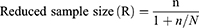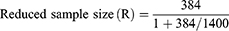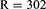Back to Journals » Patient Preference and Adherence » Volume 18
Patients’ Preference for Pharmaceutical Dosage Forms: Does It Affect Medication Adherence? A Cross-Sectional Study in Community Pharmacies
Authors Limenh LW , Tessema TA, Simegn W , Ayenew W , Bayleyegn ZW, Sendekie AK , Chanie GS , Fenta ET , Beyna AT , Kasahun AE
Received 7 February 2024
Accepted for publication 19 March 2024
Published 26 March 2024 Volume 2024:18 Pages 753—766
DOI https://doi.org/10.2147/PPA.S456117
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Liknaw Workie Limenh,1 Tewodros Ayalew Tessema,1 Wudneh Simegn,2 Wondim Ayenew,2 Zemenu Wube Bayleyegn,2 Ashenafi Kibret Sendekie,3 Gashaw Sisay Chanie,3 Eneyew Talie Fenta,4 Alemante Tafese Beyna,5 Asmamaw Emagn Kasahun1
1Department of Pharmaceutics, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Social and Administrative Pharmacy, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 3Department of Clinical Pharmacy, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 4Department of Public Health, College of Medicine and Health Science, Injibara University, Injibara, Ethiopia; 5Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Liknaw Workie Limenh, Email [email protected]
Background: Dosage forms (DF), which are primarily divided into solid, semisolid, liquid, and gaseous, are among the different factors that influence drug adherence. Thus, the purpose of this study was to evaluate how patients’ preferences for pharmaceutical DF affected their adherence to medication in community pharmacies in Gondar town.
Methods: A cross-sectional study on community pharmacies was carried out from June 25 to July 27, 2023. The statistical package for social sciences, version 26, was used for data analysis. Factors associated with patient medication discontinuation were found using both bivariate and multivariate logistic regressions.
Results: According to our study, the majority of respondents (42.4%) preferred tablet DF. Most respondents (63.9%) DF preference was affected by the size of the medication, in which small-sized were most preferable (59.6%). The oral route of administration was the most preferable (71.2%). The majority of the respondents (59.9%) had a history of discontinuation of medicines. Being male (AOR=2.21, 95% CI: 1.29, 3.79), living in rural areas (AOR=1.98, 95% CI: 1.03, 3.83), types of DF (AOR=4.59, 95% CI: 1.28, 16.52), high frequency of administration (AOR=2.22, 95% CI: 1.08, 4.57), high cost of medication (AOR=3.09, 95% CI: 1.69, 5.68), getting some improvement from illness (AOR=3.29, 95% CI: 1.10, 9.87), and high number of drugs (AOR=3.29, 95% CI: 1.67, 13.85) were significantly associated with medication discontinuation.
Conclusion: Our findings showed that tablet dosage forms, oral routes of administration, and once-daily taking of medicines were the most preferred by our respondents. Being male, living in rural areas, types of DF, high frequency of administration, high cost of medication, getting some improvement from illness, and high number of drugs were significantly associated with medication discontinuation. This provides an insight into what to consider when prescribing medicine to enhance patients’ adherence and overall therapeutic outcomes.
