Back to Journals » Cancer Management and Research » Volume 16
Pre- and Post-Treatment Quality of Life Among Patients with Advanced Stage Cervical Cancer at Tikur Anbessa Specialized Hospital, Ethiopia
Authors Teshome R , Yang I, Woldetsadik E, Girma E, Higgins M, Wells J
Received 5 December 2023
Accepted for publication 28 February 2024
Published 17 April 2024 Volume 2024:16 Pages 311—323
DOI https://doi.org/10.2147/CMAR.S451124
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Roza Teshome,1 Irene Yang,2 Edom Woldetsadik,3 Eshetu Girma,4 Melinda Higgins,2 Jessica Wells2
1Department of Midwifery, School of Nursing & Midwifery, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 2Nell Hodgson Woodruff School of Nursing, Emory University, Atlanta, GA, USA; 3Department of Oncology, School of Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 4Department of Preventive Medicine, School of Public Health, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Roza Teshome, Tel +251-911028610, Email [email protected]
Background: The development of health concepts beyond traditional markers of illness and death has made the evaluation of quality of life (QoL) crucial to patient care. Yet, there is little research evaluating the pre- and post-treatment QoL of cervical cancer survivors in Ethiopia.
Objective: This study aimed to assess the pre- and post-treatment QoL of women diagnosed with advanced-stage cervical cancer.
Methods and Materials: A cohort design was conducted at the Tikur Anbessa Specialized Hospital Oncology Center. A total of 166 cervical cancer patients were recruited consecutively. Data was collected through interviews with standardized questionnaires before and after treatment. The Wilcoxon rank test was used to assess the significant differences in pre-treatment and post-treatment quality of life. Additionally, the Mann-Whitney U-test was also employed. Statistical significance was determined with p-values < 0.05 and a 95% confidence interval.
Results: Women who were in stages IVA and IVB were 24.7% and 10.2%, respectively. Both the global health scale (66.67 [47.92– 75] to 83.33 [66.67– 83.33]) and the functional domain QoL (66.67 [40– 80] to 70 [46.67– 86.66]) showed statistically significant improvements from pre-treatment to post-treatment QoL. Women under the age of 45 were found to have higher global health QOL (P < 0.001) and functional domain QOL (P = 0.029). Women presented in stages II and III had comparatively higher global health QoL (P = 0.008) and functional domain QoL (P = 0.021).
Conclusion: Global health QOL and the majority of functional quality of life significantly improved following six months of cancer treatment. But there was no discernible change in terms of sexual enjoyment, sexual function, or activity. Age, marital status, the duration since diagnosis, the stage of the cancer, and the presence of comorbidities were the factors that affected the improvement of post-treatment quality of life.
Keywords: pre-treatment, post-treatment, quality of life, cervical cancer, Ethiopia
Background
Cervical cancer is one of the most common cancers in women worldwide, being the fourth most common after breast, colorectal, and lung cancer.1 According to GLOBOCAN 2020 estimates, there were about 604,000 new cases of cervical cancer worldwide in 2020, with 342,000 deaths annually. Approximately 85% of new cases and 90% of deaths, respectively, take place in low- and middle-income countries. Sub-Saharan Africa has the highest regional incidence and mortality, with rates higher in Eastern Africa (40.1 cases and 28.6 deaths per 100,000 population).2 Cervical cancer is the second most common type of cancer among women in Ethiopia. Reports from 2018 indicate that there were an anticipated 6294 new cases of cervical cancer each year and 4884 deaths related to the disease.3
The incidence and mortality of cancer are rising at an accelerated rate, which can be attributed to various factors such as aging and population growth, as well as shifts in the distribution and frequency of the main cancer risk factors, some of which are associated with socioeconomic status.4
The percentage of patients diagnosed with cervical cancer varies based on the disease’s stage; the majority of patients are found in the mid-to-late stages, and the majority of patients present in the early stages, when treatment is most successful. 5 The staging system for invasive cervical cancer that is most commonly used is the International Federation of Gynecology and Obstetrics (FIGO) guideline, which is separated into four stages: I (A, A1, A2, B, B1, B2, and B3), II (A, A1, A2, and B), III (A, B, and C), and IV (A and B). Patients with advanced-stage cervical cancer are in FIGO stages IIB to IVB and may have pelvic or lower back pain, flank pain, and lower limb edema. Furthermore, problems relating to the bowel or bladder, such as pressure changes or the flow of feces or urine through the vagina, indicate invasion of the rectum and bladder, respectively.6 Surgery, radiation therapy, chemotherapy, or a combination of these treatments may be used to treat patients, depending on the stage of their cervical cancer and the presence of comorbidities. While radiation therapy or surgery can be used to treat cervical cancer in its early stages, combination treatments are necessary for patients presenting with advanced stages of the disease.1,5,7
