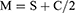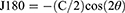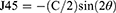Back to Journals » Clinical Optometry » Volume 16
Peripheral Eye Length Evaluation in Myopic Children Undergoing Orthokeratology Treatment for 12 Months Using MRI
Authors Low YC , Mohd-Ali B , Shahimin MM , Mohidin N, Abdul-Hamid H, Mokri SS
Received 9 November 2023
Accepted for publication 13 January 2024
Published 9 February 2024 Volume 2024:16 Pages 35—44
DOI https://doi.org/10.2147/OPTO.S448815
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Mr Simon Berry
Yu Chen Low,1 Bariah Mohd-Ali,1 Mizhanim Mohamad Shahimin,1 Norhani Mohidin,1 Hamzaini Abdul-Hamid,2 Siti Salasiah Mokri3
1Optometry and Vision Science Program and Research Centre for Community Health (REACH), Faculty of Health Sciences, UKM Jalan Raja Muda Abdul Aziz, Kuala Lumpur, Malaysia; 2Department of Radiology, Faculty of Medicine, UKM, Cheras, Kuala Lumpur, Malaysia; 3Department of Electrical, Electronics and Systems, Faculty of Engineering and Built, UKM, Bangi, Selangor, Malaysia
Correspondence: Bariah Mohd-Ali, Optometry and Vision Science Program and Research Centre for Community Health (REACH), Faculty of Health Sciences, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, Kuala Lumpur, 50300, Malaysia, Tel +603-92897602, Email [email protected]
Purpose: To investigate changes in peripheral eye length (PEL) in myopic children undergoing orthokeratology (Ortho-K) treatment for 12 months using MRI. The results were compared to single vision spectacle wearers (SVS).
Patients and Methods: A total of 70 children with myopia (aged 8– 9 years old) were recruited. A total of 45 children were fitted with Ortho-K, and 25 were fitted with SVS. The PEL and axial length (AL) were measured by using MRI 3-Tesla, whereas central and peripheral refraction (PR) measurements were conducted at ± 30 degrees horizontally with nasal (N) and temporal (T) intervals of 10°, 20°, and 30° and with an open field autorefractometer (WAM-5500 Grand Seiko). All the measurements were conducted at the baseline and 12 months.
Results: The MRI analysis indicates that at 12 months, the SVS group showed more elongation of the PEL and AL at all eccentricities than the Ortho-K group did (p < 0.05). The Ortho-K group only showed significant PEL elongation beyond 20 degrees at N20, N30, T20, and T30 (p < 0.05); however, a significant reduction in the AL was detected in the center AL, N10, and T10 (p < 0.05). All eccentricities in the relative PR of the Ortho-K group were significantly more myopic than at the baseline (p < 0.05), whereas in the SVS group, all eccentricities in the relative PR were shown to be significantly more hyperopic than at the baseline (p < 0.05). The PEL and PR showed negative correlations at 12 months in the Ortho-K group.
Conclusion: MRI analysis can be utilized to describe changes in PEL in myopic children. It appears that as myopia progressed in Ortho-K lens wearers, the PEL increased by a greater amount than the AL did; thus, the retina was reshaped to become increasingly oblate and to display peripheral myopic defocus.
Keywords: myopia, peripheral eye length, peripheral refraction, children
Introduction
Myopia is one of the most prevalent disorders of the eye, with the highest prevalence among younger generation in East and Southeast Asian countries.1,2 Even though several myopia risk factors have been identified by previous investigators such as heredity,3 ethnicity,4,5 environmental factors,6 and lack of outdoor activities,7 the exact pathogenic mechanisms underlying the etiology of myopia remain unclear. In addition, high myopia is associated with increased risk of ocular comorbidities (such as cataract and maculopathy), socioeconomic burden, and compromised quality of life. Therefore, finding means of controlling myopia progression is essential.
