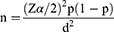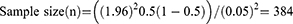Back to Journals » Clinical Optometry » Volume 15
Patients’ Satisfaction with Topical Anti-Glaucoma Medications and Associated Factors at Gondar University Tertiary Eye Care and Training Center, Northwest Ethiopia, 2021
Authors Belie NY, Ayele FA, Mengist B , Alemayehu AM , Assem AS , Fekadu SA , Yibekal BT
Received 6 March 2023
Accepted for publication 9 June 2023
Published 19 June 2023 Volume 2023:15 Pages 139—146
DOI https://doi.org/10.2147/OPTO.S411390
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Mr Simon Berry
Natnael Yeneneh Belie,1 Fisseha Admassu Ayele,2 Belayneh Mengist,3 Abiy Maru Alemayehu,1 Abel Sinshaw Assem,1 Sofonias Addis Fekadu,1 Betelhem Temesgen Yibekal1
1Department of Optometry, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Ophthalmology, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 3Department of Public Health, College of Health Sciences, Debre Markos University, Debre Markos, Ethiopia
Correspondence: Betelhem Temesgen Yibekal, Email [email protected]
Background: Glaucoma is one of the leading cause of global irreversible blindness if left untreated. Satisfaction is a multifaceted outcome based on sufficient information and encouragement from the practitioner and based on the medications’ nature. Determining patients’ satisfaction is essential to increase their courage in their long-time follow-up of medical care.
Objective: To assess Patients’ satisfaction with topical anti-glaucoma medications and associated factors among glaucoma patients at Gondar University Tertiary Eye Care and Training Center, Northwest Ethiopia.
Methods: Hospital-based cross-sectional study was conducted from June 30 to August 27, 2021, among 395 glaucoma patients at Gondar University Tertiary Eye Care and Training Center. Data was entered into Epi info version 7 and exported to SPSS version 26 software for analysis. A Binary logistic regression model was used to determine factors associated with satisfaction with topical anti-glaucoma medications. Statistical significance was considered when p-value < 0.05.
Results: A total of 395 study subjects participated in the study with a response rate of 93.38%. The overall satisfaction with topical anti-glaucoma medication was 62.5% with 95% CI: (57.5– 67.8%). The absence of ocular side effects (AOR=5.39, 95% CI: 2.35– 12.37) and the absence of ocular surface diseases (AOR=4.12, 95% CI: 1.69– 10.09) were significantly associated with patient satisfaction.
Conclusion: More than half of the study participants were satisfied with topical anti-glaucoma medications. The absence of ocular side effects and absence of Ocular surface diseases were significantly associated with patient satisfaction with anti-glaucoma medication.
Keywords: satisfaction, anti-glaucoma medication, Gondar, Northwest Ethiopia
Introduction
Glaucoma is one of the leading cause of global irreversible blindness. It affects 60.5 million people globally and this number is estimated to be 111.8 million by 2040.1–3 The prevalence of primary open-angle glaucoma (POAG) is highest in Africa (4.20%) and that of Primary angle-closure glaucoma (PACG) is highest in Asia (1.09%).3 Glaucomatous damage to the optic nerve can be prevented by effective lowering of intraocular pressure (IOP). This can be true through good patients’ compliance with treatment and regular clinical follow-ups which in turn increases patients’ satisfaction.4–8
Patient Satisfaction is a multifaceted outcome based on sufficient information and encouragement from the practitioner and based on the medications’ nature of convenience, acceptability, ease of use, efficacy, and side effect profile.5,9 Satisfaction with topical anti-glaucoma medication is the patient’s evaluation of the process of taking the medication and the outcomes associated with it.10 The impact and tolerability of treatment are significant considerations to measure satisfaction. Patients who are satisfied with treatment are more likely to continue using medical services, cooperate with healthcare providers by disclosing important medical information, and comply with treatment.9 Various manifestations of ocular surface disease (OSD) such as blepharitis, meibomian gland dysfunction, dry eye, eczema, rosacea, allergic conjunctivitis, and hyperemia are the most common adverse events of topical anti-glaucoma medications and challenging patients’ satisfaction.5,7,11 Satisfaction with medication is important since glaucoma is chronic, asymptomatic, and can lead to irreversible vision loss and predicts patients’ continuation of pharmaceutical treatment, correct medication usage, and compliance with medication regimens.11 Within the past few years, patient satisfaction with medical care showed increasing interest globally. This interest reflects the perspective that has developed over this time of the patient as an active consumer of health care services rather than merely as a passive recipient of these services.10
Patients satisfied with anti-glaucoma medications are more likely to comply with medical treatments and their follow-up appointment time because glaucoma needs strict follow-up in the patient’s lifetime. But, in Africa including Ethiopia and the study area, there are limited studies and little is known about patient satisfaction with anti-glaucoma medications.