Patients with cervical cancer experience changes in their physical and emotional health, which ultimately impact their quality of life.8 The measurement of QoL has become essential to patient treatment due to the expansion of health concepts beyond traditional indicators of morbidity and mortality. Despite advancements in cervical cancer detection and treatment, survivors of the disease face significant challenges, most notably with regard to QoL.9
The burden of cervical cancer and its effects on patients make it necessary to evaluate baseline QoL prior to treatment.10 It is established that both the illness and the treatments have an effect on QoL. Appropriate psycho-oncological care for patients with cervical cancer throughout diagnosis and therapy may be made easier with a deeper understanding of pre- and post-treatment quality of life. However, there is a dearth of research assessing the quality of life (QoL) prior to and following therapy for patients with advanced cervical cancer in Ethiopia. Thus, this study was aimed at assessing the pre- and post-treatment quality of life of women diagnosed with advanced-stage cervical cancer.
Methods and Materials
Study Design and Setting
This study was a cohort design to assess QOL among advanced-stage cervical cancer patients who received cancer treatment at the Tikur Anbessa Specialized Hospital Oncology Center. Tikur Anbessa Specialized Hospital is one of the biggest referral hospitals and the only oncology center in Ethiopia.
Population and Data Collection Procedure
A total of 166 patients with clinically diagnosed advanced cervical cancer were included. Participants were consecutively recruited just prior to the start of the cancer treatment during the period from January 10, 2022, to September 20, 2022. Data were collected using interviewer-based, standardized questionnaires. During the pre-treatment interview, sociodemographic characteristics, clinical characteristics, type of cancer treatment, and comorbidities were additionally collected. Patients were re-interviewed six months after the pre-treatment interview to evaluate post-treatment QoL. Post-treatment interviews ended on March 20, 2023. The inclusion criteria were cervical cancer patients who were diagnosed as advanced stage of cervical cancer (FIGO stage of IIB-to-stage IVB), patients who were enrolled in any cancer treatment, and patients who were willing to participate in the study. Cervical cancer patients who were in the early stages of cancer and patients who did not begin any cancer treatment were excluded. Validated questionnaires were used, and the data collection was continuously supervised. Two days of training were given to data collectors. Daily evaluation of the data for completeness and encountered difficulties at the time of data collection were attended accordingly. All completed data collection questionnaires were examined for completeness.
Data Collection Tool
A standardized tool, the European Organization for Research and Treatment of Cancer (EORTC) core questionnaire (CX 30) and the cervical cancer module (CX 24), were used. The EORTC CX30 questionnaire is an integrated tool for assessing the health-related QoL of cancer patients and has 30 items. It includes five functional scales, three symptom scales, a global health status scale, and six single items. All of the scales and single-item measures range in score from 0 to 100. A higher score on the scale indicates a higher response level. Therefore, a high score on the global health status scale indicates a good quality of life; a high score on the functional scale indicates a high or healthy level of functioning; and a high score on the symptom scale indicates a high degree of symptomatology/problems.11 Cronbach’s alpha coefficient scores for the EORTC QLQ-C30 ranged from 0.72 to 0.95.12
The cervical cancer module (EORTC QLQ-CX24) was developed in a multicultural, multidisciplinary setting to supplement the EORTC QLQ-C30 core questionnaire. It has 24 items. It incorporates 3 multi-item scales to assess symptom experience, body image, and sexual/vaginal functioning. In addition, 6 single items assess lymphoedema, peripheral neuropathy, menopausal symptoms, sexual worry, sexual activity, and sexual enjoyment. Similar to the EORTC CX30, all of the scales and single-item measures range in score from 0 to 100. A high score on the symptom scales or single items indicates a high degree of problems or symptomatology, while a high score on the functional single items indicates a high degree of functioning13 Cronbach’s alpha coefficients range from 0.72 to 0.87 for the subscales, suggesting high internal consistency (symptom experience, 0.72; body image, 0.86; sexual/vaginal functioning, 0.87).14
The English-language EORTC CX 30 and 24 tools were translated into Amharic. Cronbach’s alpha’s for the translated versions ranged from 0.70 to 0.84.15 In our study, we used this validated Amharic language version tool to assess the pre-treatment and post-treatment QoL among advanced-stage cervical cancer patients.