The role of hyperopic defocus on the peripheral retina as a risk factor for the progression of myopia remains controversial.8,9 Previous studies conducted on populations of children found that emmetropes have myopic relative peripheral refraction (PR), whereas myopes have relatively hyperopic PR.10,11 It was reported that peripheral hyperopic defocus stimulates the growth of the axial length (AL), thus increasing the central refractive error and exacerbating myopia.12,13 The relationship between peripheral eye length (PEL), PR and retinal shape in young myopic adults in horizontal and vertical has been investigated.14 The authors reported that that the effects of meridian and refraction on relative PR and relative PEL patterns are consistent with effects on retinal shape and that steeper retinas derived from peripheral eye lengths predict more positive RPR. In another study of children aged 7–11 years old, it was found that there is a significant correlation between relative PEL and central myopic shift, indicating that retinal steepness influences AL elongation through growth stimuli produced by hyperopic defocus and this may promote myopia progression in children.15
Nevertheless, studies have shown that there may not be a direct link between hyperopic defocus and myopia progression in children. The relative PR changes in 58 children aged 6–9 with different ametropias were evaluated earlier, and the authors concluded that there is a lack of direct causation between relative PR and the development of central myopia in a child’s eye.16 Similarly, in support of their findings, a larger scale longitudinal study with Chinese children concluded that central refraction changes between follow-up visits did not align with the hypothesis that central refraction change becomes more myopic as relative PR becomes more hyperopic.17 The authors also highlighted that the relationship between peripheral optics and central myopia progression is more complex than previously thought, with factors like retinal shape potentially playing a role, emphasizing the need for a more comprehensive approach to myopia research and management.
Several optical treatment options have been used in the past to reduce myopia progression in children. Orthokeratology (Ortho-K) has been proven to effectively reshape the cornea, which slows down the progression of myopia and improves the vision-related quality of life in children.18,19 Wearing Ortho-K lenses flattens the central cornea and steepens the peripheral cornea. This results in optical light rays being focused centrally on the fovea and peripheral light rays are being focused anterior to the peripheral retina, thus converting hyperopic defocus into myopic defocus in Ortho-K wearers.20 Several studies have shown that subjects who wore spectacles for corrections showed hyperopic PR after Ortho-K treatment.21–23 Studies on the growth of the posterior ocular shape—specifically on the peripheral retina length in children with myopia who underwent Ortho-K treatment—are limited. Only one study investigated the influence of Ortho-K treatment on the PEL and PR in children with myopia.24 However, the authors measured central eye length and PEL using optical biometer instead of a direct visualization of the ocular structure. According to Atchison and Rozema,25 the application of equipment intended for on axis measurement such as ocular biometry, to the periphery, may produce incorrect interpretation and artifacts. Fixation target that is attached to the equipment, as was done by previous investigators may cause artefact to occur when the device is moved laterally for corrective realignment. In this study, MRI was used to obtain a direct imaging of the posterior retina that could show a clearer picture of the actual state of the posterior retina in myopic eyes. MRI is a powerful tool that facilitates three-dimensional measurements of the eye and is suited for studies of the ocular structure, including the shape of the posterior segment of the eye.26 In previous reports, MRI was used to investigate the shapes of myopic eyes and provide comprehensive information for morphometric analysis.26–28
The main goal of this work was to determine changes in the peripheral retina influenced the progression of myopia in children wearing Ortho-K lenses for 12 months, using MRI. The results obtained in the Ortho-K group were compared with those of a single-vision spectacle (SVS) control group.
Materials and Methods
Ethical Approval and Consents
All subjects were healthy, had no history of myopia control interventions, and were free of ocular diseases. The parents of the subjects were briefed about the project and consents were obtained from them prior to the commencement of the study. The tenets of the Declaration of Helsinki were followed, and ethical clearance were obtained from the research ethics committee of Universiti Kebangsaan Malaysia (UKM PPI-800-1/1/5 JEP-2017-422).
Sample Size Calculation
The change in AL was the main outcome of this study, and convenience sampling was used to recruit the participants. The G*Power calculator (version 3.1.9.3) was used to calculate the sample size in this study based on data from previous study,29 in which the authors reported that the changes in AL were 0.36 (SD: 0.24 mm) at the end of 2 years of Ortho-K treatment. About 90% power were used to detect a 0.24 mm standard deviation in the AL measurements between the SVS group and Ortho-K group, which was equivalent to a change in refraction of approximately 0.75D,30 with a significance level at α = 0.05. Thus, the minimum sample size needed to complete the study in each group was n = 17.
