Back to Journals » Integrated Blood Pressure Control » Volume 16
Predictors of Tuberculosis and Non-Communicable Disease Comorbidities Among Newly Enrolled Tuberculosis Patients, Southern Ethiopia
Authors Nunemo MH , Gidebo KD , Woticha EW , Lemu YK
Received 25 August 2023
Accepted for publication 3 November 2023
Published 22 November 2023 Volume 2023:16 Pages 95—109
DOI https://doi.org/10.2147/IBPC.S432251
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Turgay Celik
Mengistu Handiso Nunemo,1 Kassa Daka Gidebo,2 Eskinder Wolka Woticha,2 Yohannes Kebede Lemu3
1Department of Public Health, Wachemo University, Hossana, Central Region, Ethiopia; 2Department of Public Health, Wolaita Sodo University, Wolaita, South Region, Ethiopia; 3Department of Health, Behaviour and Society, Jimma University, Jimma, Oromia Region, Ethiopia
Correspondence: Mengistu Handiso Nunemo, Department of Public Health, Wachemo University, Po.Box: 667, Hosanna, Centeral Region, Ethiopia, Tel + 251 916357401, Email [email protected]
Introduction: Non-communicable diseases are comorbid with tuberculosis, however only a few record review based studies have been conducted, which are more concentrated on elevated glucose levels. This study aimed to assess non-communicable disease comorbidity and its predictors among tuberculosis patients.
Methods: A prospective cross-sectional study design was used and the data were collected by a previously validated tool from a sample of 443 tuberculosis patients using cluster random sampling methods. Multinomial logistic regression was interpreted by relative risk to predict the association of comorbidity status with independent variables.
Results: The majority (87.81%) of TB patients were not comorbid with NCDs. The prevalence of hypertension and diabetes mellitus among tuberculosis patients were 6.55%, and 5.64%, respectively. The people who had a risk score > 8 were 6.47 times more likely to have tuberculosis comorbid with one non-communicable disease compared to those with a risk score ≤ 8. The relative risk of tuberculosis patients with BMI > 25 is 3.33 times compared to those with a BMI < 23 of being comorbid with one non-communicable disease vs tuberculosis patients without non-communicable diseases. Those tuberculosis patients with an awareness of non-communicable disease comorbidities are 9.33 times more likely to have tuberculosis with multi-comorbidities compared to those who are unaware.
Conclusion: The majority of TB patients were not comorbid with NCDs. The person’s weight, family size of more than five, monthly income > 3000 birr, risk score > 8 and BMI > 25 significantly predict comorbidity with one non-communicable disease compared to those without a comorbidity. The presence of non-communicable disease comorbidity, treatment awareness, and being aged 50+ years significantly predict the presence of multi-comorbidities compared to those without comorbidity. For early detection and management of both diseases, establishing bidirectional screening platforms in tuberculosis care programs is urgently required.
Plain Language Summary: Non-communicable diseases are comorbid with tuberculosis, however, only a few record review based studies have been conducted, which are more concentrated on elevated glucose levels.
This is a former prospective cross-sectional study of non-communicable disease comorbidities and their predictors among tuberculosis patients using the two stages of the WHO step-wise screening procedure.
The majority (87.81%) of TB patients were not comorbid with NCDs, 7.22% were comorbid with one NCD and 4.97% were multi-comorbid. The prevalence of hypertension and diabetes mellitus among tuberculosis patients were 6.55%, and 5.64%, respectively. The person’s weight, family size of more than five, monthly income > 3000 birr, risk score > 8 and BMI > 25 significantly predict the comorbidity with one non-communicable disease related to those without comorbidity. The presence of non-communicable disease comorbidity, treatment awareness, and being aged 50+ years significantly predicted the presence of multi-comorbidity compared to those without comorbidity. For early detection and management of both diseases, establishing bidirectional screening platforms in tuberculosis care programs is urgently required.