Data Analysis
Data was entered, cleaned, and analyzed using SPSS version 29 software.16 Descriptive statistics were performed to analyze sociodemographic characteristics such as age, education level, marital status, and clinical traits such as cancer stage and treatment types. Before data analysis, a normality test was performed. The Kolmogorov-Smirnov and the Shapiro-Wilk tests showed that normality assumptions were not satisfied. Therefore, non-parametric tests, such as the Wilcoxon ranked test, were used to assess differences in pre-treatment and post-treatment QoL. Values are presented in mean, median, standard deviation, and interquartile range. The Mann-Whitney U-test was used to assess differences in post-treatment QoL based on sociodemographic and clinical characteristics. Statistical significance was determined with p-values <0.05 and a 95% confidence interval.
Furthermore, global health QoL, symptom domain QoL, and functional domain QoL were categorized. Based on other published works, the functional domain, the symptom domain, and the global health status were categorized as good, “moderate, and poor for comparison’s sake.16,17 The functional domain and global health status were categorized as “good” with a median score of greater than 66.7, “moderate” with a median score of 33.4–66.6, and “poor” with a median score of less than 33.3. The symptom domain was classified as “less symptomatic” with median score of below 33.3, as “moderate symptomatic” with median score of 33.4–66.6 and as “very symptomatic” with median score of above 66.7
Results
Sociodemographic and Clinical Characteristics of Participants
A total of 166 women with advanced-stage cervical cancer participated in this study. Participants’ mean age was 52.33 years, with a standard deviation of 10.16 years and a range of 30–81 years. From these, 31.9% were 50–59 years of age. Most were housewives; more than half had no formal education, 42.2% were married, 70% reported no alcohol consumption, and 81% reported no regular exercise.
The most common histologic type was squamous cell carcinoma (96.2%). Twenty-five percent and 10.2% of women were in FIGO stages IVA and IVB, respectively. Thirty-seven percent and 53% of cervical cancer patients were treated with radiotherapy and chemoradiotherapy, respectively. Anemia and HIV/AIDS accounted for 21.1% and 12% of the co-morbidities, respectively (Table 1).
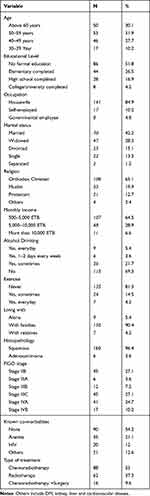 |
Table 1 Sociodemographic and Clinical Characteristics of Participants (n = 166) |
Pre-Treatment Quality of Life
Higher values in the functional and global health status domains suggest a higher quality of life, according to the scoring manual, while higher values in the symptom category indicate an a symptomatic or health problem issue. The interquartile range (IQR) for women’s global health status before to treatment was [41.67–75], with a median score of 58.33. The functional domain yielded higher scores for physical functioning (63.33 [40–80]) and cognitive functioning (66.67 [33.33–66.67]), respectively.
The EORTC QLQ-C30 tool’s symptom domain revealed that fatigue (55.56 [33.33–66.67]), constipation (66.67 [0–100]), and financial difficulties (66.67 [0–100]) scored higher than the other symptoms, whereas the median score of symptom experience using EORTC CX24 was 39.39 [21.21–54.55] (Table 2).
 |
Table 2 Pre-Treatment QOL of Women with Advanced Cervical Cancer Using EORTC CX30 and CX24 (n = 166) |
Post-Treatment Quality of Life
Six months after the pre-treatment interview, post-treatment QoL was evaluated. Thirty-two deaths were recorded within six months post-treatment. As a result, 134 individuals were interviewed in the post-treatment phase.