Subjects
All subjects were 8–9 years old, had a myopic spherical equivalent refractive error (SER) of −0.75 D to −4.00 D, had an astigmatism of less than −1.50 DC, and had best corrected log of the minimal angle of resolution (logMAR) visual acuity of 0.0 or better in each eye. The allocation of subjects to either the Ortho-K treatment group or the SVS control group in this study was decided by their parents. Written consent was obtained from the parents of all participants in the Ortho-K group and SVS group prior to the commencement of the treatment. The methods and intervention used in this study were previously reported.19 The specific methods relevant to this section of the PEL and PR measurements are described below.
Examinations and Measurements
Data collection were performed at the baseline and 12 months on all subjects. Following the instillation of a topical anaesthetic (0.5% proxymetacaine hydrochloride, Alcaine, Alcon), cycloplegia was induced with two drops of 1% cyclopentolate hydrochloride (CyclogelTM, Alcon, Geneva, Switzerland) separated by 5 min intervals. Objective central refraction and PR measurements were taken with a WAM-5500 autorefractor (Grand Seiko Co., Ltd., Hiroshima, Japan) when the pupil size exceeded 5 mm. All subjects were instructed to stabilize their heads while facing straight ahead and to rotate their eyes to fixate on high-contrast letter targets with the sizes of 6/12 (20/40, 0.3 logMAR) that were mounted on the wall at 4 m. These targets were separated by 10° intervals over the central ±30° interval across the horizontal eccentricities in the nasal and temporal visual fields (VFs, 30° N, 20° N, 10° N, centre 10° T, 20° T, 30° T). The eye rotation technique was used in this research. To obtain the PR measurements, as the eye rotated, the axis of the autorefractor was aligned with the pupil’s centre and the corneal reflex.31 When measuring the right eye, the left eye was occluded, and vice versa. Five consistent measurements were taken at each point of the target, and the mean was obtained. If an error or fixation loss was found, the reading was discarded and repeated. The results were recorded as VF eccentricities, where the nasal VF represented the temporal retina, and the temporal VF represented the nasal retina. Refractive error readings were obtained in the form of sphere (S), cylinder (C), and axis (θ). The results were then calculated by converting the spherocylindrical refractive error into power vector components—M, J180, and J45—as recommended by Thibos et al:32
Where M represents the mean spherical equivalent, J180 represents the horizontal component, and J45 represents the oblique cross-cylindrical component.32
MRI Measurement
All MRI measurements were conducted by an expert paediatric radiologist at the university’s hospital (Hospital Canselor Tuanku Muhriz (HCTM)). The subjects were ensured to be healthy and fit for MRI examinations. An explanation and videos with information about MRI were administered 30 min before the procedure. Earplugs and headphones for noise cancelling were provided to the subjects. A researcher remained in the MRI room throughout the whole MRI process to make sure that the child was not anxious and stayed still during the examination. The details of the methods used for MRI acquisition in this study have been published elsewhere.27,28
MRI Segmentation and PEL Measurement
All MRI data were exported as Digital Imaging and Communications in Medicine (DICOM) images for analysis. By using MATLABTM (MathWorks, Natick, MA, USA), the center axial images were detected, and an initial curve was defined. For a specific selected axial image slice, a level-set segmentation method based on the Chan–Vese model was used on the contour of the eyeball, which was obtained by defining the initial curve (zero-level set) for each eye, and an outline was generated to trace the outer edge of the shape of the sclera.33 The details of the methods used to develop the program in this study for analyzing the MRI images have been published elsewhere.27,28
Validation of the MRI Measurements
For the validation of the MRI measurements, Pearson’s correlation analysis was performed to yield the correlation between the AL obtained from the MRI scan and the AL obtained from an AScan (Sonomed, New Hyde Park, NY, USA). The results showed that the AL obtained from the MRI scan (23.72 ± 0.82 mm) was significantly highly correlated with the AL obtained from the AScan (23.54 ± 0.55 mm) (p < 0.05).
Statistical Analysis
Only the data for the right eye was included in this study. A statistical analysis was performed by using the Statistical Package for the Social Sciences (SPSS, version 21.0), and values were reported as the mean ± standard deviation. All data was normally distributed. A paired t-test was performed to evaluate the differences in the baseline data and 12-month data within the Ortho-K group and SVS group. The differences in the baseline and 12-month data between the Ortho-K group and SVS group were compared through an independent t-test. Spearman correlation was performed to examine the relationship between PEL and PR.