Keywords: adherence, associated factors, dosage form preference, routes of administration
Introduction
The effectiveness of pharmaceutical treatments is influenced by both the chemical makeup of the drug and its formulation and administration. Patient perceptions of pharmaceutical therapy can be significantly impacted by variations in formulations. Improving the acceptability of dosage forms (DF) for patients is currently of great interest for improving adherence.The majority of DF are classified based on their state, such as solid, liquid, semisolid, or gaseous.Gaseous dosage forms include aerosols, inhalations, and sprays. Liquid DF is mainly classified into two categories: internal and external liquid preparations.2,3 Semisolid DF is subcategorized as ointments, creams, pastes, and jellies.3,4 There are various solid DF, including tablets, capsules, pills, etc. Tablets are the most popular solid DF available today due to their ease of administration, compactness, and ease of manufacturing.4,5
Oral, parenteral, topical, ophthalmic, pulmonary, vaginal, otic, rectal, nasal, and transdermal were the classifications of the routes of administration (RoA).6 Each RoA has associated benefits and drawbacks.1,6 The majority of products (73.0%) were oral RoA, with parenteral (17.7%) and topical (4.2%) products following.6,7
It has been demonstrated that medication adherence, or taking prescription drugs as directed at the appropriate times and doses, improves health outcomes and lowers medical expenses. However, medication-related issues may hinder adherence, which in turn affects the efficacy of drug treatment. Certain DF, additional handling instructions such as splitting tablets, multiple dosages per day, and reliance on food intake are medication-related factors that negatively affect patient adherence. Therefore, since many medication-related factors can be readily changed or corrected, they may be especially attractive targets for patient adherence improvement strategies.8,9 According to various findings, oral medications’ color, shape, and size in solid DF appear to be significant predictors of patients’ acceptance. It can be quantified not only in monetary terms but also potentially provide valuable guidance to pharmaceutical companies developing new products or to legislators working to increase patient adherence through improved prescription or dispensing practices based on patient preference.10 When designing the final drug product, manufacturers should consider the practical issues that elderly consumers may face. This includes factors like size, palatability, and appearance. To improve swallowability and visual identification, these qualities ought to be maximized.5,11
Patients on long-term therapy plans frequently do not take their prescriptions as directed, which is linked to a higher risk of hospitalization and death. Patients with long-term therapeutic needs for diseases like the human immune virus, diabetes, hypertension, and asthma are frequently seen to have complex regimens.12
The percentage of a patient prescribed medication doses actually taken over a given period of time is typically used to report the patient’s rate of adherence.10 Individuals suffering from chronic illnesses have trouble with medication adherence. According to a thorough analysis of studies on medication compliance, about 50% of patients do not take their prescribed medications as directed by their healthcare providers.13,14 The World Health Organization estimated that the annual cost of drug therapy noncompliance in Europe was €125 million, which included costs for avoidable hospitalizations, nursing home admissions, and early deaths.15,16 Therefore, the aim of this study was to assess patients’ preferences for various pharmaceuticals and their impact on medication adherence in Gondar town community pharmacies.
Methodology
Setting and Design
The study was carried out in the community pharmacies of Gondar town. Gondar town is located in the central Gondar administration zone of the Amhara regional state, in northwest Ethiopia. It is located 738 km from Addis Ababa to the north and 180 km from Bahir Dar to the north. In Gondar town, there are 102 community pharmacies, which provide for an estimated 1400 patients per month. Using a self-administered questionnaire, an a cross-sectional study designed for community pharmacies was carried out from June 25 to July 27, 2023.
Population and Eligibility Criteria
The study population consisted of sampled patients who took medication during the data collection period, while all patients who were taking medication in Gondar town community pharmacies were the source population. All patients who were at least 18 years old and both mentally and physically capable of communicating were included in our study. Patients with physical or mental health issues, as well as those who were not of consenting age, were not included in our study.
Sample Size Determination
Fisher’s formula was used to calculate the total sample size because the population size of Gondar Town community pharmacy users was unknown. Consequently, 1400 patients were expected to be seen each month. The sample size can be determined using the following formula:
Where n is the desired sample size and p is the prevalence since the estimate proportion is unknown. p=50%=0.5, whereas q=1-p = 0.5, Z is the standard normal deviation of 1.96 at 95% CI, and D is an acceptable margin of error of 0.05 at 95% CI. Then,
Since the population size is less than 10,000, the required sample size can be reduced by using the reduction formula as follows:
Where N is the estimated number of patients who visit Gondar town community pharmacies within a month.
These 302 patients were chosen using a systematic random sampling technique.
Data Collection Instrument and Procedure
Data were collected using interview-based questionnaires adapted from previous literature. International organizations, national studies like the Demographic and Health Survey, and publications in peer-reviewed journals in pharmacy all used the questionnaire items.4,10,16–19 The final questionnaire consisted of various sections, including: demographic characteristics; history of chronic diseases; dosage form use; and allergy to a specific dosage form; history of discontinuing taking a medication and associated reasons; preference for different dosage forms; preference for routes and frequency of administration; level of adherence; and variables influencing respondents’ adherence to their prescribed regimen. For the purpose of gathering data, a total of three pharmacists with prior experience gathering data were chosen. The data for the study were gathered through in-person interviews conducted in the working areas of pharmacies in order to minimize any potential research bias.