The median global health score after cancer treatment was 83.33 [66.67–83.33]. Physical functioning had the highest score (70 [46.67–86.66]) among the functional domains of the EORTC QLQ-C30 tool. Pain (50 [16.67–66.67]) and fatigue (55.55 [22.22–77.78]) scored highest in the symptom domain. According to the EORTC QLQ-CX24, sexual/vaginal functioning had the highest value of 33.33 [33.33–41.67] among the functional domains, and symptom experience had the highest value of 15.15 [6.06–36.36] in the symptom domain (Table 3).
 |
Table 3 Post-Treatment QOL of Women with Advanced Cervical Cancer Using EORTC CX30 and CX24 at TASH, 2022 (n = 134) |
Comparison of Pre-Treatment and Post-Treatment Quality of Life
In order to assess the quality of life before and after treatment, individuals with cervical cancer who died within the first six months (n = 32) were not included in the analysis of pre-treatment QoL.
A comparison of the global health status prior to and after cancer treatment showed statistically significant improvement in their median score, which was from 66.67 [47.92–75] to 83.33 [66.67–83.33]. This difference was significant according to the Wilcoxon rank test (p < 0.001).
Based on the EORTC QLQ-C30 functional domain, women showed statistically significant improvement on all functional scales from the pre-treatment period. The functional domain increased from 66.67 [40–80] to 70 [46.67–86.66].
On the other hand, it was found that the value in the body image functional domain significantly decreased from the pre-treatment phase (33.33 [11.11–66.67]) to the post-treatment phase (11.11 [0–44.44]), rather than increasing. Furthermore, sexual enjoyment and sexual activity did not show any improvement (Table 4).
 |
Table 4 Comparison of Pre-Treatment and Post-Treatment Quality of Life Among Women with Advanced Stage of Cervical Cancer |
Before receiving treatment, the global health status of over half of participants (52.1%) was moderate, and 29.1% was good. Following treatment, participants with good global health status increased to 62.7% and participants with moderate global health status decreased to 34.2% (Figure 1).
 |
Figure 1 Comparing Pre-treatment and Post-treatment global health, functional domain and symptom experience among women with advanced stage of cervical cancer. |
Participants were asked for their sexual engagement to respond as “not at all”, “a little bit”, “a quite bit” and “very much”. During pre-treatment interview, most of women (80.6%) reported “not at al”, 5.7% of them responded as “a little bit”, 2.2% of them said “quite bit” and only 1.5% of them said “very much”. Similarly, during post-treatment interview, 74.1% of them responded as “not at all”, and 1.2% said as “very much”. Women who reported no sexual enjoyment were 93.2% during pre-treatment interview and 88.6% during post-treatment interview (Figure 2).
 |
Figure 2 Comparing Pre-treatment and Post-treatment by sexual activity and sexual enjoyment. |
Factors Associated with Post-Treatment Quality of Life
Age of Participants
Among the 134 individuals who underwent assessment after cancer treatment, 65.7% were older than 45 years. Women under the age of 45 were found to have higher global health status (P < 0.001) and functional domain of quality of life (P 0.029). Likewise, there was a significant difference in the symptoms of fatigue and pain. It was also shown that women under 45 experienced more sexual worry than those over 45 (P 0.038). However, age did not associate with sexual activity or sexual enjoyment (Table 5).
 |
Table 5 Post-Treatment Quality of Life by Age, Presence of Anemia and FIGO Stage |
Comorbidities
Forty-five percent of the participants overall had at least one comorbidity. Anemia, HIV/AIDS, diabetes, heart disease, kidney disease, and liver disease were among the comorbidities that were mentioned. Anemia accounted for 21.1% and HIV/AIDS for 12.1% of cases. The global health and functional quality of life of women without comorbidities was significantly higher than that of women with comorbidities (Table 5).