Keywords: comorbidity, non-communicable disease, tuberculosis, prevalence, predictors
Introduction
Tuberculosis disease (TB) is a major public health problem and tops the list of causes of death in the world.1 95% of tuberculosis deaths occur in low-middle-income countries (LMICs), with two-thirds of deaths happening in 8 LMICs and Ethiopia is one of the top 22 counties affected by tuberculosis (TB)2 with a 2.1/1000 incidence and 2.0/1000 prevalence rate.3
The most common comorbid non-infectious diseases with TB are diabetes mellitus (DM), hypertension, heart diseases, chronic obstructive pulmonary diseases (COPD), and cancer.4 However, TB and non-infectious diseases share many essential socio-economic and socio-demographic determinants.5–8 Moreover, concomitantly tuberculosis and non-communicable diseases (NCDs) increase the occurrence or consequence of each other.9
The common and shared risk factors for both diseases are cigarette smoking, alcohol drinking, physical inactivity, and inadequate fruit and vegetable consumption.10–12 In Ethiopia, the estimated number of TB cases attributed to common TB risk factors are malnutrition, alcohol consumption, and smoking.13 Comorbid patients are not simple to diagnose and manage on a timely basis, contributing to TB transmission.14
In India, the prevalence of hypertension was 7%, DM at 8%, alcohol use at 34%, and use of smokeless tobacco at 33% were screened from routine tuberculosis patients.15 A total of 9,651 patients with TB were identified in China, of whom approximately 61.4% had no chronic conditions, 17.4% had 1 chronic condition, and 21.3% had ≥2 chronic conditions.16
In South Africa, The prevalence of NCDs, included alcohol-use disorder (24.3%), daily tobacco use (15.0%), hypertension (8.9%), ischemic heart disease or angina (7.5%), arthritis (4.5%), type 2 diabetes (4.1%), asthma (3.5%), cancer or malignant neoplasms (2.1%), chronic lung disease (1.9%) and dyslipidemia (1.6%). The overall comorbidity (with one NCD) was 26.9% and multi-morbidity (with two or more NCDs) was 20.9%.17 The prevalence of DM among TB in the Amhara region of Ethiopia was 8.3%.18
Despite the early detection of NCDs from TB patients being highly recommended by the Global Action Plan of NCDs,19,20 WHO14 and SDG,21 few studies have reported the non-infectious disease comorbidity status among TB patients, most of which are based on record reviews and concentrated mostly on elevated glucose levels.22–24 However, the current study aimed to determine the prevalence of common non-infectious diseases comorbidity with tuberculosis and its predictors using the two stages of the World Health Organization (WHO)-stepwise screening procedure, which is not well known. Conducting this study is crucial for policymakers and program managers to establish an early detection platform of NCDs in TB care programs.
Methods and Materials
This study was carried out at the Hadiya Zone tuberculosis treatment centre from January 2022 to April 2023 for three months at 3 district hospitals, 1 comprehensive referral hospital, and 3 health centres, which were selected by eligibility criteria from 4 hospitals and 61 health centres. The Hadiya Zone Administration is one of the 14 South Nation Nationalities People Representative States (SNNPRS). Hosanna Town is the capital town which is located on the main road from Addis Ababa to Hosanna just 192 km from the capital city of Ethiopia. The administration of the Hadiya Zone has 7 urban towns administered with 29 kebeles and 303 rural kebeles within 10 rural districts. The zone has more than 1.7 million population of whom 848,695 are males and 857,225 are females.
The Zone was to detect and treat tuberculosis patients based on WHO guidelines, in 2020 the total number of pulmonary culture-positive TB cases was 781, and pulmonary culture-negative TB cases was 579, extra pulmonary TB 270, and the total of all forms of TB was 1630. The TB detection rate for all forms is 192/100,000. Currently, the Zone has a new initiative chronic care program which was started in four selected hospitals and three health centres. In 2021, of 73,333 total individuals screened for hypertension, 397 new patients enrolled for hypertension care.
Study Design and Population
A prospective cross-sectional study design was used, to determine the prevalence of the common non-infectious disease comorbidity and its predictors among adult TB patients aged ≥20 years by applying the two stages of the WHO Stepwise screening procedure.
The study population were all sampled tuberculosis patients in the selected study health facility, and they were enrolled on the DOT program from the Hadiya Zone catchment, However, those unable to give informed consent, pregnant women, the severely ill, those comorbid with HIV, MDR-TB, those who transfered out from the catchment and those referred for further investigation were excluded from the study.
Study Variable
TB-NCD comorbidity status (not comorbid, comorbid and multi-comorbid were categorized as multinomial dependent variables). Behavioural factors (overweight/obesity (BMI, waist circumference), smoking, inadequate physical exercise, fruit and vegetable consumption, and alcohol drinking), socio-demography factors, TB clinical variables, and awareness of NCD comorbidities and their treatment were independent variables.
Sample Size and Sampling Procedure
The required sample size was determined by using a single population proportion formula (n) = [z2 *p (1-p)]/w2 and considering a 95% confidence interval (CI) and 5% significance level. Due to the absence of similar research, we used 50% for the TB patients’ comorbid with common NCDs. Based on these assumptions, the expected sample size was 403. By adding 10% of the total sample size for the nonresponse rate (NR) which was 40 participants, 443 TB patients were screened for common non-infectious diseases.
