Back to Journals » Research and Reports in Urology » Volume 15
Predictors of Long-Term Urinary Incontinence After Robot–Assisted Laparoscopic Prostatectomy
Authors Yamashita K , Kijima Y , Sekido E, Nagasaka N, Inui M
Received 4 May 2023
Accepted for publication 16 August 2023
Published 21 August 2023 Volume 2023:15 Pages 387—393
DOI https://doi.org/10.2147/RRU.S419903
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Guglielmo Mantica
Kaori Yamashita, Yu Kijima, Eri Sekido, Naoki Nagasaka, Masashi Inui
Department of Urology, Tokyo Women’s Medical University Yachiyo Medical Center, Yachiyo-city, Japan
Correspondence: Kaori Yamashita, Department of Urology, Tokyo Women’s Medical University Yachiyo Medical Center, 477-96, Owadashinden, Yachiyo-shi, Chiba, 276-8524, Japan, Tel +81 474506000, Fax +81 474587047, Email [email protected]
Introduction: Urinary incontinence is a major complication after radical prostatectomy. We analyzed the predictors of urinary incontinence after robot-assisted radical prostatectomy.
Material and Methods: One hundred twenty-one patients, whose urinary continence status was evaluable at 3 months, 6 months, and 12 months after robot-assisted radical prostatectomy, were included from October 2016 to September 2021. Data were retrospectively collected from electronic medical records. The continence status was evaluated by interviewing the patients about the number of urinary pads used per day. We compared the patients’ age, body mass index, prostate volume, membranous urethral length on preoperative magnetic resonance imaging, surgeon experience, and pathological findings between patients with and without regained continence at 12 months after robot-assisted radical prostatectomy.
Results: The urinary continence rates were 30%, 57.8% and 79.3% at 3 months, 6 months, and 12 months, respectively, after robot-assisted radical prostatectomy. Twelve months after robot-assisted radical prostatectomy, 96 patients had regained continence and did not require urinary pads, whereas 25 patients had persistent urinary incontinence and required urinary pads. Membranous urethral length and surgeon experience were significantly different between patients with and without regained continence at 12 months after robot-assisted radical prostatectomy (P=0.05). However, no significant differences existed in age, body mass index, prostate volume, and pathological findings between patients with and without regained continence at 12 months after robot-assisted radical prostatectomy.
Conclusion: Membranous urethral length and surgeon experience are predictors of urinary incontinence after robot-assisted radical prostatectomy. Measuring the membranous urethral length is recommended before performing the operation.
Keywords: cancer, incontinence, magnetic resonance imaging, prostate, robotic surgery
Introduction
Prostate cancer was the second most common cancer in men worldwide in 2020, according to the World Health Organization website. Radical prostatectomy and radiation therapy for prostate cancer patients in stage 2 or stage 3 are common treatments, and each treatment has various complications. Urinary incontinence (UI) is a considerable and common complication after radical prostatectomy. In addition, male stress UI is iatrogenic after surgery. Men find the need to use urine pads troublesome; therefore, male patients may hesitate using pads and regret undergoing the surgery. Analyzing the predictors of UI after robot-assisted radical prostatectomy (RARP) may be useful, and some predictors of UI have been analyzed such as age,1–3 body mass index,4 overactive bladder,2 membranous urethral length (MUL),1,5–9 surgical experience and technique,10 and a positive margin in pathology.1,2 In this paper, we analyzed the predictors of UI after RARP and report the relationship between UI and MUL.
Materials and Methods
This study was conducted at Tokyo Women’s Medical University Yachiyo Medical Center (Yachiyo City, Chiba Prefecture, Japan). It was approved by the Institutional Review Board of Tokyo Women’s Medical University (registration no. 2022-0146). The patient’s informed consent to review the medical record was not required by the committee due to the retrospective of the study. The study and protocol were in accordance with the Declaration of Helsinki. Patient data were extracted and stored on a password-protected shared drive, which could only be accessed by the personnel named in this study; therefore, patient confidentiality is protected. Of the 193 male patients who underwent RARP from October 2016 to September 2021, 121 patients, whose continence status was evaluable at 3 months, 6 months, and 12 months after RARP, were included. We performed RARP, based on the technique described by Su et al in Campbell-Walsh-Wein Urology (12th edition):11 we used the da Vinci Xi system and we placed 6 trocars for a transperitoneal anterior approach. Nerve-sparing was not undertaken in the 121 patients, no surgeon utilized a method to improve the early return of urinary continence after RARP.12 The patients were interviewed about the number of pads they used daily for UI after RARP. The patient’s data were retrospectively collected from the electronic medical records. We compared age, body mass index, prostate volume, and MUL on magnetic resonance imaging (MRI) as the patient parameters; surgeon experience; and pathological findings (eg, tumor stage, extraprostatic extension, resection margin) between the group of patients who regained continence and did not require urinary pads, and the group of patients who had persistent UI and required urinary pads at 12 months after RARP.