Results
A total of 80 children with myopia were recruited at the beginning of the study. However, only 70 of the children (45 in the Ortho-K group and 25 in the SVS group) completed the study. Of the five (5) children in the Ortho-K group who did not complete the study, one was unable to control his frequent eye-rubbing habit, two showed poor compliance, and another two dropped out due to COVID-19 infection. Five children in the SVS group were unable to comply with the follow-up appointments and dropped out of the study. There were no significant differences in any baseline characteristics between the Ortho-K and SVS groups (p > 0.05). The A-scan and MRI results also showed no significant differences in LAL and PEL measurements (p > 0.05). The participants’ demographic data are presented in Table 1.
 |
Table 1 Demographic Data of the Subjects |
Measurements at 12 Months
Table 2 provides a summary of baseline and 12-month data measurements for both the Orthokeratology (Ortho-K) and Single Vision Spectacles (SVS) groups. For the Ortho-K subjects, there was significant reduction in the SER after 12 months of Ortho-K wear (p<0.05). There was also significant slowing of eye growth in the Ortho-K group, which was reflected by the decrease in Cornea FK, Axial length and LAL at the centre at 12 months (p<0.05). Whereas for SVS subjects, SER, AL and LAL at the centre increased significantly (p<0.05).
 |
Table 2 Mean and Standard Deviation (±SD) of Ocular Parameters at Baseline and 12 Months |
Figure 1 shows that SVS group had significantly increased PEL at all the measured horizontal eccentricities (p < 0.05), except N30. By contrast, the Ortho-K group only showed significant PEL elongation beyond 20° eccentricities, N20, N30, T20, and T30 (p < 0.05) but demonstrated no significant elongation at N10 and T10. Rather, a significant reduction in LAL was detected in the Ortho-K group (p < 0.05), Figure 2.
 |
Figure 1 Peripheral eye length (PEL) at the baseline and 12 months in single vision (SVS) wearers. |
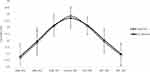 |
Figure 2 Peripheral eye length (PEL) at the baseline and 12 months in Orthokeratology (Ortho-K) wearers. |
Central Refraction and Relative Peripheral Refraction (RPR)
The mean central refraction and relative peripheral refraction at the baseline and 12 months are shown in Table 3.
 |
Table 3 Mean Relative Peripheral Refraction (RPR) in the Orthokeratology (Ortho-K) and Single Vision (SVS) Wearers at the Baseline and 12 Months |
All subjects in both groups showed hyperopic RPR at the baseline, and the RPR did not significantly vary between the Ortho-K and SVS groups (p > 0.05). However, the RPR at all eccentricities in the Ortho-K group was significantly more myopic at 12 months than at the baseline (p < 0.05), which was evident with the increase in myopia beyond 20° of the visual field. By contrast, in the SVS group, the RPR at 12 months was significantly more hyperopic than that at the baseline (p < 0.05). The difference in the RPR measurements between the nasal and temporal parts of the retina in both groups was also analyzed and was found to be nonsignificant (p > 0.05).
Correlation analysis between the peripheral refraction (PR) and peripheral eye length (PEL) in Ortho-K subjects were performed and revealed significant correlation ranged from 0.31 to 0.65 (all <0.05) at each eccentricity. Linear regression showed negative correlation at each eccentricity, indicating a more hyperopic or less myopic refractive error centrally and peripherally with shorter eye length (Table 4).
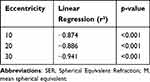 |
Table 4 Linear Regression Between Changes in SER at 12 Months for a Given Peripheral Eccentric Location [(M Nasal + M Temporal)/2] and the SER at Baseline |
Discussion
The role of peripheral eye length (PEL) and peripheral refraction (PR) as potential factors in myopia development has been reported. This study described and compared the peripheral eye length PEL and peripheral refraction PR in children with myopia who wore Ortho-K lenses and SVS for 12 months by using Magnetic resonance imaging (MRI) measurement. MRI is a powerful tool that facilitates the three-dimensional measurement of the eye and has been used in several studies of myopia to define the ocular shape.31,34
To our knowledge, this is the first report to demonstrate the effect of Ortho-K treatment on the PEL and its correlation with PR in myopic children using MRI measurements.