A single-stage cluster random sampling was used. Initially, Hadiya Zone health facilities were identified as a sample cluster, based on both NCDs and TB continuous detection, diagnosis and treatment services availability criteria. By using this eligibility criterion 3 health centres, 3 general hospitals and 1 comprehensive referral hospital were selected from 4 hospitals and 61 health centres. Due to a small sample size availability in an eligible health facility, we included all TB patients who started the treatment and enrolled on the DOT program in the study clusters during the study period. The study participants were enrolled by consecutively simple random sampling until the final sample size adequacy (Figure 1).
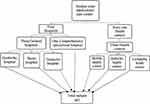 |
Figure 1 Cluster sampling schematic presentation of Hadiya Zone tuberculosis care centre. |
Data Collection Methods and Techniques
After the study cluster site and a total number of TB patients were identified, NCDs and risk factors were screened by the WHO Step-wise screening procedure. This tool was formerly validated and tested in different health settings.25,26
The data were collected by applying the two-stage WHO Step-wise screening procedure, in the first stage all study participants were screened for behavioural risk factors using nine (9) scaling items with a score of 20. If the score was more than 8 of the 20 total score, the participants were moved to the second stage. In the second stage, biological risk factors were screened and if the participants had a score more than 12 over the two stages they were linked to the chronic clinic for further investigation of diabetes mellitus. However, for those tuberculosis patients who scored less than or equal to 12 an awareness of NCD prevention and control was given. All physical assessments were conducted in a private area and they measured and determined its’ cut-off points in a standardized manner. From each of seven (7) health facilities at least one senior TB focal, who had at least six months of work experience, was recruited and trained for five (5) days on the study screening procedure before the commencement of the study and they were allocated to the respective study site. The data were collected for three months from January 5, 2022 up to April 7, 2023.
Operational Definitions of Common NCDs and Risk Factors
Comorbidity status: defined as absence of any common NCDs (not comorbid), presence of one common NCD (comorbid) and presence of at least two NCDs (multi-comorbidity).
Shared risk factors were overweight/obesity and smoking, inadequate physical exercise, fruit and vegetable consumption, and alcohol drinking.27
Overweight and obesity were measured by standard methods for body mass index (BMI) and waist circumference based on National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) recommendations.28
Adequate consumption of fruit and vegetables were measured by using the previous study standards.29 Alcohol drinking, physical inactivity and smoking were also assessed using the former study categorizations,30 similarly hypertension31 and DM.15
Data Management and Analysis Plan
The data were analyzed by STATA v.14 and descriptive statistics of the proportion, the average, the reference value, and the median were presented by tables and figures. A multinomial logistic regression model was developed to identify the relative risk ratio (RRR) of multinomial outcome variables on tuberculosis NCD comorbidity status (0 = not comorbid, 1 = comorbid with one NCD, 2 = comorbid with at least two NCDs). And candidate variables for multiple multinomial logistic regressions were identified by crude RRR association at PV<0.05 at 95% CI. Test command was used to see the overall effect, significant on outcomes and margins command was used to calculate the predicted probability of choosing each outcome at each level of independent variable, holding all other variables in the model at their means. The margins plot and graph combined command were used to plot the expected probabilities by independent variable for each output variable category. Basically, before statistical model development their assumptions, model fitness and model comparing were made and the result was summarized and displayed using tables and figures.
Data Quality Assurance
A standard structured questionnaire was used, which was translated, trained with for 5 days and pretested on 22 participants in the Gabo health centre. The collected data were verified by the daily supervisor to ensure that it was complete and modified and then cleaned up before analysis. Before analysis, the data were explored to see the data distribution and checked for missing data.
Result
Sociodemographic Characteristics
Among a total of 443 participants with a 100% response rate, 234 (52.82%) were male, 265 (59.82%) were in the age range of 20–34, 214 (48.31%) lived in rural dwellings, and almost half (236, 53.27%) the respondent's monthly income was ≤1000 Ethiopian birr (ETB). The majority (270, 60.95%) were married, 122 (27.54%) in occupation were students and 150 (33.86%) were illiterate (could neither read or write) (Table 1).
 |
Table 1 Socio-Demographic Characteristics of Participants in Implementing NCD and Risk Factor Screening Intervention in Southern Ethiopia |
Two Stage NCDs and Risk Factor Screening Intervention on TB Patients
Out of 443 participants who were screened by using a two-stage WHO step-wise procedure, 53 (11.96%) had a score >8 and were recruited to stage two of the screening. Among the total who were screed in the second stage (53), 17 (32.08%) had a score of more than 12, and 16 (94.12%) were linked to NCDs. From the total linked patients, the majority (87.5%) started the treatment (Figure 2).