MRI examinations were conducted using a 1.5-T or 3.0-T machine. MUL was estimated in the sagittal plane on T2-weighted images on preoperative MRI. MUL was defined as the distance from the apex of the prostate to the urethra at the level of the penile bulb.
With regard to surgeons, the Japanese Urological Association certifies a urologist who, as a proctor, has operated on more than 40 patients by using RARP or robot-assisted radical cystectomy. Our university hospital had only one proctor. We also compared the rate of patients with persistent UI after RARP performed by a proctor versus the rate after RARP performed by nonproctors (ie, 13 urologists).
Statistical analysis was conducted by using JMP Pro 16 (SAS Institute Japan Co, Ltd, Minato-ku, Tokyo). Qualitative parameters were compared by using the χ2 test, and quantitative parameters were compared by using the unpaired two-sample t-test. A value of P < 0.05 was significant.
Results
The mean age of the patients was 70.5 years, the mean body mass index of the patients was 27.1 kg/m2, the mean prostate volume of the patients was 34.7 cm3 and the mean MUL of the patients was 12.1 mm. The rates of urinary continence (ie, patients required no urinary pads) were 30%, 57.8%, and 79.3% at 3 months, 6 months, and 12 months, respectively, after RARP (Figure 1). At 12 months after RARP, 96 patients regained continence and did not require urinary pads; this group of patients was allocated to the Continence (+) group. By contrast, 25 patients had persistent UI and required urinary pads; this group of patients was allocated to the Continence (−) group. MUL on preoperative MRI and surgeon experience (ie, proctor vs nonproctor) differed significantly between the Continence (+) group and the Continence (−) group (P=0.05). However, no significant difference existed in age, body mass index, prostate volume, and pathological findings (eg, tumor stage, extraprostatic extension, resection margin) between the Continence (+) group and the Continence (−) group (Table 1).
 |
Table 1 Clinical Characteristics of Patients, Based on Continence Status at 12 Months After RARP |
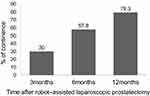 |
Figure 1 Serial changes in urinary recovery after robot–assisted laparoscopic prostatectomy. |
Discussion
Table 2 summarizes the rate of continence after RARP as 30–86% at 3 months, 53–94% at 6 months, and 72–96% at 12 months. The patients initially had UI and could have regained continence gradually. Pelvic floor muscle training is useful for patients experiencing UI after RARP.13 We recommended that patients perform pelvic floor muscle training after RARP, and the patients tried doing pelvic floor muscle training. Therefore, the rate of patients who regained continence may increase as the time progresses.
 |
Table 2 Urinary Continence Rates Reported in RARP |
UI is a considerable and common complication after radical prostatectomy. Some useful predictors of UI have been analyzed such as age,1–3 body mass index,4 overactive bladder,2 MUL,1,5–9 surgical experience and technique,10 and positive margin in pathology.1,2 We concluded that MUL was a useful predictor of UI after RARP. Table 3 summarizes the finding of the comparison of the mean MUL between the Continence (+) group and the Continence (–) group. Thus, all authors concluded that a relationship existed between MUL and UI. The mean MUL in the Continence (+) group ranged from 12.1 mm to 16.1 mm, and the mean MUL in the Continence (−) group ranged from 9.3 mm to 13.9 mm. To speculate the MUL cutoff value that predicts UI, based on the previous data, is difficult. However, MUL should be measured by using preoperative prostate MRI to predict UI after RARP. If the patient’s MUL is short, then UI may occur. Basourakos et al16 reported that the MUL is significantly shorter in Asian men (7.9 mm, 95% confidence interval [CI] 7.5–8.3) than in non-Asian men (10.9 mm, 95% CI 10.2–11.7) with a mean difference of 3.0 mm (95% CI 2.15–3.87; P<0.01) and concluded that Asian men had worse UI after the removal of the prostate. The impact of race differences in MUL is unclear. Measurements of the MUL are needed globally to understand the relationship between the race differences in MUL and UI after RARP.