The findings of this study support the theory that the Ortho-K lens treatment can effectively reshape the eyeball and temporarily reduce the refractive error and AL elongation, hence retard the progression of myopia in children.26,35–38 AL shortening of −0.18 ± 0.38mm has been observed in Ortho-K treatment group. It was also reported in a previous study, where subjects aged 7–11 years old who underwent Ortho-K treatment had a 0.026mm AL shortening.37 This phenomenon is attributed to central corneal thinning, which occurs due to the redistribution of corneal epithelial tissue when optimal correction is reached. However, AL reduction is a transient effect and typically reverts to the baseline condition once Ortho-K treatment is discontinued.
It was observed that after 12 months of treatment, although the whole eyeball was elongated in SVS and Ortho-K subjects, eyeball elongation in SVS group has appeared to become increasingly prolate when PEL elongates at all eccentricities with AL being the longest. On the contrary, Ortho-K subjects showed a flatter retinal plane with increased PEL increased at every eccentricity compared to AL, rather, a significant reduction in AL was detected in the Ortho-K group.
In an earlier study, the investigators used an optical biometer to investigate the effect of Ortho-K treatment for 13 months on PEL and PR in children with myopia and found similar results.24 In the study, children in the Ortho-K group showed that PEL-N20° grew faster than all the other PELs and ALs (all p<0.05), whereas PEL-T10 grew slower than all other PELs and ALs (all p<0.05), which is also found in this study where the Ortho-K group only showed significant PEL elongation beyond 20 eccentricities, N20, N30, T20, and T30 (p < 0.05). Their study reported that the eye shape in the SVS group became more prolate with the progression of myopia, whereas in the Ortho-K group, the subjects showed a less prolate eye shape after 12 months. The reverse geometry rigid gas permeable design of Ortho-K lenses is hypothesized to cause a flattening effect on the central cornea and steepening effect on the mid-peripheral cornea, which caused changes in the status of peripheral defocus.
The results of this study aligned with previous studies who reported that individuals exhibit myopia reduction in the central field and the relative peripheral refraction become myopic after 12 months of Ortho-K treatment.20–24 Although peripheral refraction showed that there are hyperopic changes in the retina at the central refractive error within the central ± 20° and a myopic shift beyond 20°, the correlations shown between absolute PR and PEL at every eccentricity were not significant. Further analysis on the peripheral myopia defocus induced by Ortho-K as a function of individual baseline M component were carried out and the regression analysis showed that the peripheral values obtained were being the highest at the most peripheral eccentricities and progressively lower at the centre, 30°(r²= −0.941), 20°(r²=−0.886) and 10°(r²=−0.874), (all p<0.05) respectively. This finding concurred with Queiros et al23 who reported that the ratio of the highest myopic relative PR induced in the periphery by Ortho-K to baseline SER was 1:1, suggesting that the induced myopic defocus is influenced by the degree of myopia correction achieved in the central field. Therefore, it is crucial to emphasize that the alterations in relative peripheral refraction by Ortho-K are likely primarily to be caused by the amount of myopia reduction in the central vision area, rather than the cumulative effect arising from the deliberate induction of myopic defocus in the peripheral vision area.
Limitations of Study
The limitations of the present study must be acknowledged. First, the most significant limitation of this study is the short follow-up period. Given the short 12 months follow-up period of the current study, it is difficult to determine if the peripheral hypermetropia provides such a stimulus for growth or is simply a resultant effect of the increased axial growth. A longer follow-up period would be likely to include more variability within the observed myopia control efficacy from the Ortho-K lens and thus improve the ability to evaluate the impact of the magnitude of the PR myopic shift on the degree of myopia control.
Secondly, like most studies, this study investigated only the peripheral refraction at the horizontal meridian, and the vertical meridian was not assessed. Mutti et al reported that vertically, myopic subjects demonstrated myopic defocus relative to the fovea. It is particularly interesting to find out if the existing relative peripheral myopia in the vertical meridian would exert some effective myopia control or restrict the expansion of the globe in the vertical meridian relative to the horizontal meridian.39
Conclusion
This study concludes that wearing Ortho-K lens alters eyeball shape, reduces myopia and AL elongation and retards myopia progression. It appears that as myopia progressed in Ortho-K lens wearers, the PEL increased by a greater amount than the AL did; thus, the retina was reshaped to become increasingly oblate and to display peripheral myopic defocus. Nevertheless, more data is needed to confirm these findings.