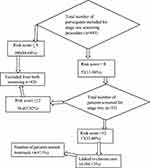 |
Figure 2 Classic flow chart diagram of the two stages of WHO step-wise NCDs and risk factor screening procedure from routine tuberculosis patients in Hadiya Zone, southern Ethiopia. |
NCDs and Risk Factor Awareness Among Tuberculosis Patients
Among the total participants (443), the total mean time delay for treatment was (60.05 days), of this health-seeking delay was 50.67 ± 3.15 days, treatment initiation delay was 16.38 ± 2.52 days. The majority 290 (65.46%) were pulmonary tuberculosis and 279 (62.98%) and 245 (55.30%) had an awareness of the common NCDs and risk factors, respectively. The majority of participants, 328 (74.04%) and 327 (73.81%), did not have awareness about stroke and COPD, respectively. Concerning the comorbidity, 287 (64.79%), and 248 (55.98) did not have awareness of tuberculosis comorbidity with NCDs and risk factors, respectively (Table 2).
 |
Table 2 Distribution of Tuberculosis Patient’s Awareness Towards NCDs and Risk Factors in Southern Ethiopia |
Prevalence of Risk Factors Among Tuberculosis Patients
Among the total (443) screened by the WHO Step-wise screening procedure, the prevalence of smokers was 39 (8.80%), and we are 95% confident the true population prevalence will fall between 6.5% and 11.8%. Concerning alcohol use, 47 (10.61%) were alcohol drinkers and the true population prevalence will fall within a 95% confidence interval of 8.1% and 13.9%. The majority of participants consumed inadequate fruit 391 (88.26%), 95% CI [0.849, 0.910] and inadequate vegetables 368 (83.07%), 95% CI [0.793, 0.863], had BMIs <23,330 (74.49%) we are 95% confident the true population prevalence of BMI <23 will fall within 70% and 78% (Table 3).
 |
Table 3 Prevalence of Risk Factors Among Routine Tuberculosis Patients, in Southern Ethiopia |
Prevalence of Common NCDs Among Tuberculosis Patients
The majority (389, 87.81%) of TB patients were not comorbid with NCDs, but 32 (7.22%) and 22 (4.97%) of TB patients were comorbid with one NCD and ≥2 NCDs (multi-comorbidity), respectively. The prevalence of DM was 25 (5.64%), and we are 95% confident the true population prevalence will be found within 3.8% and 8.2% intervals. The prevalence of hypertension among tuberculosis patients was 29 (6.55%), in which the population prevalence will be found between 4.6% and 9.3% intervals, we are 95% confident (Table 4).
 |
Table 4 Prevalence of Common NCDs Among Routine Tuberculosis Patients, in Southern Ethiopia |
Predictors of Comorbid NCDs and Risk Factors with Tuberculosis
At the beginning, we checked the association between 30 explanatory variables with response variables in bivariate multinomial logistic regression, half (15) the variables showed a significant association with outcome variables, namely, income category, family size category, sex category, knowledge of common NCDs, the weight of patients, awareness of risk factors, awareness of comorbidity and awareness of the presence of NCD treatments, risk scores of >8 and >12, alcohol use, physically inactivity, waist circumference, BMI, and age category. From fifteen variables, only eight variables were significantly associated with outcome variables in multiple multinomial logistic regression, and risk score >8, family size, monthly income, BMI, and weight were independent variables that significantly predicted whether the person fell into the TB comorbid with one NCD category (comparing group) versus the not comorbid tuberculosis group (baseline). The remaining variables, age category, awareness of NCD comorbidity and NCD treatment, were the independent variables that significantly predict whether the person falls into the TB with multi-comorbid NCDs category (comparing group) versus TB without an NCD comorbidity (baseline).
The overall model is statistically significant (chi-square = 105.63, p=<.001), and based on McFadden’s, we might say that the full model containing our predictors represents 26% (pseudo-R-square), improving in fit relative to the null model. We used the test command to check whether one of the independent variables predicted equally or not the outcome variable, and all predictors PV<0.05, and the test showed that the effects were statistically different from each other.
The relative risk ratio (RRR) for weight indicates that for each one-unit increase in weight, the relative risk of being in the TB comorbid with one NCD related to the risk of belonging to the TB comorbid with one NCD changed by a factor of 1.06. This means that as weight increases, the risk of falling into the TB comorbid with one NCD group is predicted to increase while the risk of falling into TB without a comorbid group is expected to decrease ([95% CI, ARR (1.01, 1.11)].
A family size >five is 2.81 times more likely to fall into tuberculosis comorbid with one NCD in relation to not being comorbid with NCDs, compared to a family size <five. 95% CI, ARR 2.81 (1.05, 7.60)].
Subsequently, the monthly income variable was treated as three categories, the higher incomes category was compared to the lower income category (less than or equal to 1001; baseline category). Although the two dummy variables were positive, only the last dummy variable was positively significantly associated (PV = 0.050). Those participants with monthly income >3000 birr are 3.12 times more likely to fall into tuberculosis comorbid with one NCD compared to TB without being comorbid with NCDs, compared to those with monthly income <1000 ETB.