 |
Table 3 Articles That Analyzed the Relationship Between the MUL in Preoperative MRI and UI at 12 Months After RARP |
We did not perform nerve-sparing radical prostatectomy in this study. Suardi et al17 report that a nerve-sparing approach during radical prostatectomy is strongly associated with the rate of postoperative urinary continence recovery. If we had performed nerve-sparing radical prostatectomy, the outcome of UI would have improved. Various techniques have been developed to improve UI after RARP.12,18–20 Kojima et al12 performed various techniques to improve the outcomes of UI after RARP. They reported that carrying out three steps may be necessary to achieve an improvement in the early return of UI after RAPR: (1) preservation (bladder neck, neurovascular bundle, puboprostatic ligament, pubovesical complex, and/or urethral length, etc), (2) reconstruction (posterior and/or anterior reconstruction and/or reattachment of the arcus tendinous to the bladder neck, etc), and (3) reinforcement (bladder neck plication and/or sling suspension, etc). They concluded that further modifications should be developed to improve UI, based on these steps. Choi et al18 performed a bladder neck plication stitch to prevent UI after RARP as the reinforcement for the urethral sphincter, but concluded that this technique had no effect on time to recovery from postoperative UI after RARP and acquiring the technical skills for reinforcing the urethral sphincter may be difficult. Preservation of the MUL is the first step of three steps regarding the improvement of UI after RARP, preservation of the MUL may be easier to achieve than reconstruction and reinforcement during RARP. Clinicians should take care to avoid using energy devices near the urethra or cutting the margin of the apex of the prostate in order to preserve the MUL.
Our study also demonstrated that surgical experience is a predictor of UI after RARP. Fossati et al,10 Samadi et al,21 and Zorn et al22 concluded that surgeon experience is a predictor of urinary continence recovery after RARP. Fossati et al10 reported that the surgical learning curve of urinary continence recovery did not reach a plateau, even after more than 100 cases. Therefore, the Japanese Urological Association certifies urologists who have operated on more than 40 patients by using RARP or robot-assisted radical cystectomy as a proctor. However, 40 patients may be insufficient to acquire the technical skills to improve the early return of UI after RARP. Clinicians need to continue learning the technique to improve the early return of UI after RARP, even after becoming a proctor.
Our results revealed no significant difference in age between the Continence (+) group and the Continence (−) group; however, the age of the patients between the two groups was 70.4 years and 70.5 years, which was nearly the same. Therefore, the conclusion that age was not significantly different is not valid. Yamada et al2 also revealed no significant difference between the Continence (+) group and the Continence (−) group in age. The mean age was 67 years and 70.5 years in Yamada’s group and in our group, respectively. However, Song et al1 and Lee et al3 reported that patient age was a predictor of the early return of continence after RARP. The mean age was 64.5 years and 59.2 years in Song’s group and in Lee’s group, respectively. The individuals in the Song and Lee study groups were younger than the individuals in Yamada study group and in our group. Therefore, age may be a predictor of the early return of continence after RARP, but only in individuals younger than 65 years old.
The results by Song et al,1 Yamada et al,2 and our group revealed no significant difference between the Continence (+) group and the Continence (−) group in body mass index. However, Wolin et al4 reported that body mass index is a predictor of UI after RARP and concluded that decreasing weight in patients with prostate cancer may improve quality of life by offsetting the negative side effects of treatment. Overweight and obesity increase the risk of UI in female individuals;23 however, whether body mass index is or is not a predictor of UI after RARP remains a controversial issue.
Our results revealed no relationship between prostate volume and UI. Jo et al24 reported that intravesical prostatic protrusion on ultrasound had an impact on UI and that patients with a shorter intravesical prostatic protrusion had a significantly higher chance of recovering continence. Urethral length such as intravesical prostatic protrusion, rather than prostate volume, may be associated with UI after RARP.
Pathological extraprostatic extension and the resection margin were not predictors of UI after RARP in our findings. Yamada et al2 reported that extraprostatic extension and the resection margin did not have a statistically significant difference between the Continence (+) and Continence (−) groups at 6 months and 12 months after RARP. Song et al1 reported that pathological characteristics such as extracapsular extension, seminal vesicle invasion, and positive surgical margin were not significantly different between the Continence (+) and Continence (−) groups at 12 months after RARP. Choi et al18 reported that the pathological stage (ie, pT2, pT3a or pT3b/N1) was not associated with the recovery of urinary continence within 3 months and 6 months after RARP. Pathological findings may not be a predictor of UI after RARP.