Acknowledgments
The authors acknowledge Menicon Ltd, Japan, for funding this study (NN-2022-019).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Pan CW, Dirani M, Cheng CY., et al. The age-specific prevalence of myopia in Asia: a meta-analysis. Optom Vis Sci. 2015;92(3):258–266. doi:10.1097/OPX.0000000000000516
2. Grzybowski A, Kanclerz P, Tsubota K, et al. A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol. 2020;20(1):27–32. doi:10.1186/s12886-019-1220-0
3. Li J, Zhang Q. Insight into the molecular genetics of myopia. Mol Vis. 2017;23:1048–1080.
4. Kaur S, Ramli NI, Narayanasamy S. Heredity factor in myopia development among a sample in Klang Valley, Malaysia. Chin Med J. 2012;125:3522–3525.
5. Gifford KL, Richdale K, Kang P, et al. IMI—clinical management guidelines report. Invest Ophthalmol Vis Sci. 2019;60(3):M184–M203. doi:10.1167/iovs.18-25977
6. Siddharth K, Ashwini DL, Priyanka M, et al. Physical activity, time spent outdoors, and near work in relation to myopia prevalence, incidence, and progression: an overview of systematic reviews and meta-analyses. Indian J Ophthalmol. 2022;70(3):728–739. doi:10.4103/ijo.IJO_1564_21
7. Muralidharan AR, Lança C, Biswas S, et al. Light and myopia: from epidemiological studies to neurobiological mechanisms. Ther Adv Ophthalmol. 2021;13:1–45.
8. Atchison DA, Rosén R. The possible role of peripheral refraction in development of myopia. Optom Vis Sci. 2016;93(9):1042–1044. doi:10.1097/OPX.0000000000000979
9. Smith EL, Kee CS, Ramamirtham R, et al. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2005;46(11):3965–3972. doi:10.1167/iovs.05-0445
10. Mutti DO, Sholtz RI, Friedman NE, et al. Peripheral refraction and ocular shape in children. Invest Ophthalmol Vis Sci. 2000;41(5):1022–1030.
11. Sng CC, Lin XY, Gazzard G. Peripheral refraction and refractive error in Singapore Chinese children. Invest Ophthalmol Vis Sci. 2011;52(2):1181–1190. doi:10.1167/iovs.10-5601
12. Damani JM, Annasagaram M, Kumar P, et al. Alterations in peripheral refraction with spectacles, soft contact lenses and orthokeratology during near viewing: implications for myopia control. Clin Exp Optom. 2021;105(7):761–770. doi:10.1080/08164622.2021.1970480
13. Zheng X, Cheng D, Lu X, et al. Relationship between peripheral refraction in different retinal regions and myopia development of young Chinese people. Front Med. 2022;8:02706. doi:10.3389/fmed.2021.802706
14. Verkicharla PK, Suheimat M, Schmid KL, et al. Peripheral refraction, peripheral eye length, and retinal shape in myopia. Optom Vis Sci. 2016;93(9):1072–1078. doi:10.1097/OPX.0000000000000905
15. Schmid GF. Association between retinal steepness and central myopic shift in children. Optom Vis Sci. 2011;88(6):684–690. doi:10.1097/OPX.0b013e3182152646
16. Lee TT, Cho P. Relative peripheral refraction in children: twelve-month changes in eyes with different ametropias. Ophthalmic Physiol Opt. 2013;33(3):283–293. doi:10.1111/opo.12057
17. Atchison DA, Shi-Ming L, Li H, et al. Relative peripheral hyperopia does not predict development and progression of myopia in children. Invest Ophthalmol Vis Sci. 2015;56(10):6162–6170. doi:10.1167/iovs.15-17200
18. Zhong Y, Chen Z, Xue F, et al. Central and peripheral corneal power change in myopic orthokeratology and its relationship with 2-year axial length change. Invest Ophthalmol Vis Sci. 2015;56(8):4514–4519. doi:10.1167/iovs.14-13935
19. Mohd-Ali B, Low YC, Shahimin MM, et al. Comparison of vision-related quality of life between wearing Orthokeratology lenses and spectacles in myopic children living in Kuala Lumpur. Contact Lens Anterior Eye. 2022;46:1–8.