Stage of Cancer
Of the 134 women assessed after treatment, 34 (25.4%) were in FIGO stage IV. For the majority of domain measurements, there was a statistically significant difference in post-treatment quality of life by stage presentation. Compared to women presenting in FIGO stage IV, those in stages II and III had comparatively higher global health (P = 0.008) and functional quality of life (P = 0.021). When it came to sexual enjoyment, there was no discernible difference between women in stage IV compared to stages II and III (P = 0.334), although women presenting in the lower stages experienced higher levels of sexual activity (P = 0.039) (Table 5).
Income
The mean monthly family income was 5,000 ETB with a range of 500 to 22,000 Ethiopian Birr (ETB). Global health quality of life was lower for women with monthly incomes of less than 5,000 ETB compared to those with incomes above 5,000 ETB (P < 0.001). However, their sexual activity, sexual enjoyment, and emotional functioning did not differ statistically (Table 6).
 |
Table 6 Post-Treatment Quality of Life by Family Monthly Income, Marital Status and Time Since Diagnosis |
Marital Status
Of the 134 women who were assessed after treatment, 43.3% were married, 11.2% were single, 15.7% had divorced, 28.4% were widowed, and 1.5% were separated. Global health quality of life did not differ by marital status; however, married women scored higher in the emotional functioning domain of quality of life. Married women also had more sexual worry and higher levels of sexual activity, but there was no significant difference in sexual enjoyment between marital status groups (Table 6).
Time Since Diagnosis
Time since diagnosis was over a year for 37.3% of participants and was associated with global health quality of life. Longer time diagnosis was significantly associated with quality of life. Women with time of diagnosis longer than a year had lesser global health quality of life (P < 0.001) (Table 6).
Discussions
Quality of life (QOL) is defined as a person’s self-reported perception of physical, psychosocial, and sexual well-being. Survivors’ health-related QOL is becoming an increasingly important consideration as the rate of cervical cancer patients’ survival increases, and improving QOL is crucial as advancements in the diagnosis and treatment of cancer have increased the life expectancy of cancer survivors.17
This study examined the pre- and post-treatment QoL of patients with advanced cervical cancer. Pre-treatment global health median score in the current study was comparable to results reported in South India.8 In our study, following cancer treatment, there was a considerable improvement in global health status of quality of life. This is consistent with studies conducted in India.9,10 On the other hand, a study conducted in Austria revealed that following the initial (less than three months) cancer treatment, there was a considerable reduction in global health status QOL. This inconsistency may be due to the fact that the majority of patients in their study underwent surgery and the length of the post-treatment evaluation varied. The same authors, however, reported that after a six-month and year-long follow-up, global health status had improved.18
Following cancer treatment, 53% of participants in our study had good global health status scores. This is similar to the results of studies conducted in Tanzania, China, and Iran.19–22 The present investigation revealed a considerable improvement in physical, emotional, and social functioning following cancer therapy. These findings are in line with research conducted in Austria, Poland, and India.9,10,18,23 Pain decreased significantly post-treatment; however, fatigue symptoms remained the same which was consistent with a study in Denmark24 but differed from a study in India where fatigue symptoms also decreased.9
Sexual dysfunction problems, such as feeling unable to satisfy their partner’s demands or perceiving changes in their partner’s sexual interest, are significant sources of distress for women who have survived cervical cancer.25,26 For cervical cancer survivors, both radiation therapy and major surgery may have medium- and long-term effects on their sexual functioning.27 In our study, most participants, both pre- and post-treatment, did not have sexual activity. Sexual/vaginal functioning also remained the same between pre- and post-treatment periods. These findings were in line with several studies.8–10,21,27–30 In the present study very few participants engaged in sexual activity similar to what was found in a study done in Korea.31 Similarly, a study conducted in Lithuania found that the sexual activity and enjoyment of cervical cancer survivors were particularly low.32 Related factors have been suggested by numerous studies and include dyspareunia, orgasmic difficulties, dry vagina, and vaginal atrophy, all brought on by the illness and side effects of cancer treatments.33–36
Radiotherapy has a greater adverse effect on sexual function than radical hysterectomy with pelvic lymphadenectomy, but more recent research suggests that new radiotherapy methods may not so negatively affect sexual function. Additionally, a study in Tanzania and a systematic review found that the use of combination radiation therapy (external plus brachytherapy) can considerably reduce sexual concern, increase sexual activity, and improve an individual’s body image.35,37