The relative risk of tuberculosis patients with BMI >25 is 3.33 times that of BMI <23 to being TB comorbid with one NCD vs being TB without being comorbid with NCDs. [95% CI, ARR 3.33 (1.12, 9.90)].
In comparison to having TB not comorbid with NCDs, a risk score >8 is 6.47 times more likely to have TB comorbid with one NCD compared to a risk score <8. [95% CI, ARR 6.47 (1.50, 27.97)].
In comparison to not having an awareness of TB NCDs comorbidity, the presence of awareness of TB-NCDs comorbidity is 9.33 times more likely to have tuberculosis with multi-comorbidity compared to tuberculosis not comorbid with NCDs. [95% CI, ARR 9.33 (2.02, 43.17)]. Similarly, the presence of awareness of NCD treatment is 52.32 times more likely to fall to tuberculosis with multi-comorbidity compared to tuberculosis without comorbidity. [95% CI, ARR 52.32 (1.890, 175.0)]
Since the age variable was treated as categorical, the two older categories were compared with the youngest category (20–34; baseline category). Although from the two dummy variables, only the last dummy variable was positively significantly associated (PV = 0.031). Persons aged 50+ are at a 22.13 times greater risk than the 20–34 years aged category of falling into the TB with multi-comorbid NCDs compared to TB comorbid without NCDs [95% CI, ARR 22.13 (3.46, 141.41)] (Table 5).
 |
Table 5 Predictors of Non-Communicable Disease Comorbidity Status with Tuberculosis Disease in Southern Ethiopia, Hadiya Zone |
Discussion
Our study revealed that 87.81% of TB patients were not comorbid with NCDs, 7.22% were comorbid with one NCD and 4.97% multi-comorbid. This prevalence is less than that from South Africa (26.9%),17 Indonesia (35.5%)32 and the Philippines (40%).33 This difference might be due to a sample size difference, our study sample size is 443, but the South Africa sample size was 4207, and because of non-communicable disease comorbidity assessment methods variations.
In this study, out of the total screened, 11.96% had a risk score of more than 8 of which 32.08% had a risk score of more than 12, 94.12% had links to NCD clinics, the majority (87.5%) had started the treatment. This result is higher and contradicts the study conducted in Delhi, India (82% reached the clinic, and 83% started the treatment).15 This might be, because of the difference in the study setting and sample size, in India 403 sample size and two DOT centres, but in our study 443 sample size and seven DOT centres.
In the current study, the prevalence of diabetes mellitus and elevated blood pressure among tuberculosis patients were 5.64% and 6.55%, respectively. This finding varies from the studies conducted in India,15 the hypertension prevalence is 7% and 8% for DM, and is lower than Amhara, Ethiopia (8.3%)18 and Nigeria (12%).34 The variation might be because of different study populations and sample sizes, in Nigeria 4000 persons aged above 12 years were included. However, it is similar to the study conducted in Luanda, Angola,22 where the diabetes mellitus prevalence among tuberculosis patients is 6%, this might be due to having a similar number of DOT centres and an equal sample size.
In a study in Luanda, Angola, the prevalence of hypertension among newly diagnosed TB patients was 19.6%22 and in northern Angola it was 23%,35 it contradicts our finding and might be different due to the study design (community-based) and large sample size (1464 adults).
Our study showed that, among the total TB patients, the prevalence of smokers 8.80%, alcohol drinkers 10.61%, consuming inadequate fruit (88.26%), and vegetables (83.07%) and BMI >25 /obesity (9.71%), which is lower and contradicted with other setting studies. In Semnan City, cigarette smokers were 16.9% and consumed alcohol 1.3%,6 in Nepal tobacco use was 23.7%, alcohol 19.1%, obesity 24.8%36 and in Delhi, India, inadequate vegetables consumed by 80% and inadequate fruits consumed were 72%.15 This variation might be due to the difference in risk factor measurement techniques, follow-up time and enrollment strategies of TB patients for the study, for example, other studies enrol new TB patients from outpatient departments, but for our study, we selected TB patients from routine care.