20. Nti AN, Berntsen DA. Optical changes and visual performance with orthokeratology. Clin Exp Optom. 2020;103(1):44–54. doi:10.1111/cxo.12947
21. Kang P, Swarbrick H. Time course of the effects of orthokeratology on peripheral refraction and corneal topography. Ophthalmic Physiol Opt. 2013;33(3):277–282. doi:10.1111/opo.12027
22. Lin Z, Martinez A, Chen X, et al. Peripheral defocus with single vision spectacle lenses in myopic children. Optom Vis Sci. 2010;87(1):4–9. doi:10.1097/OPX.0b013e3181c078f1
23. Queiros A, Gonzalez-Meijome JM, Jorge J, et al. Peripheral refraction in myopic patients after orthokeratology. Optom Vis Sci. 2010;87(5):323–329. doi:10.1097/OPX.0b013e3181d951f7
24. Huang Y, Li X, Ding C, et al. Orthokeratology reshapes eyes to be less prolate and more symmetric. Contact Lens Anterior Eye. 2021;1:101532.
25. Atchison DA, Rozema JJ. Technical notes on peripheral refraction, peripheral eye length and retinal shape determination. Ophthalmic Physiol Opt. 2023;43(3):584–594. doi:10.1111/opo.13097
26. Moriyama M, Ohno-Matsui K, Modegi T, et al. Quantitative analyses of high resolution 3D MR Images of highly myopic eyes to determine their shapes. Invest Ophthalmol Vis Sci. 2012;53(8):4510–4518. doi:10.1167/iovs.12-9426
27. Mohd Ali B, Low YC, Shahimin MM, et al. Ocular dimensions by three-dimensional magnetic resonance imaging in emmetropic versus myopic school children. Med Hypothesis Discov Innov Ophthalmol. 2022;11(2):64–70. doi:10.51329/mehdiophthal1447
28. Mohd-Ali B, Low YC, Shahimin MM, et al. Ocular dimensions, refractive error, and body stature in young Chinese children with myopia in Kuala Lumpur, Malaysia. Clin Optom. 2022;14:101–110. doi:10.2147/OPTO.S368672
29. Cho P, Cheung SW. Retardation of myopia in orthokeratology (ROMIO) Study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci. 2012;53(11):7077–7085. doi:10.1167/iovs.12-10565
30. Rabbetts RB. Bennett and Rabbetts’ Clinical Visual Optics.
31. Ehsaei A, Chisholm CM, Mallen EA, et al. The effect of instrument alignment on peripheral refraction measurements by automated optometer. Ophthalmic Physiol Opt. 2011;31(4):413. doi:10.1111/j.1475-1313.2011.00838.x
32. Thibos LN, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci. 1997;74(6):367–375. doi:10.1097/00006324-199706000-00019
33. Chan TF, Vese LA. Active contours without edges. IEEE Trans Image Process. 2001;10(2):266–277. doi:10.1109/83.902291
34. Rayan J, Mokri S, Low YC, et al. Eyeball segmentation and measurement in MRI images of myopic children. J Phys Conf Ser. 2022;2312(1):12028. doi:10.1088/1742-6596/2312/1/012028
35. Chen C, Cheung SW, Cho P. Myopia Control Using Toric Orthokeratology (TO-SEE Study). Invest Ophthalmol Vis Sci. 2013;54(10):6510–6517. doi:10.1167/iovs.13-12527
36. Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res. 2005;30(1):71–80. doi:10.1080/02713680590907256
37. Jason K, Lau K, Sin-Wan W, et al. Weekly changes in axial length and choroidal thickness in children during and following orthokeratology treatment with different compression factors. Trans Vision Sci Technol. 2019;8(4):9–12. doi:10.1167/tvst.8.4.9
38. Cho P, Tan Q. Myopia and orthokeratology for myopia control. Clin Exp Optom. 2019;102(4):364–377. doi:10.1111/cxo.12839
39. Mutti DO, Loraine TS, Kathleen S, et al. Bifocal lenses In Nearsighted Kids (BLINK) Study Group; peripheral refraction and eye lengths in myopic children in the bifocal lenses In Nearsighted Kids (BLINK) Study. Trans Vision Sci Technol. 2019;8(2):17–20. doi:10.1167/tvst.8.2.17
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.