Back to Journals » Journal of Inflammation Research » Volume 17
Piezo1 Knockout Improves Post-Stroke Cognitive Dysfunction by Inhibiting the Interleukin-6 (IL-6)/Glutathione Peroxidase 4 (GPX4) Pathway
Authors Tang L , Xie D, Wang S, Gao C , Pan S
Received 8 November 2023
Accepted for publication 19 March 2024
Published 13 April 2024 Volume 2024:17 Pages 2257—2270
DOI https://doi.org/10.2147/JIR.S448903
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Lujia Tang,* Di Xie,* Shangyuan Wang, Chengjin Gao, Shuming Pan
Department of Emergency, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200092, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shuming Pan; Chengjin Gao, Email [email protected]; [email protected]
Background: Cerebral infarction often results in post-stroke cognitive impairment, which impairs the quality of life and causes long-term disability. Astrocytes, the most abundant glial cells in the central nervous system, have a crucial role in cerebral ischemia and neuroinflammation. We explored the possible advantages of interleukin-6 (IL-6), a powerful pro-inflammatory cytokine produced by astrocytes, for post-stroke cognitive function.
Methods: Mendelian randomization was applied to analyze the GWAS database of stroke patients, obtaining a causal relationship between IL-6 and stroke. Further validation of this relationship and its mechanisms was conducted. Using a mouse model of cerebral infarction, we demonstrated a significant increase in IL-6 expression in astrocytes surrounding the ischemic lesion. This protective effect of Piezo1 knockout was attributed to the downregulation of matrix metalloproteinases and upregulation of tight junction proteins, such as occludin and zonula occludens-1 (ZO-1).
Results: Two-step Mendelian randomization revealed that IL-6 exposure is a risk factor for stroke. Moreover, we conducted behavioral assessments and observed that Piezo1 knockout mice that received intranasal administration of astrocyte-derived IL-6 showed notable improvement in cognitive function compared to control mice. This enhancement was associated with reduced neuronal cell death and suppressed astrocyte activation, preserving ZO-1.
Conclusion: Our study shows that astrocyte-derived IL-6 causes cognitive decline after stroke by protecting the blood-brain barrier. This suggests that piezo1 knockout may reduce cognitive impairment after brain ischemia. Further research on the mechanisms and IL-6 delivery methods may lead to new therapies for post-stroke cognition.
Keywords: cerebral infarction, neuroinflammation, astrocytes, blood-brain barrier, tight junctions
Introduction
Cerebral infarction, commonly referred to as a stroke, is a leading cause of cognitive dysfunction worldwide. The pathophysiology of cognitive impairment following cerebral infarction is multifactorial, involving various cellular and molecular mechanisms. Amongst the key players in this process are astrocytes, the most abundant glial cells in the central nervous system.1,2 Astrocytes are crucial for maintaining brain homeostasis and supporting neuronal function. However, following cerebral infarction, they undergo phenotypic changes, becoming reactive astrocytes. Reactive astrocytes release inflammatory cytokines, chemokines, and reactive oxygen species, contributing to neuroinflammation and exacerbating neuronal damage.3,4
The role of astrocytes in cognitive impairment has gained increasing recognition in recent years. Astrocytes are a type of glial cell that provide support and nourishment to neurons in the brain. While traditionally viewed as primarily supportive cells, emerging evidence has revealed their active involvement in cognitive processes. Astrocytes play a critical role in maintaining the integrity of the blood-brain barrier (BBB), a highly selective barrier that regulates the entry of substances into the brain. Dysfunction of the BBB has been implicated in various cognitive disorders, including Alzheimer’s disease.5 Astrocytes contribute to BBB maintenance by forming end-feet processes that surround the blood vessels and release molecules that regulate the permeability of the barrier. In addition to their role in the BBB, astrocytes are involved in neurotransmitter balance and synaptic transmission. They regulate the levels of important neurotransmitters, such as glutamate and gamma-aminobutyric acid (GABA), which are crucial for cognitive functions, including learning and memory.6 Dysregulation of astrocytic glutamate uptake, for example, has been implicated in neurodegenerative diseases and cognitive impairments.