The patients who had a risk score >8 are at 6.47 times higher risk for TB being comorbid with one NCD compared to not comorbid, compared to risk score ≤8. They might be using alcohol, smoking or other risk factors which can expose TB patients to developing NCDs and the majority of our participants consume inadequate fruit (88.26%) and inadequate vegetables (83.07%). This finding is similar to the Gabon, predictors of comorbidity of elevated blood pressure with TB,37 and the Delhi India study, among TB patients the major risk factors are inadequate vegetable and fruit consumption.15
Those who had a family size greater than 5 are at a 2.81 times greater risk for TB comorbid with one NCD, compared to not comorbid with NCDs, and in comparison with a monthly income ≤1000 ETB, those persons who had a monthly income of more than 3000 ETB are at a 3.12 times high risk of having TB comorbid with one NCD related to without a comorbidith. This might be due to the presence of social and lifestyle stress with a large family and the person having more money might be exposed to luxurious activity like alcohol drinking, cigarette smoking and using more spicy food being at risk of NCDs. It is similar to the report by the WHO,38 determinants of tuberculosis incidence rate are low income, disparity and food deficiency, living style, crowded environment and ageing.
Those tuberculosis patients’ increase of one unit in weight is positively predicted with a 0.08 increase in the relative log odds of being TB comorbid with one NCD compared to without NCDs. Similarly, those persons who had a BMI of 25+ are 3.33 times more likely at risk for TB comorbid with one NCD opposed to without NCDs, compared to a BMI <23 (baseline). This may be because increasing uncontrolled body weight can increase risk factors for TB patients to be affected by NCDs. This report is in line with the Luanda, Angola study report, the risk factors of elevated blood pressure were old age and obesity or overweight.22,37
On the other hand, those TB patients aged >50 years are 22.13 times more likely comorbid with multi-comorbidities related to without comorbid, compared to those aged 20–34 years. The reason might be as age increases, the immunity status of the patients decreases, due to this TB patients in the long run can develop NCDs. This finding is similar to predictors of non-communicable disease multi-morbidity with tuberculosis including age.22,39
Those TB patients who had awareness of NCD comorbidity were 9.33 times more likely to fall into TB with multi-comorbidity category compared to those without comorbidity, related to having no awareness of comorbidity. This might be due to the health workers’ education and counselling of the comorbid TB-NCD patients on comorbidity, treatment and self-management, however, those not comorbid TB patients mightn’t be aware of NCDs. The awareness of TB and NCDs comorbidity can be given by health workers, due to this those TB patients comorbid with NCDs, have more awareness of it. This means only comorbid patients have adequate awareness of NCDs, but those not comorbid TB patients may not have adequate awareness. This finding was supported in Delhi, India,40 Australia, and the LMIC review study,41–43 for most integration efforts, a broader set of clinical skills may be required, and training in healthcare workers is an important facilitator to increase consciousness of the worth of integrating specific programs, and for building working proficiency, self-confidence, and working drive.
Limitation
The positive aspects of the study wwere that we collected data by standard measurement tools prospectively through interviewing TB patients and screening non-communicable diseases so that the validity of the data is better than record review-based data. However, we included only routine TB patients in the DOTs centres for screening TB patients. Suspected TB in the outpatient department was not included and we screened most risk factors by self–report, which might introduce biases.
Conclusion
In general, the majority of tuberculosis patients were not comorbid with NCDs. The person’s weight, family size of more than five, monthly income >3000 birr, risk score >8 and BMI >25 significantly predict the comorbidity with one non-communicable disease in relation to those without a comorbidity. The presence of non-communicable disease comorbidity and treatment awareness and being aged 50+ years significantly predict NCD multi-comorbidity compared to those without a comorbidity. For early detection and management of both diseases, establishing bidirectional screening platforms in tuberculosis care programs is urgently required.
Abbreviations
NCDs, non-communicable diseases; TB, tuberculosis; DM, diabetes mellitus; CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease; HMIS health management information system; ETB, Ethiopian birr; WHO, World Health Organization; HCW, healthcare worker; LMIC, low and middle-income country.
Data Sharing Statement
Datasets, interview and screening procedure tools, and participant informed sheets can be accessed through the primary author email online system based on reasonable justification.
Ethics Consideration
Ethical principles related to protecting human subjects in research were followed as outlined in the Declaration of Helsinki. Before the study was conducted, Wolaita Sodo University’s ethical review committee approved the study protocol (with approval number: Ref. no WSU/41/33/1356). Using this approval letter the research team communicated with the study site administration Hadiya Zone office to secure permission before starting the data collection. Informed verbal consent, which was witnessed and documented, was obtained from the participants after ensuring the confidentiality and rights of participants.
Acknowledgments
The authors acknowledge Wolaita Sodo University for overall coordination and financial support from preparation to finalizing the research project. Gratitude extends to Wachemo University and Hadiya Zone health office managers and coordinators for their partnership, and to the implementer and patients for providing pertinent information for this project achievement.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data; or in all these areas; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declared that they have no competing interests in this work.
References
1. World Health Organisation. WHO Consolidated Guidelines on Tuberculosis: Tuberculosis Preventive Treatment. World Health Organization; 2020.