In the current study, common significant factors associated with post-treatment QoL were age, time since diagnosis, marital status, presence of comorbidity and stage of cervical cancer. This is inconsistent with certain published studies that found no statistically significant variations in QoL ratings between age groups and cervical cancer stages.32,38 According to this study, time since diagnosis had an impact on cancer survivors’ self-reported health state and QOL. Another study found a similar relationship between the time since diagnosis and global health, functional health, and symptom scale.21,39
Implication to Clinical Practice
Improving quality of life (QoL) for patients with cervical cancer in low- and middle-income countries is crucial. In order to effectively care for patients with cervical cancer and to tailor their treatment, it is imperative to assess their QOL.40 Furthermore, current research indicates that QoL data can offer unique predictive information in addition to being useful for evaluating patient well-being and aiding clinicians in making decisions.41 When planning and evaluating a cancer patient’s therapy course, QoL should be taken into account in addition to clinical characteristics.38 Because this is the first study in Ethiopia to study pre- and post-treatment impacts of QOL, our results provide baseline findings for comparing pre- and post-treatment effects. Numerous studies have been conducted in other countries, however when evaluating participants’ self-reported QoL, it is crucial to take into account sociodemographic, cultural, and religious backgrounds.
Limitations and Future Research Recommendations
Particularly in a population such as Ethiopia, where discussing sexuality is not an open conversation, evaluating sexual function, sexual engagement, and sexual enjoyment would be difficult. To comprehend and explore more about sexual issues and experiences, further research using methodologies such as qualitative approaches is encouraged. Furthermore, sexual counselling must to be taken into account in every facet of cervical cancer treatment. The observational method was used to conduct the current investigation. Additional study methodologies beyond observational design should be encouraged to evaluate the pre- and post-treatment impacts involving control groups.
Conclusion
In conclusion, following six months of cancer treatment, there was a significant improvement in global health quality of life. There were also improvements in physical functioning, role functioning, social and emotional functioning. Symptoms of pain and nausea/vomiting did not change, but fatigue decreased. Additionally, there were no noticeable differences in sexual function, sexual activity and sexual enjoyment from pre- to post-treatment phases. The factors that were linked to post-treatment QoL were age, marital status, time since diagnosis, cancer stage, and the presence of comorbidities. In addition to clinical parameters, QoL should be considered while planning and assessing a cancer patient’s course of treatment course.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Statement
Data was collected after ethical clearance was obtained from the Institutional Review Boards of Addis Ababa University College of Health Sciences (IRB (057/21/Nursing). After that, consent was obtained from the oncology center. Eligible participants were informed, by trained data collector, about the objectives and purposes of the study, and the benefits of the study. The study was conducted in accordance with the Declaration of Helsinki. Study participants were included when they stated their willingness to participate in the study and signed on the informed consent.
Acknowledgment
We would like to express our gratitude to all data collectors. We would like to thank all Nurses at Tikur Anbessa Specialized hospitals for their co-operations during data collection.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
The study was funded by Addis Ababa University College of Health sciences.
Disclosure
The authors declared that there is no conflict of interest.
References
1. Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri: 2021 update. Int J Gynecol Obstet. 2021;155(S1):28–44.
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Bruni L, Albero G, Serrano B, et al. Human papillomavirus and related diseases report in world. ICO/IARC Inf Cent HPV Cancer (HPV Inf Centre); 2021. Available from: www.hpvcentre.com.
4. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever‐increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–3030. doi:10.1002/cncr.33587
5. Gopu1 U on systemic therapy for cervical cancer P. Febin antony1, sunu cyriac2 KK& AMOD. updates on systemic therapy for cervical cancer. J Med Res. 2022;293–302.
6. Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynecol Obstet. 2018;143(S2):22–36. doi:10.1002/ijgo.12611
7. Burmeister CA, Khan SF, Schäfer G, et al. Cervical cancer therapies: current challenges and future perspectives. Tumour Virus Res. 2022;13(April):200238. doi:10.1016/j.tvr.2022.200238
8. Somanna SN, Sastry NB, Cheluvarayaswamy R, Malila N. Quality of life and its determinants among cervical cancer patients in south India. Asian Pacific J Cancer Prev. 2022;23(8):2727–2733. doi:10.31557/APJCP.2022.23.8.2727
9. Dahiya N, Acharya AS, Bachani D, et al. Quality of life of patients with advanced cervical cancer before and after chemo-radiotherapy. Asian Pacific J Cancer Prev. 2016;17(7):3095–3099.