Therefore, this study aimed to assess patients’ satisfaction with topical anti-glaucoma medications and associated factors among glaucoma patients at Gondar University tertiary eye care and training center, Northwest Ethiopia.
Methods
Study Design, Period, and Setting
A Hospital-based cross-sectional study was conducted from June 30 to August 27, 2021, at Gondar university comprehensive and specialized hospital tertiary eye care and training center (UGCSHTECTC), Gondar, Northwest Ethiopia. Gondar city is the capital of the central Gondar administrative zone and it is located 738 km away from Addis Ababa, the capital city of Ethiopia, and 180 km from Bahir-Dar, the administrative capital city of Amhara National Regional State.
The tertiary eye care and training center is the only eye care center in Gondar city and was established in 2004 by Orbis international and light for the world in collaboration with the department of ophthalmology and optometry. It provides eye care services for a large number of people in Northwest Ethiopia. Glaucoma clinic is one of the different service provision areas in this tertiary eye care and training center and it is open for patients three days a week (Monday, Wednesday, and, Friday). Based on daily logbook registration, 26 to 70 patients get services per day in the glaucoma clinic.
Source and Study Population
The source population was all adult glaucomatous patients with anti-glaucoma medications attending UGCSHTECTC and the Study population was all adult glaucomatous patients with anti-glaucoma medications attending UGCSHTECTC during the data collection period.
Inclusion and Exclusion Criteria
All adult glaucomatous patients with anti-glaucoma medication follow-up in the outpatient department for at least six months4 before the data collection time were included in the study. Patients who were unable to communicate due to mental illness were excluded from the study.
Sample Size and Sampling Procedures
Since there was no previous study done on patient satisfaction with topical anti-glaucoma medications and associated factors in Ethiopia including in the study area, the sample size was calculated by assuming 50% of the proportion of the study participants had satisfaction with topical anti-glaucoma medications. With a precision of 5%, at a 95% confidence level and an additional 10% to compensate for nonresponse rate, the calculated sample size was 423 by using the single population proportion formula.
Where; n = sample size
Z = Value of z statistic at 95% confidence interval = 1.96
P – Proportion satisfied patients with topical anti-glaucoma medications (50%)
The calculated sample size became 384 and by considering a 10% non-response rate, the final sample size was 423.
A systematic random sampling method was used to select the required samples. According to glaucoma patients’ logbook registration in UGCSHTECTC, the number of patients attending the glaucoma clinic per day was about 48 on average. This implies that the total number of patients attending the glaucoma clinic during the data collection period was estimated as 1248. The “K” value (interval between each study participant) for this study was 3 (ie, 1248 divided by total sample size, of 423). The first eligible study subject was selected by using a lottery method then every “K” (every 3) participant was interviewed to get the required data set.
Operational Definition
Satisfaction: Participants scoring greater than or equal to the median (32.00) of the sum of all the eight satisfaction questions were categorized as satisfied whereas, participants scoring below the median were categorized as unsatisfied.12
Knowledge: Participants who scored the median (11.00) and above of the eleven knowledge questions were considered to have good knowledge while those who scored below the median were considered as having poor knowledge.13,14
Data Collection Procedures and Quality Control
A semi-structured and interviewer-administered questionnaire developed from different reviewed literatures9,15–17 was used in data collection. The questionnaire was prepared in English language and then translated to the local (Amharic) language. A pretest was done on 5% of study samples (22 patients) at Felege-Hiwot Comprehensive Specialized Hospital Eye Care Center in Bahir Dar and Cronbach’s alpha (α=0.89) was used to check for its internal consistency in all eight patient satisfaction questions. Data were collected by two optometrists after one day of training was given by the principal investigator on the objective of the study, on how to obtain consent, and on how to record. The questionnaire contains the main socio-demographic characteristics of the study participants, satisfaction questions, and clinical characteristics. All satisfaction questions consisted of a 5‐level Likert scale ranging from Very unsatisfied (1 point) to Very satisfied (5 points) and from Very difficult (1 point) to Very easy (5 points). Clinical questions were reviewed from medical registration charts after they finish their examination in the glaucoma clinic.