Astrocytes secrete neurotrophic factors and neuroprotective molecules, promoting neuronal survival and regeneration. Additionally, they contribute to the clearance of neuronal metabolic waste and regulate neuronal activity, maintaining the stability of neural circuits. In conclusion, astrocytes play a dual role in the context of cerebral infarction and cognitive dysfunction. On one hand, activated astrocytes exacerbate brain damage and contribute to cognitive impairment through their inflammatory response. However, the mechanism by which Piezo1 contributes to cognitive dysfunction after cerebral infarction remains unclear. We hypothesize that Piezo1 knockout alleviates BBB damage by regulating neuroinflammation, thereby mitigating endothelial cell ferroptosis. This study aims to explore the specific mechanisms by which Piezo1 knockout improves BBB damage, potentially providing new therapeutic strategies for cognitive recovery after stroke.
Materials and Methods
Study Design
We first conducted two-sample Mendelian randomization analysis (TSMA) on the following causal associations: the association between inflammatory factors and stroke. We applied multiple testing significance thresholds to evaluate the associations between blood metabolites and diseases, resulting in significant causal associations and potential causal associations. Subsequently, we performed MR-BMA analysis on the TSMR results that met the criteria: the quantity of TSMR results≥2 and passed sensitivity analysis to select potential causal associations and significant causal associations.7
Data Source
The GWAS data on immune cell used in this study came from a large metabolomics dataset recently released by the UK Biobank. The GWAS data for stroke came from a large-scale multi-ethnic meta-analysis based on the 1000 Genomes Project. The stroke database, labeled ebi-a-GCST90018864, comprises 11,929 stroke patients and 472,192 controls. The database contains information on a total of 24,174,314 SNPs.
Genetic Instrumental Variable Selection
The core component of Mendelian randomization studies involves utilizing single nucleotide polymorphisms (SNPs) as instrumental variables (IVs). SNPs, serving as IVs, can overcome confounding factors inherent in observational research, provided that we obtain effective IVs through stringent selection criteria. For immune cell and inflammatory factors, P<5×10−6 represents the threshold for selecting genetic predictive factors. Secondly, we computed the F-statistic for each genetic instrumental variable and selected IVs with F>10. To minimize bias introduced by linkage disequilibrium (LD), we grouped all SNPs based on a linkage disequilibrium threshold of r2<0.001 within a distance of ±10,000 kb. This grouping process was performed in 800 genomes of the European reference panel separately, limited to SNPs with a minor allele frequency >0.01. Finally, we ensured that the impact of a SNP on a specific outcome and exposure is adjusted to the same allele, ensuring allele-specific harmonization. Additionally, we excluded palindromic SNPs from the analysis.8
MCAO Mice Model
The model creation involved the use of male C57 mice with a weight range of 15–20g. Administer pentobarbital sodium anesthesia to fully anesthetize the mice, ensuring complete relaxation. Make a skin incision and superficial cut on the neck of the mouse to expose the carotid artery and jugular vein. Use a surgical microscope to separate and ligate the external and internal carotid arteries adjacent to the carotid artery to block blood flow to the brain. Carefully remove the ligature after confirming the blockage of blood flow, ensuring it no longer obstructs the blood flow. Suture the wound to ensure complete closure and allow the mouse to recover in a warm and dry environment. Perform neuroimaging techniques and behavioral tests to validate the effectiveness of the brain ischemia model.
To verify the regulatory relationship between Piezo1 and IL-6, it was divided into Control group, MCAO group, MCAO group+Piezo1 knockout group, and Piezo1 group.
To verify the regulatory relationship between Piezo1 and IL-6 and GPX4, IL-6R alpha was added to the body and divided into Control group, MCAO group, MCAO group+Piezo1 knockout group, MCAO group+Piezo1 knockout+IL-6Ra group, IL-6Ra group (IL-6 receptor agonist, sIL-6R group).