2. ABABA A. Federal Democratic Republic of Ethiopia Ministry of Health CLTSH Verification and Certification Protocol. Federal Democratic Republic of Ethiopia Ministry of Health CLTSH Verification and Certification Protocol; 2012.
3. World Health Organisation, Staff WHO. Global Tuberculosis Report 2013. World Health Organization; 2013.
4. Wang Y, Wang J. Modelling and prediction of global non-communicable diseases. BMC Public Health. 2020;20(1):1–13. doi:10.1186/s12889-020-08890-4
5. Penaloza R, Navarro JI, Jolly PE, Junkins A, Seas C, Otero L. Health literacy and knowledge related to tuberculosis among outpatients at a referral hospital in Lima, Peru. Res Rep Trop Med. 2019;Volume 10:1–10. doi:10.2147/RRTM.S189201
6. Tilahun D, Abera A, Nemera G. Communicative health literacy in patients with non-communicable diseases in Ethiopia: a cross-sectional study. Trop Med Health. 2021;49(1):1–9. doi:10.1186/s41182-021-00345-9
7. Hargreaves JR, Boccia D, Evans CA, Adato M, Petticrew M, Porter JD. The social determinants of tuberculosis: from evidence to action. Am J Public Health. 2011;101(4):654–662. doi:10.2105/AJPH.2010.199505
8. Marmot M, Bell R. Social determinants and non-communicable diseases: time for integrated action. BMJ. 2019;364. doi:10.1136/bmj.l251
9. Marais BJ, Lönnroth K, Lawn SD, et al. Tuberculosis comorbidity with communicable and non-communicable diseases: integrating health services and control efforts. Lancet Infect Dis. 2013;13(5):436–448. doi:10.1016/S1473-3099(13)70015-X
10. Begum N, Nawshin I, Rahman MA. Behavioural risk factors of non-communicable diseases among rural population in a selected area of Dhaka City. J Curr Adv Med Res. 2022;9(1):9–15. doi:10.3329/jcamr.v9i1.59738
11. World Health Organisation. Noncommunicable diseases country profiles 2018; 2018.
12. Esmailnasab N, Moradi G, Delaveri A. Risk factors of non-communicable diseases and metabolic syndrome. Iran J Public Health. 2012;41(7):77.
13. World Health Organisation. Global tuberculosis report 2020: executive summary; 2020.
14. Puchner KP, Rodriguez-Fernandez R, Oliver M, Solomos Z. Non-communicable diseases and tuberculosis: anticipating the impending global storm. Glob Public Health. 2019;14(9):1372–1381. doi:10.1080/17441692.2019.1580760
15. Anand T, Kishore J, Isaakidis P, et al. Integrating screening for non-communicable diseases and their risk factors in routine tuberculosis care in Delhi, India: a mixed-methods study. PLoS One. 2018;13(8):e0202256. doi:10.1371/journal.pone.0202256
16. Chen Q, Che Y, Xiao Y, et al. Impact of multimorbidity subgroups on the health care use and clinical outcomes of patients with tuberculosis: a population-based cohort analysis. Front Public Health. 2021;9:756717.
17. Peltzer K. Tuberculosis non-communicable disease comorbidity and multimorbidity in public primary care patients in South Africa. Afr J Prim Health Care Fam Med. 2018;10(1):1–6. doi:10.4102/phcfm.v10i1.1651
18. Workneh MH, Bjune GA, Yimer SA. Prevalence and associated factors of diabetes mellitus among tuberculosis patients in South-Eastern Amhara Region, Ethiopia: a cross sectional study. PLoS One. 2016;11(1):e0147621. doi:10.1371/journal.pone.0147621
19. Bates M, Marais BJ, Zumla A. Tuberculosis comorbidity with communicable and noncommunicable diseases. Cold Spring Harb Perspect Med. 2015;5(11):a017889. doi:10.1101/cshperspect.a017889
20. Rehm J, Samokhvalov AV, Neuman MG, et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health. 2009;9(1):1–12. doi:10.1186/1471-2458-9-450
21. Magee M, Narayan KV. Global confluence of infectious and non-communicable diseases—the case of type 2 diabetes. Prev Med. 2013;57(3):149–151. doi:10.1016/j.ypmed.2013.05.027
22. Segafredo G, Kapur A, Robbiati C, et al. Integrating TB and non-communicable diseases services: pilot experience of screening for diabetes and hypertension in patients with Tuberculosis in Luanda, Angola. PLoS One. 2019;14(7):e0218052. doi:10.1371/journal.pone.0218052
23. Lin Y-H, Chen C-P, Chen P-Y, et al. Screening for pulmonary tuberculosis in type 2 diabetes elderly: a cross-sectional study in a community hospital. BMC Public Health. 2015;15(1):1–8. doi:10.1186/1471-2458-15-3
24. Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9(12):737–746. doi:10.1016/S1473-3099(09)70282-8
25. Amarchand R, Krishnan A, Saraf DS, Mathur P, Shukla DK, Nath LM. Lessons for addressing noncommunicable diseases within a primary health-care system from the Ballabgarh project, India. WHO-SEAJPH. 2015;4(2):130–137. doi:10.4103/2224-3151.206682