Data Processing and Analysis
The collected data were entered into Epi info version 7 and exported to SPSS version 26 software for analysis. The data were made ready for analysis after cleaning and coding into categories. Descriptive statistics of the main socio-demographic and clinical characteristics of the study participants were presented in tables and overall patients’ satisfaction with topical anti-glaucoma medications was presented in a pie chart. Candidate variables for multivariable analysis were selected with a 0.2 level of significance by using bivariable logistic regression. Model fitness was checked by the Hosmer and Lemeshow Test (0.201). A multivariable logistic regression model was used to determine factors associated with patient satisfaction with topical anti-glaucoma medications. Variables (factors) having a p-value less than 0.05 with a 95% confidence interval in multivariable logistic regression were considered as significantly associated with the dependent variable and AOR was used to measure the strength of association between predictor variables and the outcome variable.
Ethical Considerations
The study was conducted in accordance with the Declaration of Helsinki and approved by the ethical review committee of the University of Gondar, college of medicine and health science, school of medicine. Permission was also obtained from the ophthalmology department at University of Gondar. Study participants were informed by the data collectors about the objective of the study and they were able to know that there is no harm to them in participating in the study except devoting some time for an interview. Written informed consent was obtained from each participant. Confidentiality was kept secure by avoiding personal identifiers in the data collection tool.
Result
Socio-Demographic Characteristics
A total of 395 study subjects participated in the study with a response rate of 93.38%. The median age of participants was 63 years with an interquartile range (IQR; 54–70). The majority of (66.6%) the participants were males, (Table 1).
 |
Table 1 Socio-Demographic Characteristics of the Study Participants at Gondar University Tertiary Eye Care and Training Center, Northwest Ethiopia, 2021 (n=395) |
Clinical Characteristics and Knowledge of the Study Participants
The study participants’ median time since diagnosis of glaucoma in either eye was 3 years with IQR: 1.50–5.33. The majority (40.8%) of the study participants’ follow-up time was every two months and about 13.9% of the study participants missed their appointment time at least once in their glaucoma follow-up time in the last year. Twenty-seven (6.8%) participants had ocular surface diseases in either eye of which more than half, (59.25%) of them were with blepharitis. The majority, 253 (64.1%) of the study participants had good knowledge about glaucoma. Almost all study participants, (99.7%) were using timolol. About 8.90% of the participants reported using eye drops other than anti-glaucoma medications (Table 2).
 |
Table 2 Medication, Follow-Up, and Clinical Characteristics of the Study Participants at Gondar University Tertiary Eye Care and Training Center, Northwest Ethiopia, 2021 (n=395) |
Patients’ Satisfaction with Topical Anti-Glaucoma Medications
The overall patients’ satisfaction with topical anti-glaucoma medication was 62.5% with a 95% CI: (57.5–67.8%).
Factors Associated with Topical Anti-Glaucoma Medication Satisfaction
Binary logistic regression model was used to identify factors associated with patients’ satisfaction with topical anti-glaucoma medications. Variables like patients’ age, marital status, type of glaucoma, the severity of glaucoma, ocular surface diseases, and frequency and side effects of topical anti-glaucoma medications were selected for multivariable analysis based on a 0.2 level of significance. Finally, the factors listed in the table below were used to compute the adjusted odds ratio (AOR) in multivariable logistic regression analysis. The absence of ocular side effects of topical anti-glaucoma medications upon instillation and ocular surface diseases (OSD) were found to be statistically significantly associated with patients’ topical anti-glaucoma medication satisfaction at p <0.05 level of significance with 95% CI. The odds of satisfaction with topical anti-glaucoma medications were 5.39 times higher among patients without ocular side effects than patients who had side effects (AOR=5.39, 95% CI: 2.35–12.37). The odds of satisfaction with topical anti-glaucoma medications were 4.12 times higher among patients without OSD than patients who had OSD (AOR=4.12, 95% CI: 1.69–10.09) (Table 3).
 |
Table 3 Factors Associated with Patient Satisfaction with Topical Anti-Glaucoma Medications at Gondar University Tertiary Eye Care and Training Center, Northwest Ethiopia, 2021 (n=395) |
Discussion
This institution-based cross-sectional study was conducted to assess satisfaction with topical anti-glaucoma medications and associated factors among glaucoma patients at GUCSHTECTC glaucoma clinic, Gondar Northwest Ethiopia.
This study showed that the overall patients’ satisfaction with topical anti-glaucoma medications was 62.5%. The absence of ocular side effects of topical anti-glaucoma medications and OSD were found to be statistically significantly associated with patients’ overall satisfaction with topical anti-glaucoma medications.
The overall patient satisfaction of this study was in line with the study done in Korea (67.0%).18 This might be due to the similarities in the study setting and design since both studies were hospital-based cross-sectional studies.
