in Ethiopia. BMC Genomic Data. 2024;25(1):12. doi:10.1186/s12863-024-01195-0
18. Gebretsadik S, Kassa T, Alemayehu H, Huruy K, Kebede N. Isolation and characterization of Listeria monocytogenes and other Listeria species in foods of animal origin in Addis Ababa, Ethiopia. J Infect Public Health. 2011;4(1):22–29. doi:10.1016/j.jiph.2010.10.002
19. Mengesha D, Zewde BM, Toquin MT, Kleer J, Hildebrandt G, Gebreyes WA. Occurrence and distribution of Listeria monocytogenes and other Listeria species in ready-to-eat and raw meat products. Berl Munch Tierarztl Wochenschr. 2009;122(1–2):20–24.
20. Ahimed HM, Hiko A, Abdellah A. Isolation and multidrug drug resistance profile of Listeria species in selected Dairy Farm’s Operational stages in Oromia Regional State. Ethiopia Sci Af. 2022;16. doi:10.1016/j.sciaf.2022.e01167
21. Teshome Y, Giragn F, Gudeta D, Desa G, Bekele D. Isolation and prevalence of listeria species in milk and milk product samples collected from bishoftu and Dukem towns, Oromia. Ethiopia World J Dairy Food Sci. 2019;14(2):196–201.
22. Hiwot D, Savoinni G, Cattaneo D, et al. Bacteriological quality of milk in raw bovine bulk milk in the selected milk collection centers: smallholder dairy processing Ethiopia; 2016. Available from: https://bit.ly/2IrUO80.
23. Muhammed W, Diriba M, Yoseph D, Abebaw G, Bitew M. Studies on occurrence of listeria monocytogenes and other species in milk and milk products in retail Market of Jimma Town, Ethiopia. Asian J Dairy Food Res. 2013;32:35–39.
24. Seyoum ET, Woldetsadik DA, Mekonen TK, Gezahegn HA, Gebreyes WA. Prevalence of Listeria monocytogenes in raw bovine milk and milk products from central highlands of Ethiopia. J Infect Dev Ctries. 2015;9(11):1204–1209. doi:10.3855/jidc.6211
25. Mulu S, Pal M. The prevalence risk factors public health implication and antibiogram of listeria monocytogenes in sheep meat collected from municipal abattoir and butcher shops In Addis Ababa. J Foodbor Zoonot Dis. 2016;4(1):01–14.
26. Welekidan LN, Bahta YW, Teklehaimanot MG, et al. Prevalence and drug resistance pattern of Listeria monocytogenes among pregnant women in Tigray region, Northern Ethiopia: across-sectional study. BMC Res Notes. 2019;12(1):538. doi:10.1186/s13104-019-4566-36
27. Girma Y, Abebe B. Isolation, identification and antimicrobial susceptibility of listeria species from raw bovine milk in Debre-Birhan Town, Ethiopia. J Zoonotic Dis Public Health. 2018;2(1):4.
28. Gebremedhin EZ, Hirpa G, Borana BM, et al. Listeria species occurrence and associated factors and antibiogram of listeria monocytogenes in beef at abattoirs, butchers, and restaurants in ambo and Holeta in Ethiopia. Infect Drug Resist. 2021;14:1493–1504. doi:10.2147/IDR.S304871
29. Girma L, Geteneh A, Amenu D, Kassa T. Isolation and characterization of Listeria monocytogenes among women attending Jimma University medical center, Southwest Ethiopia. BMC Infect Dis. 2021;21(1):1–6. doi:10.1186/s12879-021-06254-w
30. Borena BM, Dilgasa L, Gebremedhin EZ, et al. 2022 listeria species occurrence and associated risk factors and antibiogram of listeria monocytogenes in milk and milk products in Ambo, Holeta, and Bako Towns, Oromia Regional State, Ethiopia. Vet Med Int. 2022;2022:5643478. doi:10.1155/2022/5643478
31. Garedew L, Taddese A, Biru T. Prevalence and antimicrobial susceptibility profile of Listeria species from ready-to-eat foods of animal origin in Gondar Town Ethiopia. BMC Microbiol. 2015;15(1):100. doi:10.1186/s12866-015-0434-4
32. Hiko A, Asrat D, Zewde G. Occurrence of Listeria monocytogenes in retail raw meat products in Ethiopia. J Infect Public Health. 2008;1(3):189–195. doi:10.1016/j.jiph.2008.09.003
33. Derra FA, Karlsmose S, Monga DP, et al. Occurrence of Listeria spp. in retail meat and dairy products in the area of Addis Ababa, Ethiopia. Foodborne Pathog Dis. 2013;10(6):577–579. doi:10.1089/fpd.2012.1361
34. Kebede A, Tassew H, Habebo F, Belina D. Prevalence and antimicrobial resistance profile of Listeria monocytogenes isolated from ready-to-eat foods of animal origin in Gondar town, Ethiopia. African J Microbiol Res. 2014;8(21):2186–2192. doi:10.5897/AJMR2013.6529
35. Ghidey Z, Habte-Gabr E. Isolation and identification of Listeria species from Awash milk and Domiat cheese in Ethiopia. Ethiopian Med J. 1989;27(1):17–21.
36. Pinner RW, Schuchat A, Swaminathan B, et al. Role of foods in sporadic listeriosis. II. Microbiologic and epidemiologic investigation. JAMA. 1992;267(15):2046–2050. doi:10.1001/jama.1992.03480150062034
37. Hawaz H, Taye M, Muleta D. Characterization and Antimicrobial Susceptibility Patterns of Listeria monocytogenes from Raw Cow Milk in the Southern Part of Ethiopia. Hindawi J Food Qual. 2023;6:11. doi:10.1155/2023/5590136
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