Immunofluorescence
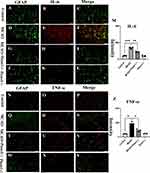
For tissue immunofluorescence, brain tissue sections (20um) were deparaffinized and hydrated prior to antigen retrieval. The renal sections were then blocked with 10% bovine serum albumin for 1 hour at room temperature. The slides were incubated overnight at 4°C with anti-IL-6 (1:100, AiFang, AF06790), anti-TNF-α (1:100, AiFang, AF06294), and AE1 (Santa Cruz, sc-81,714).9,10
Enzyme-Linked Immunosorbent Assay (ELISA)
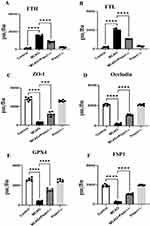
Elisa kits were employed to detect the levels of FTH, FTL, ZO-1, Occludin, GPX4, and FSP1. Follow the specific operating instructions provided in the reagent kit manual, and record the results using an excel spreadsheet.
Morris Water Maze
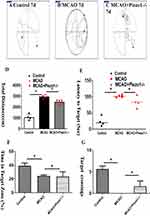
The Morris water maze has been instrumental in studying spatial learning and memory deficits, as well as the effects of genetic manipulations, drug treatments, and environmental factors on cognitive function. Escape latency: The time it takes for the animal to find and climb onto the hidden platform. It is typically measured for each trial and can provide information about the animal’s learning curve over time. Path length: The distance traveled by the animal to reach the hidden platform. It can indicate the efficiency of navigation and the ability to find the platform. Probe trial: This is a separate trial conducted after the learning phase, where the hidden platform is removed. The animal’s exploration and search for the platform’s previous location are observed. This measure assesses memory retention and spatial memory consolidation.
Statistics
Statistical analysis Statistical analysis was conducted using Graphpad prism 9.0. Data were expressed as the means ± standard error (SE). Comparisons between multiple groups were performed using one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
Results
Instrument Variables Included in Analysis
The selection criteria mentioned above were used to extract valid instrumental variables from immune cell and inflammatory factors GWAS. All Single Nucleotide Polymorphisms (SNPs) utilized in the analysis had an F-statistic greater than 10.
Two-Sample Mendelian Randomization (MR) Analysis
Establishing significance thresholds for multiple testing for each level of immune cell categories. The significance thresholds for immune cell categories were determined as follows: Fibroblast growth factor 5 levels, Interleukin-6 levels, Neurturin levels, Caspase 8 levels.
Significant Causal Association
In the MR analysis of immune cell, inflammatory factors and stroke, upon applying the multiple testing significance threshold, we identified 2 immune cell displaying significant causal associations with stroke. Fibroblast growth factor 5 levels (OR=0.9, CI=0.83–0.99, IVW), Interleukin-6 levels (OR=1.11, CI=1.07–1.14, IVW), Neurturin levels (OR=0.90, CI=0.83–0.97, IVW), Caspase 8 (OR=1.13, CI=1.02–1.26, IVW) levels showed a significant association with stroke. In Two-Sample Mendelian Randomization (TSMR), we have identified inflammatory factors that exhibit potential causal associations with stroke (Figures1–3).
 |
Figure 1 Forest plots showed the causal associations between stroke and inflammation factor traits. Abbreviations: IVW, inverse variance weighting; CI, confidence interval. |
Sensitivity Analyses
In subsequent studies, we excluded the potentially causal associations with inconsistent directions but retained those with significant causal associations despite the inconsistency. We approached these results cautiously in further analyses. No evidence of horizontal pleiotropy was found in the causal associations based on the intercept from MR-Egger analysis. The Cochran’s Q statistic indicated heterogeneity, but as previously mentioned, the inverse variance-weighted method with multiplicative random effects can account for the heterogeneity in the causal estimates. The leave-one-out analysis identified causal associations that are influenced by specific SNP variants. After removing these causal associations, we performed MR-PRESSO analysis on the remaining associations.