The proportion of patient satisfaction in this study was higher than in other study done in Vietnam (49.2%)19 but lower than in studies done in the United States of America (77.9%),20 Birmingham (100%),21 and the United Kingdom (90.4%).22 The difference in the socio-demographic and /or economic status of the study participants, and differences in health systems and medication accessibility may bring such discrepancies between the results of this study.
In the present study, among variables analyzed in multivariable regression, only two variables were statistically significantly associated with patient satisfaction with topical anti-glaucoma medications.
Participants who did not have ocular side effects upon the instillation of topical anti-glaucoma patients are more likely to be satisfied than those who had ocular side effects. This result is in agreement with other studies done in the USA15 and Europe.5,7,9 This might be due to that side effects upon instillation of topical anti-glaucoma medications may overweigh in some individuals by inducing discomfort and inconvenience. Repeated allergies represent a load to patients and are a factor discouraging compliance and satisfaction.12 These may increase the proportion of patients declaring being dissatisfied with their current glaucoma treatment.21 As a result, patients without ocular side effects upon instillation may show more satisfaction than patients who had side effects.
Participants without ocular surface disease are more likely to be satisfied with topical anti-glaucoma medications than those who have ocular surface disease. This finding is consistent with other studies.5,23,24 This could be resulted from preservatives in topical anti-glaucoma medication trigger OSD and impact treatment compliance and satisfaction.24 Ocular surface diseases are common among glaucoma patients receiving topical anti-glaucoma medications. The severity increases in older patients and those receiving multiple treatments. Besides the presence of OSD, glaucomatous patients need to take multiple treatments on top of anti-glaucoma medications.25 Moreover, the severity of OSD has been associated with low effective control of IOP.23,24
Limitation of the Study
The study was not able to explore the effects of different classes of medications on patients’ satisfaction because medications other than timolol were often used by only a small number of patients, making it difficult to show differences in satisfaction between drugs. Only small domains of patient satisfaction questions were used in this study. Recall bias was observed among study participants in answering some study questions.
In addition, this study has selection bias since it is a hospital-based study participants who came for their regular follow-up were included making those who cannot come because of different reasons less represented. A follow-up study could reveal long-term ocular and systemic side effects as well as some rare side effects of topical anti-glaucoma medications which were not accomplished with this study design.
Conclusion and Recommendation
Though more than half of the study participants were satisfied with topical anti-glaucoma medications in this study, a non-negligible number of individuals were dissatisfied with anti-glaucoma medications. The absence of ocular side effects of topical anti-glaucoma medications and the absence of OSDs were areas identified to be significantly associated with patients’ satisfaction with topical anti-glaucoma medications. Efforts to improve patient satisfaction should focus on ocular side effects and OSD. It is better to increase the availability of different classes of anti-glaucoma medications with preservative-free types since almost all patients reported using only preserved timolol eye drops in GUCSHTECTC.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chow JT, Hutnik CM, Solo K, Malvankar-Mehta MS. When is evidence enough evidence? A systematic review and meta-analysis of the trabectome as a solo procedure in patients with primary open-angle glaucoma. J Ophthalmol. 2017;2017. doi:10.1155/2017/2965725
2. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi:10.1136/bjo.2005.081224
3. Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi:10.1016/j.ophtha.2014.05.013
4. Foo VHX, Tan SEM, Chen DZ, et al. Areas and factors associated with patients’ dissatisfaction with glaucoma care. Clin Ophthalmol. 2017;11:1849. doi:10.2147/OPTH.S138668
5. Lemij HG, Hoevenaars JG, van der Windt C, Baudouin C. Patient satisfaction with glaucoma therapy: reality or myth? Clin Ophthalmol. 2015;9:785. doi:10.2147/OPTH.S78918
6. Peterson KM, Huisingh CE, Girkin C, Owsley C, Rhodes LA. Patient satisfaction with care in an urban tertiary referral academic glaucoma clinic in the US. Patient Prefer Adher. 2018;12:775. doi:10.2147/PPA.S162439
7. Stalmans I, Lemij H, Clarke J, Baudouin C, Ophthalmology Gsg JC. Signs and symptoms of ocular surface disease: the reasons for patient dissatisfaction with glaucoma treatments. Clin Ophthalmol. 2020;14:3675. doi:10.2147/OPTH.S269586
8. Kim JM, Kim T-W, Kim CY, Kim HK, Park KH. Comparison of the intraocular pressure-lowering effect and safety of brimonidine/timolol fixed combination and 0.5% timolol in normal-tension glaucoma patients. Japanese J Ophthalmol. 2016;60(1):20–26. doi:10.1007/s10384-015-0420-2
9. Kerr NM, Patel HY, Chew SS, et al. Patient satisfaction with topical ocular hypotensives. Clin Exper Ophthalmol. 2013;41(1):27–35. doi:10.1111/j.1442-9071.2012.02823.x
10. Shikiar R, Rentz AMJVi H. Satisfaction with medication: an overview of conceptual, methodologic, and regulatory issues. Value Health. 2004;7(2):204–215. doi:10.1111/j.1524-4733.2004.72252.x
11. Atkinson MJ, Stewart WC, Fain JM, et al. A new measure of patient satisfaction with ocular hypotensive medications: the Treatment Satisfaction Survey for Intraocular Pressure (TSS-IOP). Health Qual Life Outcomes. 2003;1(1):1–13.