Based on the American Urological Association guidelines for UI after prostate treatment, for patients with bothersome stress UI after prostate treatment, surgery may be considered as early as 6 months if incontinence is not improving, despite conservative therapy.25 Rahnamai et al26 reported various surgical devices such as fixed sling, adjustable sling, artificial urinary sphincter, and noncircumferential compression for treating UI after RARP. Their devices anatomically support the urethra and are supposed to prolong the MUL.
Our study has several limitations. First, approximately 37% of patients were not included in the study because of missing information, which is a weakness of our study. Second, the study was based on retrospective information and the evidence level was lower than that in prospective studies. Third, the MUL was measured on only the preoperative MRI image; however, we did not routinely take MRI images after the operation. Therefore, the number of postoperative MRI images of the MUL was small. We hope to collect long-term MUL data on postoperative MRI. Fourth, nearly all previous investigators used the term “urinary incontinence” to include urgency UI, stress UI, or mixed UI. However, UI should be divided into three types: urgency UI, stress UI, or mixed UI. As a matter of convenience, we did not divide UI into these types to match the methods used in previous papers.
Conclusion
MUL and surgical experience are the predictors of UI after RARP. Measuring the MUL with MRI before RARP is recommended. Surgeons should continue learning techniques for improving the early recovery of UI after RARP.
Abbreviations
UI, urinary incontinence; RARP, robot-assisted radical prostatectomy; MUL, membranous urethral length; MRI, magnetic resonance imaging.
Disclosure
The authors have no conflict of interest directly relevant to the content of this article.
References
1. Song W, Kim CK, Park BK, et al. Impact of preoperative and postoperative membranous urethral length measured by 3 Tesla magnetic resonance imaging on urinary continence recovery after robotic-assisted radical prostatectomy. Can Urol Assoc J. 2017;11(3–4):93. doi:10.5489/cuaj.4035
2. Yamada Y, Fujimura T, Fukuhara H, et al. Overactive bladder is a negative predictor of achieving continence after robot-assisted radical prostatectomy. Int J Urol. 2017;24(10):749–756. doi:10.1111/iju.13411
3. Lee DJ, Cheetham P, Badani KK. Predictors of early urinary continence after robotic prostatectomy. Can J Urol. 2010;17:5200–5205.
4. Wolin KY, Luly J, Sutcliffe S, Andriole GL, Kibel AS. Risk of urinary incontinence following prostatectomy: the role of physical activity and obesity. J Urol. 2010;183(2):629–633. doi:10.1016/j.juro.2009.09.082
5. Munoz-Calahorro C, Garcia-Sanchez C, Barrero-Candau R, Garcia-Ramos JB, Rodriguez-Perez AJ, Medina-Lopez RA. Anatomical predictors of long-term urinary incontinence after robot-assisted laparoscopic prostatectomy: a systematic review. Neurourol Urodyn. 2021;40:1089–1097. doi:10.1002/nau.24652
6. Sadahira T, Mitsui Y, Araki M, et al. Pelvic magnetic resonance imaging parameters predict urinary incontinence after robot-assisted radical prostatectomy. Low Urin Tract Symptoms. 2019;11:122–126. doi:10.1111/luts.12245
7. Regis L, Salazar A, Cuadras M, et al. Preoperative magnetic resonance imaging in predicting early continence recovery after robotic radical prostatectomy. Actas Urol Esp. 2019;43:137–142. doi:10.1016/j.acuro.2018.07.003
8. Schmid FA, Wettstein MS, Kessler TM, et al. Contrast media kinetics in multiparametric magnetic resonance imaging before radical prostatectomy predicts the probability of postoperative incontinence. World J Urol. 2020;38(7):1741–1748. doi:10.1007/s00345-019-02952-y
9. Mungovan SF, Sandhu JS, Akin O, Smart NA, Graham PL, Patel MI. Preoperative membranous urethral length measurement and continence recovery following radical prostatectomy: a systematic review and meta-analysis. Eur Urol. 2017;71(3):368–378. doi:10.1016/j.eururo.2016.06.023
10. Fossati N, Trapani ED, Gandaglia G, et al. Assessing the impact of surgeon experience on urinary continence recovery after robot-assisted radical prostatectomy: results of four high-volume surgeons. J Endourol. 2017;31(9):872–877. doi:10.1089/end.2017.0085
11. Su LM, Otto BJ, Costello AJ. Laparoscopic and robotic-assisted laparoscopic radical prostatectomy. In: Partin AW, Dmochowski RR, Kavoussi LR, Peters CA, editors. Campbell-Walsh-Wein Urology.