Piezo1 Knockout Improves Cognitive Dysfunction Following MCAO
The water maze experiment was conducted to assess the behavioral performance of mice. The pathway of crossing the platform is illustrated in (Figure 4A–C). The measurements included escape latency, number of platform crossings, distance in the target zone, and percentage of distance in the target zone. In comparison to the control group, the MCAO group demonstrated prolonged escape latency, decreased number of platform crossings, and reduced distance in the target zone. However, treatment with Piezo1 knockout improved these conditions (Figure 4D–G).
Piezo1 Knockout Alleviates Astrocyte-Induced Neuroinflammation Following MCAO
In comparison to the control group (Figure 5A–C), the protein expression levels of IL-6 were upregulated in the hippocampus region of brain tissue at 3 days after MCAO (Figure 5D–L). However, administration of Piezo1 knockout inhibited the expression of IL-6 (Figure 5M). The results demonstrated that MCAO treatment induced TNF-α immunoreactivities in mice. Nevertheless, Piezo1 knockout therapy reduced the expression levels of TNF-α. Furthermore, compared to the control group, the MCAO group exhibited a significant increase in the protein expression level of TNF-α, but Piezo1 knockout treatment restored the expression of TNF-α (Figure 5N–Z).
Piezo1 Knockout Improves Ferroptosis and Blood-Brain Barrier Permeability
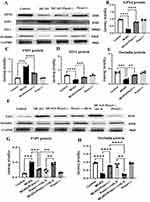
Elisa quantified the ferroptosis by endothelial cells. The MCAO group reduced the expression levels of ZO-1, occludin, GPX4, FSP1 and increased the expression levels of FTH, and FTL in comparison to the control group. Following intervention with Piezo1 knockout, the expression levels of ZO-1, occludin, GPX4, FSP1 increased and the expression levels of FTH and FTL decreased (Figure 6A–F).
Piezo1 Knockout Did Not Affect the Changes in ROS in Lung Tissue
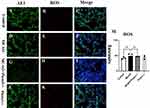
Figure 7A–L depict the ROS expression in lung tissues across various groups. The bar chart M revealed that the MCAO group did not induce significant alterations in lung ROS levels compared to the control group.
The Knockout of Piezo1 Did Not Induce Noticeable Damage to the Target Organs
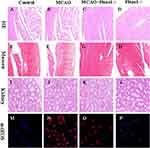
Figure 8A–D display the outcomes of HE staining performed on myocardial tissue. MCAO can lead to architectural disarray in myocardial tissue, whereas Piezo1 knockout does not interfere with myocardial fiber structure. Figure 8E–H present the findings from Masson’s staining of cardiac tissue. MCAO can aggravate myocardial cell fibrosis, whereas Piezo1 knockout intervention does not exacerbate it. Figure 8I–L show that Piezo1 knockout has no effect on the kidneys, while 8M-8P shows that Peizo1 has no effect on oxidative stress in the kidneys.
Piezo1 Knockout Improves ZO-1 and Occludin by Inhibiting the IL-6/GPX4 Pathway
We assume that Piezo1 protects the blood-brain barrier by inhibiting the IL-6/GPX4 pathway. To determine this hypothesis, the effects of Piezo1 on the blood-brain barrier and iron death were first verified. At 3 days, the protein expression levels of GPX4, FSP1, ZO-1, and Occludin were measured in the brain using Western blotting. Compared with the control group, after 3 days of MCAO modeling, FSP1 levels were significantly upregulated in optical density, while GPX4, ZO-1, and Occludin levels were significantly downregulated. However, Piezo1 knockout significantly reduced FSP1 expression and increased GPX4, ZO-1, and Occludin levels (Figure 9A–E). After adding additional sIL-6R, a specific IL-6 receptor agonist, compared to the MCAO+Piezo1 knockout group, FSP1 expression was upregulated, while occludin levels were significantly downregulated. Therefore, in vivo data confirms that Piezo1 protects the blood-brain barrier by inhibiting the IL-6/GPX4 pathway (Figure 9F–H).