10. Kumar S, Rana ML, Verma K, et al. PrediQt-cx: post treatment health related quality of life prediction model for cervical cancer patients. PLoS One. 2014;9:2.
11. Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A EORTC QLQ-C30 scoring manual the EORTC QLQ-C30 introduction. EORTC QLQ-C30 Scoring Man; 2001:1–67.
12. Davda J, Kibet H, Achieng E, Atundo L, Komen T. Assessing the acceptability, reliability, and validity of the EORTC Quality of Life Questionnaire (QLQ-C30) in Kenyan cancer patients: a cross-sectional study. J Patient Rep Outcomes. 2021;51.
13. Greimel ER, Vlasic KK, Waldenstrom C, et al. the European organization for research and treatment of cancer (EORTC) quality-of-life questionnaire cervical cancer module. Int J Med. 2006:1–2. doi:10.1002/cncr.22217
14. Greimel ER, Vlasic KK, Waldenstrom AC, et al. The European organization for research and treatment of cancer (EORTC) quality-of-life questionnaire cervical cancer module: EORTC QLQ-CX24. Cancer. 2006;107(8):1812–1822. doi:10.1002/cncr.22217
15. Araya LT, Gebretekle GB, Gebremariam GT, Fenta TG. Reliability and validity of the amharic version of European organization for research and treatment of cervical cancer module for the assessment of health related quality of life in women with cervical cancer in Addis Ababa, Ethiopia 11 medical and health. Health Qual Life Outcomes. 2019;17(1):1–7. doi:10.1186/s12955-019-1089-x
16. Corporation IBM. IBM SPSS Statistics Version 29; 2023.
17. Vistad I, Fosså SD, Dahl AA. A critical review of patient-rated quality of life studies of long-term survivors of cervical cancer. Gynecol Oncol. 2006;102(3):563–572. doi:10.1016/j.ygyno.2006.03.050
18. Greimel E, Thiel I, Peintinger F, Cegnar I, Pongratz E. Prospective assessment of quality of life of female cancer patients. Gynecol Oncol. 2002;85(1):140–147. doi:10.1006/gyno.2002.6586
19. Torkzahrani S, Rastegari L, Khodakarami N, Akbarzadeh-Baghian A, Alizadeh K. Quality of life and its related factors among Iranian cervical cancer survivors. Iran Red Crescent Med J. 2013;15(4):320–323. doi:10.5812/ircmj.4410
20. Khalil J, Bellefqih S, Sahli N, et al. Impact of cervical cancer on quality of life: beyond the short term (Results from a single institution). Gynecol Oncol Res Pract. 2015;2(1):2–7. doi:10.1186/s40661-015-0011-4
21. Thapa N, Maharjan M, Xiong Y, et al. Impact of cervical cancer on quality of life of women in Hubei, China. Sci Rep. 2018;8(1):2–10. doi:10.1038/s41598-018-30506-6
22. Mvunta DH, August F, Dharsee N, et al. Quality of life among cervical cancer patients following completion of chemoradiotherapy At Ocean Road Cancer Institute (ORCI) in Tanzania. BMC Womens Health. 2022;22(1):426. doi:10.1186/s12905-022-02003-6
23. Babiarczyk B, Fraś M, Ulman-Włodarz I, Jarosova D. Analiza poziomu satysfakcji zawodowej oraz jej zwia{ogonek}zku z subiektywnie oceniana{ogonek} jakościa{ogonek} życia położnych. Med Pr. 2014;65(1):99–108. doi:10.13075/mp.5893.2014.011
24. Klee M, Thranov I, Machin D. The patients’ perspective on physical symptoms after radiotherapy for cervical cancer. Gynecol Oncol. 2000;76(1):14–23. doi:10.1006/gyno.1999.5642
25. Bergmark K, Åvall-Lundqvist E, Dickman PW, Henningsohn L, Steineck G. Patient-rating of distressful symptoms after treatment for early cervical cancer. Acta Obstet Gynecol Scand. 2002;81(5):443. doi:10.1034/j.1600-0412.2002.810512.x