12. Kojima Y, Takahashi N, Haga N, et al. Urinary incontinence after robot-assisted radical prostatectomy: pathophysiology and intraoperative techniques to improve surgical outcome. Int J Urol. 2013;20:1052–1163. doi:10.1111/iju.12214
13. Aydin Sayilan A, Ozbas A. The effect of pelvic floor muscle training on incontinence problems after radical prostatectomy. Am J Mens Health. 2018;12:1007–1015. doi:10.1177/1557988318757242
14. Greco KA, Meeks JJ, Wu S, Nadler RB. Robot-assisted radical prostatectomy in men aged > or = years. BJU Int. 2009;104:1492–1495. doi:10.1111/j.1464-410X.2009.08718.x
15. Patel VR, Sivaraman A, Coelho RF, et al. Pentafecta: a new concept for reporting outcomes of robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2011;59:702–707. doi:10.1016/j.eururo.2011.01.032
16. Basourakos SP, Ramaswamy A, Yu M, Margolis DJ, Hu JC. Racial variation in membranous urethral length and postprostatectomy urinary function. Eur Urol Open Sci. 2021;27:61–64. doi:10.1016/j.euros.2021.03.001
17. Suardi N, Moschini M, Gallina A, et al. Nerve-sparing approach during radical prostatectomy is strongly associated with the rate of postoperative urinary continence recovery. BJU Int. 2012. doi:10.1111/j.1464-410X.2012.11315.x
18. Choi SK, Park S, Ahn H. Randomized clinical trial of a bladder neck plication stitch during robot-assisted radical prostatectomy. Asian J Androl. 2015;17:304–308. doi:10.4103/1008-682X.139258
19. Rosenberg JE, Jung JH, Edgerton Z, et al. Retzius-sparing versus standard robotic-assisted laparoscopic prostatectomy for the treatment of clinically localized prostate cancer. Cochrane Database Syst Rev. 2020;8(8). doi:10.1002/14651858.CD013641.pub2
20. Checcucci E, Pecoraro A, Cillis SDE, et al. The importance of anatomical reconstruction for continence recovery after robot assisted radical prostatectomy: a systematic review and pooled analysis from referral centers. Minerva Urol Nephrol Dial. 2021;73(2). doi:10.23736/S2724-6051.20.04146-6
21. Samadi DB, Muntner P, Nabizada-Pace F, Brajtbord JS, Carlicci J, Lavery HJ. Improvements in robot-assisted prostatectomy: the effect of surgeon experience and technical changes on oncologic and functional outcomes. J Endourol. 2010;24(7):1105–1110. doi:10.1089/end.2010.01.36
22. Zorn KC, Wille MA, Thong AE, et al. Continued improvement of perioperative, pathological and continence outcomes during 700 robot-assisted radical prostatectomies. Can J Urol. 2009;16:4742–4749.
23. Aune D, Mahamat-Saleh Y, Norat T, RiboliBody E. Body mass index, abdominal fatness, weight gain and the risk of urinary incontinence: a systematic review and dose-response meta-analysis of prospective studies. BJOG. 2019;126(12):1424–1433. doi:10.1111/1471-0528.15897
24. Jo JK, Hong SK, Byun SS, Zargar H, Autorino R, Lee SE. Urinary continence after robot-assisted laparoscopic radical prostatectomy: the impact of intravesical prostatic protrusion. Younsei Med J. 2016;57(5):1145–1151. doi:10.3349/ymj.2016.57.5.1145
25. Sandhu JS, Breyer B, Comiter C, et al. Incontinence after prostate treatment: AUA/SUFU guideline. J Urol. 2019;202(2):369–378. doi:10.1097/JU.0000000000000314
26. Rahnamai MS, Marcelissen T, Geavlete B, Tutolo M, Husch T. Current management of post-radical prostatectomy urinary incontinence. Font Sug. 2021. doi:10.3389/fsurg.2021.647656
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