Discussion
Stroke results in severe disabilities and mobility issues worldwide, and the development of effective new drugs is urgently needed for stroke-related cognitive dysfunction.11,12 In this study, we demonstrated that Piezo1 knockout can improve neurological deficits in a mouse model of MCAO and inhibit oxidative stress and inflammation in the damaged hippocampus, which is caused by ischemic stroke. Importantly, Piezo1 knockout does not have any adverse effects on the heart, lungs, kidneys, or other organs.
In recent years, several studies have highlighted the crucial role of astrocytes in ischemic brain injuries.13,14 For example, reactive astrocytes can produce and release pro-inflammatory mediators, which may lead to neuronal death and the progression of infarction. In this study, our focus was on astrocytes to gain insights into novel mechanisms through which Piezo1 affects neurological function. Our Mendelian randomization study revealed a correlation between IL-6 and cognitive function prognosis after ischemic stroke, and animal experiments showed increased IL-6 expression after MCAO, which was reduced after Piezo1 knockout. This represents the first evidence of the IL-6/GPX4 pathway triggering an inflammatory response in astrocytes in the presence of Piezo1. Importantly, Piezo1 knockout leads to reduced IL-6 and TNF-α production in astrocytes, which subsequently activates ferroptosis in endothelial cells, resulting in the reduction of tight junction proteins and the disruption of the blood-brain barrier.
Endothelial cell ferroptosis play a significant role in cerebral ischemia injury.15,16 Numerous studies have investigated the safety and therapeutic efficacy of the iron chelator and antioxidant drugs, such as deferoxamine (DFO) or N-acetylcysteine (NAC), in patients experiencing ischemic stroke. In individuals with moderate hematoma volume (HV) after intracerebral hemorrhage (ICH), a higher proportion of those treated with DFO attained a modified Rankin Scale score of 0–2 compared to those receiving a placebo, although this effect was not statistically significant for patients with small or large HVs.17,18 Early oral administration of NAC following acute ischemic stroke has demonstrated improved outcomes in terms of acute neurological deficits and disability, primarily attributable to its antioxidant and anti-inflammatory properties.19 Furthermore, selenium supplementation has been shown to effectively inhibit GPX4-dependent iron-related cell death and endoplasmic reticulum stress-induced cell death by enhancing GPX4 expression subsequent to ischemic stroke.20 These findings propose that targeting ferroptosis following IRI may hold promise as a potential therapeutic approach for ameliorating neuronal injury.
Ferroptosis, mediated by reactive oxygen species (ROS)-induced lipid peroxidation, has been implicated in the progression of cerebral infarction in recent years.21 Given the significant role of ferroptosis in the development of cerebral infarction, we examined the markers of ferroptosis in the mouse hippocampus and observed that ferroptosis occurs during cerebral infarction. GPX4, as an antioxidant enzyme, plays a crucial role in neutralizing lipid peroxidation and protecting cell membrane fluidity.22 Increasing evidence suggests that GPX4 reduces the accumulation of lipid hydroperoxides, thereby inhibiting ferroptosis. Some studies have found that GPX4 maintains the activation of Treg cells and suppresses anti-tumor immunity by preventing lipid peroxidation and iron deficiency anemia. Another study indicated that cAMP response element-binding protein (CREB) inhibits iron deficiency anemia by stimulating GPX4 transcription.23 Therefore, enhancing GPX4 expression in cerebral infarction may be an effective approach to inhibit its progression.
Recent findings have identified the role of the voltage-gated channel Piezo1 in the regulation of neuroinflammation.24 We hypothesized that Piezo1 may improve cerebral infarction by regulating GPX4 expression levels through the secretion of the inflammatory factor IL-6, thus inhibiting endothelial cell ferroptosis. As expected, our research revealed a significant decrease in GPX4 expression in a mouse model of cerebral infarction, which was reversed by Piezo1 knockout. It is widely believed that the excessive release of inflammatory factors is closely associated with disease occurrence. Increasing evidence suggests that FSP1 is regulated by inflammatory factors. In this study, we found that the levels of FSP1 are regulated by IL-6, and additional IL-6 receptor agonist (sIL-6R) downregulated FSP1 expression while upregulating Occludin expression.