26. Misra A, Chowbey P, Makkar B, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. Japi. 2009;57(2):163–170.
27. Reddy KS. Global burden of disease study 2015 provides GPS for global health 2030. The Lancet. 2016;388(10053):1448–1449. doi:10.1016/S0140-6736(16)31743-3
28. Dayspring T, Toh S-A, Sellers LA. National Cholesterol Education Program Adult Treatment Panel III NCEP ATP III. Fin Rep Circ. 2004;110(10):1219–1225. doi:10.1161/01.CIR.0000140676.88412.CF
29. Shah B, Mathur P. Surveillance of cardiovascular disease risk factors in India: the need & scope. Indian J Med Res. 2010;132(5):634. doi:10.4103/0971-5916.73420
30. Sharma U, Kishore J, Garg A, Anand T, Chakraborty M, Lali P. Dyslipidemia and associated risk factors in a resettlement colony of Delhi. J Clin Lipidol. 2013;7(6):653–660. doi:10.1016/j.jacl.2013.06.003
31. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. doi:10.1001/jama.2013.284427
32. Asturiningtyas IP, Mulyantoro DK, Kusrini I, Ashar H. Non-communicable disease comorbidity and multimorbidity among people with tuberculosis in Indonesia. Ann Trop Med Parasitol. 2021;24(01). doi:10.36295/ASRO.2021.24191
33. White LV, Edwards T, Lee N, et al. Patterns and predictors of co-morbidities in tuberculosis: a cross-sectional study in the Philippines. Sci Rep. 2020;10(1):1–12. doi:10.1038/s41598-020-60942-2
34. Ogbera AO, Kapur A, Abdur-Razzaq H, et al. Clinical profile of diabetes mellitus in tuberculosis. BMJ Open Diabetes Res Care. 2015;3(1):e000112. doi:10.1136/bmjdrc-2015-000112
35. Pires JE, Sebastião YV, Langa AJ, Nery SV. Hypertension in Northern Angola: prevalence, associated factors, awareness, treatment and control. BMC Public Health. 2013;13(1):1–10. doi:10.1186/1471-2458-13-90
36. Poudyal IP, Khanal P, Mishra SR, et al. Cardiometabolic risk factors among patients with tuberculosis attending tuberculosis treatment centers in Nepal. BMC Public Health. 2020;20(1):1–9. doi:10.1186/s12889-020-09472-0
37. Adegbite B, Edoa J, Abdul JAA, et al. Non-communicable disease co-morbidity and associated factors in tuberculosis patients: a cross-sectional study in Gabon. Eclinicalmedicine. 2022;45:101316. doi:10.1016/j.eclinm.2022.101316
38. Uplekar M, Weil D, Lonnroth K. WHO’s new End TB Strategy. Lancet 2015 World Health Organisation Global Tuberculosis report 2014; 2015.
39. Reis-Santos B, Gomes T, Macedo LR, Horta BL, Riley LW, Maciel EL. Prevalence and patterns of multimorbidity among tuberculosis patients in Brazil: a cross-sectional study. Int J Equity Health. 2013;12(1):61. doi:10.1186/1475-9276-12-61
40. Schmidt B, Campbell S, McDermott R. Community health workers as chronic care coordinators: evaluation of an Australian Indigenous primary health care program. Aust N Z J Public Health. 2016;40(Suppl 1):S107–14. doi:10.1111/1753-6405.12480
41. Fuller J, Koehne K, Verrall CC, Szabo N, Bollen C, Parker S. Building chronic disease management capacity in General Practice: the South Australian GP Plus Practice Nurse Initiative. Collegian J R Coll Nurs Aust. 2015;22(2):191–197. doi:10.1016/j.colegn.2014.02.002
42. Petersen I, van Rensburg A, Kigozi F, et al. Scaling up integrated primary mental health in six low- and middle-income countries: obstacles, synergies and implications for systems reform. BJPsych Open. 2019;5(5):e69. doi:10.1192/bjo.2019.7
43. Kia NS, Zavareh MN, Sarkheil E, Ghods E. Prevalence of biologic, behavioral and psychosocial determinant of tuberculosis in tuberculosis patients of Semnan city; a five-year cross-sectional study. J Prev Epidemiol 2020;5(2):e24–e. doi:10.34172/jpe.2020.24
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