12. Beckers HJ, Schouten JS, Webers CA, van der Valk R, Hendrikse F. Side effects of commonly used glaucoma medications: comparison of tolerability, chance of discontinuation, and patient satisfaction. Graefe’s Archiv Clin Exper Ophthalmol. 2008;246(10):1485–1490. doi:10.1007/s00417-008-0875-7
13. Alemu DS, Gudeta AD, Gebreselassie KL. Awareness and knowledge of glaucoma and associated factors among adults: a cross sectional study in Gondar Town, Northwest Ethiopia. BMC Ophthalmol. 2017;17(1):1–12.
14. Assem AS, Fekadu SA, Yigzaw AA, Nigussie ZM, Achamyeleh AA. Level of glaucoma drug adherence and its associated factors among adult glaucoma patients attending Felege Hiwot specialized hospital: Bahir Dar City, Northwest Ethiopia. Clin Optometry. 2020;12:189.
15. Day D, Sharpe E, Atkinson M, Stewart J, Stewart W. The clinical validity of the treatment satisfaction survey for intraocular pressure in ocular hypertensive and glaucoma patients. Eye. 2006;20(5):583–590. doi:10.1038/sj.eye.6701932
16. Ruiz MA, Pardo A, Martinez de la Casa JM, Polo V, Esquiro J, Soto J. Development of a specific questionnaire measuring patient satisfaction with glaucoma treatment: glausat. Ophthalmic Epidemiol. 2010;17(3):131–143.
17. Nordmann J-P, Denis P, Vigneux M, Trudeau E, Guillemin I, Berdeaux G. Development of the conceptual framework for the Eye-Drop Satisfaction Questionnaire (EDSQ©) in glaucoma using a qualitative study. BMC Health Serv Res. 2007;7(1):1–9.
18. Kim CY, Park KH, Ahn J, et al. A path analysis of effects of patients’ underlying conditions, treatment satisfaction, and adherence on quality of life among Korea glaucoma patients. J Glaucoma. 2019;28(9):785–789. doi:10.1097/IJG.0000000000001312
19. Van Huy N, Dung NN, Thang CD, Hanh LT. Patient satisfaction with health care services at a national institute of ophthalmology. Int J Health Plan Manag. 2018;33(1):e251–e62. doi:10.1002/hpm.2449
20. Rahmatnejad K, Myers JS, Falls ME, Myers SR, Waisbourd M, Hark LA. Factors associated with patient satisfaction in an outpatient glaucoma population. In: Seminars in Ophthalmology. Taylor & Francis; 2018.
21. Dreer LE, Owsley C, Campbell L, Gao L, Wood A, Girkin CA. Feasibility, patient acceptability, and preliminary efficacy of a culturally informed, health promotion program to improve glaucoma medication adherence among African Americans: “Glaucoma Management Optimism for African Americans Living with Glaucoma”(GOAL). Cur Eye Res. 2016;41(1):50–58. doi:10.3109/02713683.2014.1002045
22. Levy S, Booth A. Patient satisfaction with peninsula optometry community glaucoma scheme. Eye. 2015;29(10):1395. doi:10.1038/eye.2015.67
23. Negrete FJM, Lemij HG, Erb C. Switching to preservative-free latanoprost: impact on tolerability and patient satisfaction. Clinical Ophthalmol. 2017;11:557.
24. Erb C, Stalmans I, Iliev M, Muñoz-Negrete F. Real-world study on patient satisfaction and tolerability after switching to preservative-free latanoprost. Clin Ophthalmol. 2021;15:931. doi:10.2147/OPTH.S295821
25. Wong AB, Wang MT, Liu K, Prime ZJ, Danesh-Meyer HV, Craig JP. Exploring topical anti-glaucoma medication effects on the ocular surface in the context of the current understanding of dry eye. Ocular Surf. 2018;16(3):289–293. doi:10.1016/j.jtos.2018.03.002
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.