Limitation
Our study provides valuable insights for future clinical and animal experiments. However, our research has some limitations. Firstly, GWAS summary data represent lifetime genetic exposure. Therefore, further clinical studies and animal experiments are needed to investigate causality inferences derived from Mendelian randomization analysis, whether they can represent the impact of short-term changes in inflammatory factors on the host. Secondly, the data we analyzed only included individuals of European descent, and caution should be exercised when extrapolating our results to other populations, necessitating validation in diverse ethnic groups. Thirdly, larger-scale prospective, randomized controlled clinical trials on Piezo1 inhibitors and cognitive impairment need to be conducted in the future.
Conclusion
In summary, in this study, we discovered that Piezo1 may inhibit endothelial cell ferroptosis by suppressing IL-6/GPX4, thereby preventing the progression of cerebral infarction.
Abbreviations
IL-6, interleukin-6; GPX4, glutathione peroxidase 4; ZO-1, zonula occludens-1; BBB, blood-brain barrier; GABA, glutamate and gamma-aminobutyric acid; SNPs, single nucleotide polymorphisms; IVs, instrumental variables; LD, linkage disequilibrium; SE, standard error;ANOVA, analysis of variance; TSMA, Two-Sample Mendelian Randomization Analysis.
Data Sharing Statement
Data can be provided if necessary. The data is available from the first authors (Lujia Tang and Di Xie).
Ethics Approval and Consent to Participate
This study was approved by the Animal Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, with an ethics number of 2021-028. The researchers stated they strictly comply with the national laws (the People’s Republic of China National Standard GB/T 35892-2018).
Consent to Publish
All authors agreed to publish.
Funding
This work was Sponsored by Program of Shanghai Academic Leader, Grant Number 21XD1402200; the Shanghai Committee of Science and Technology, Grant No. 23YF1425200; and National Natural Science Foundation of China, Grant Number 82072207.
Disclosure
The authors have declared that no competing interest exists.
References
1. Brett BL, Gardner RC, Godbout J, Dams-O’Connor K, Keene CD. Traumatic brain injury and risk of Neurodegenerative Disorder. Biol Psychiatry. 2022;91:498–507. doi:10.1016/j.biopsych.2021.05.025
2. Daugherty J, Zhou H, Sarmiento K, Waltzman D. Differences in state traumatic brain injury-related deaths, by principal mechanism of injury, intent, and percentage of population living in rural areas - United States, 2016–2018. MMWR Morb Mortal Wkly Rep. 2021;70(41):1447–1452. doi:10.15585/mmwr.mm7041a3
3. Vergne-Salle P. Bertin P.Chronic pain and neuroinflammation. Joint Bone Spine. 2021;88(6):105222. doi:10.1016/j.jbspin.2021.105222
4. Wu H, Zheng J, Xu S, et al. Mer regulates microglial/macrophage M1/M2 polarization and alleviates neuroinflammation following traumatic brain injury. J Neuroinflammation. 2021;18:2. doi:10.1186/s12974-020-02041-7
5. Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron. 2017;95:1246–1265. doi:10.1016/j.neuron.2017.07.010
6. Bolte AC, Shapiro DA, Dutta AB, et al. The meningeal transcriptional response to traumatic brain injury and aging. Elife. 2023:
7. Jin Q, Ren F, Dai D, et al. The causality between intestinal flora and allergic diseases: insights from a bi-directional two-sample Mendelian randomization analysis. Front Immunol. 2023;14:1121273. doi:10.3389/fimmu.2023.1121273
8. Gao Y, Fan ZR, Shi FY. Hypothyroidism and rheumatoid arthritis: a two-sample Mendelian randomization study. Front Endocrinol. 2023;14:1179656. doi:10.3389/fendo.2023.1179656
9. Xie D, Ge X, Ma Y, et al. Clemastine improves hypomyelination in rats with hypoxic-ischemic brain injury by reducing microglia-derived IL-6 via P38 signaling pathway. J Neuroinflammation. 2020;17:57. doi:10.1186/s12974-019-1662-6
10. Xie D, Shen F, He S, et al. IL-6 induces hypomyelination in the periventricular white matter through inhibition of oligodendrocyte progenitor cell maturation via FYN/MEK/ERK signaling pathway in septic neonatal rats. Glia. 2016;64:583–602. doi:10.1002/glia.22950
11. Ting SMV, Ding CH, Wahab AA. Brain abscess caused by salmonella enteritidis following craniotomy for meningioma: a case report and literature review. Malays J Pathol. 2021;43(2):333–336.