26. Abbott-Anderson K, Kwekkeboom KL. A systematic review of sexual concerns reported by gynecological cancer survivors. Gynecol Oncol. 2012;124(3):477–489. doi:10.1016/j.ygyno.2011.11.030
27. Pfaendler KS, Wenzel L, Mechanic MB, Penner KR. Cervical cancer survivorship: long-term quality of life and social support. Clin Ther. 2015;37(1):39–48. doi:10.1016/j.clinthera.2014.11.013
28. Wu X, Wu L, Han J, et al. Evaluation of the sexual quality of life and sexual function of cervical cancer survivors after cancer treatment: a retrospective trial. Arch Gynecol Obstet. 2021;304(4):999–1006. doi:10.1007/s00404-021-06005-x
29. Shajahan Ahamed M, Degu A. Health-related quality of life among cervical cancer patients at Kenyatta National Hospital. J Oncol Pharm Pract. 2023;29(2):393–400. doi:10.1177/10781552211073886
30. De Arruda F N, Da Costa S, Bonadio R, et al. Quality of life of locally advanced cervical cancer patients after neoadjuvant chemotherapy followed by chemoradiation versus chemoradiation alone (CIRCE trial): a randomized Phase II trial. Int J Gynecol Cancer. 2020;30(6):749–756. doi:10.1136/ijgc-2019-001134
31. Lee Y, Lim MC, Kim SI, Joo J, Lee DO, Park SY. Comparison of quality of life and sexuality between cervical cancer survivors and healthy women. Cancer Res Treat. 2016;48(4):1321–1329. doi:10.4143/crt.2015.425
32. Stuopelytė R, Žukienė G, Breivienė R, Rudaitis V, Bartkevičienė D. Quality of Life in Cervical Cancer Survivors Treated with Concurrent Chemoradiotherapy. Med. 2023;59:4.
33. Kluwgant D, Homer C, Dahlen H. “Never let a good crisis go to waste”: positives from disrupted maternity care in Australia during COVID-19. Midwifery. 2022;110:103340. doi:10.1016/j.midw.2022.103340
34. Ying L, Fitzpatrick JM, Philippou J, Huang W, Rafferty AM. The organisational context of nursing practice in hospitals in China and its relationship with quality of care, and patient and nurse outcomes: a mixed-methods review. J Clin Nurs. 2021 301–2: 3–27. doi: 10.1111/jocn.15486.
35. Tramacere F, Lancellotta V, Casà C, et al. Assessment of Sexual Dysfunction in Cervical Cancer Patients after Different Treatment Modality: a Systematic Review. Med. 2022;58(9):1–13.
36. Tangjitgamol S, Manusirivithaya S, Hanprasertpong J, et al. Sexual dysfunction in Thai women with early-stage cervical cancer after radical hysterectomy. Int J Gynecol Cancer. 2007;17(5):1104–1112. doi:10.1111/j.1525-1438.2007.00907.x
37. Lindau ST. Sexual Morbidity in Very long-term Survivors of Vaginal and Cervical Cancer: a Comparison to National Norms. Gynecol Oncol. 2007;83(4):413–418.
38. Rahman Z, Singh U, Qureshi S, Nisha N, Srivastav K, Nishchal A. Assessment of quality of life in treated patients of cancer cervix. J Mid Health. 2017;8(4):183–188. doi:10.4103/jmh.JMH_40_17
39. Kim MK, Sim JA, Yun YH, et al. Health-related quality of life and sociodemographic characteristics as prognostic indicators of long-term survival in disease-free cervical cancer survivors. Int J Gynecol Cancer. 2016;26(4):743–749. doi:10.1097/IGC.0000000000000665
40. Pasek M, S L, K U, Ski A. Quality of life in cervical cancer patients treated with radiation therapy. J Clin Nurs. 2012;2222(5–6):690–697. doi:10.1111/j.1365-2702.2012.04350.x
41. Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26(8):1355–1363. doi:10.1200/JCO.2007.13.3439
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