12. Al-Zaidi AS, Mohtarrudin N, Chupri J, et al. Differential time course of glycogen synthase kinase-3 inhibition in experimental autoimmune encephalomyelitis. Malays J Pathol. 2021;43(3):413–424.
13. Spitzer D, Guérit S, Puetz T, et al. Profiling the neurovascular unit unveils detrimental effects of osteopontin on the blood-brain barrier in acute ischemic stroke. Acta Neuropathol. 2022;144(2):305–337. doi:10.1007/s00401-022-02452-1
14. Jurcau A, Simion A. Neuroinflammation in cerebral ischemia and ischemia/reperfusion injuries: from pathophysiology to therapeutic strategies. Int J Mol Sci. 2021;23(1):14. doi:10.3390/ijms23010014
15. Zheng D, Liu J, Piao H, Zhu Z, Wei R, Liu K. ROS-triggered endothelial cell death mechanisms: focus on pyroptosis, parthanatos, and ferroptosis. Front Immunol. 2022;13:1039241. doi:10.3389/fimmu.2022.1039241
16. Chu C, Wang X, Yang C, et al. Neutrophil extracellular traps drive intestinal microvascular endothelial ferroptosis by impairing Fundc1-dependent mitophagy. Redox Biol. 2023;67:102906. doi:10.1016/j.redox.2023.102906
17. Kosyakovsky J, Fine JM, Frey WH 2nd, Hanson LR. Mechanisms of intranasal deferoxamine in Neurodegenerative and Neurovascular disease. Pharmaceuticals. 2021;14(2):95. doi:10.3390/ph14020095
18. Guo X, Jin X, Han K, et al. Iron promotes neurological function recovery in mice with ischemic stroke through endogenous repair mechanisms. Free Radic Biol Med. 2022;182:59–72. doi:10.1016/j.freeradbiomed.2022.02.017
19. Turkmen S, Cekic Gonenc O, Karaca Y, et al. The effect of ethyl pyruvate and N-acetylcysteine on ischemia-reperfusion injury in an experimental model of ischemic stroke. Am J Emerg Med. 2016;34(9):1804–1807. doi:10.1016/j.ajem.2016.06.003
20. Liu C, Li Z, Xi H. Bioinformatics analysis and in vivo validation of ferroptosis-related genes in ischemic stroke. Front Pharmacol. 2022;13:940260. doi:10.3389/fphar.2022.940260
21. Liang D, Feng Y, Zandkarimi F, et al. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell. 2023;186(13):2748–2764.e22. doi:10.1016/j.cell.2023.05.003
22. Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med. 2020;152:175–185. doi:10.1016/j.freeradbiomed.2020.02.027
23. Xue Q, Yan D, Chen X, et al. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy. 2023;19(7):1982–1996. doi:10.1080/15548627.2023.2165323
24. Velasco-Estevez M, Rolle SO, Mampay M, Dev KK, Sheridan GK. Piezo1 regulates calcium oscillations and cytokine release from astrocytes. Glia. 2020;68(1):145–160. doi:10.1002/glia.23709
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.