Data Management and Quality Assurance
Before collecting the data, the literatures related to the questioners were reviewed. For face validation, the questionnaire was sent to academics and subject-matter experts; after their input, the questionnaire was simplified and made to be more objectively focused. A pretest was done with 10 respondents out of the study area (at Bahir Dar, Ethiopia). Based on the pretest, a few amendments were integrated into the final version of the questionnaires. The data collectors received a half-day of training to acquaint them with the goals and significance of the study as well as the process of gathering data through in-person interviews. Lastly, the principal investigator collected data and reviewed it daily to ensure its quality and completeness. Following data collection, appropriate categorization and coding were carried out, and the gathered data were checked for accuracy and completeness by comparing the recorded data. The accuracy of the data was verified once more after it was input into the Statistical Package for Social Sciences (SPSS).
Data Analysis
After being verified and cleaned, the data was entered in Epi Data version 4.2 and imported into SPSS version 26.0 for analysis. Descriptive statistics like frequency, percentage, mean, and standard deviation were used to summarize the findings. The relationships between dosage form preference and medication adherence were examined using bivariate and multivariate logistic regression models. To assess statistical significance, a p-value of 0.05 with a 95% confidence interval (CI) was employed.
Results
Socio-Demographic Characteristics of Respondents
A 100% response rate was achieved with a total of 302 respondents. The majority of the respondents (59.3%) were male, while females accounted for 40.7%. The respondents age distribution of 25–34 was predominant (33.1%), followed by 18–24 (26.5%). The religion of most respondents (89.1%) was Orthodox, which was followed by Muslim (9.6%). About 75.5% of the respondents lived in urban areas. The educational status of the respondents was dominated (43.7%) by tertiary, followed by secondary (22.2%). Most respondents (30.1%) were students, which was followed by civil servants (20.2%). The monthly income of the majority (39.7%) of respondents was greater than 3000 Ethiopian birr (ETB). Their monthly income was 4271 ETB on average (Table 1).
 |
Table 1 Socio-Demographic Characteristics of the Patients (n=302) |
Respondents’ History of Chronic Diseases and Dosage Forms Use
About 43.4% of the respondents had a known chronic disease. Most of the respondents (60.3%) had a history of taking tablets, followed by capsules (16%), and had no history of taking suppositories. Allergy for different DF was not a history for most of the respondents (86.1%), and only 13.9% of them had an allergy history. From the types of allergies and adverse drug reactions that respondents experienced, rash and swelling were the predominant, which were 45.2% and 11.9%, respectively. Shock and gynecomastia were low, which were seen in 2.4% of the respondents (Table 2).
 |
Table 2 Respondents History of Chronic Diseases, Dosage Form Use, and Experience of Allergy for Specific Dosage Form (n=302) |
Respondents’ Preference for Different Dosage Forms
According to our study findings, the majority of respondents (42.4%) preferred tablet DF, with capsule DF coming in second (19.9%). Suppositories were the least preferable DF, only preferred by 0.3% of the respondents (Figure 1).
 |
Figure 1 Participants preference (%) vs types of dosage forms. |
About 23.5% of respondents’ medication preferences were affected by color. White and orange colors were the most preferable, at 59.2 and 16.9%, respectively. More than half of the respondents (63.9%) DF preference was affected by the size of the medication. Small-sized formulations were the most preferred (59.6%), followed by medium-shaped formulations (33.7%). The shape of the medication affected about 33.1% of respondents’ DF preferences. From those respondents whose DF preference was affected by shape, about 64% of them preferred round formulations. Some of the respondents (22.8%) encountered difficulties taking medicines orally. Lack of appropriate flavor on the tablet (85.9%) was the main problem of taking medicines orally, which was followed by problems related to the large size of the tablets (10.1%) (Table 3).
 |
Table 3 Respondents’ Preference for Different Dosage Forms and Formulation Characteristics (n=302) |
Respondents’ Preference for Routes and Frequency of Administration
Oral RoA was the most preferable (71.2%), followed by the topical route (12.3%) (Figure 2).
 |
Figure 2 Participants preference (%) vs types of routes of administration. |
The majority of the respondents (74.2%) medication preference was affected by the frequency of administration. Weekly drug administration was the most preferable (37.7%), followed by daily administration (14.9%) (Table 4).
 |
Table 4 Preference of Respondents for Frequency of Administration (n=302) |
History and Factors of Respondents’ Medication Discontinuation
The majority of the respondents (59.9%) had a history of discontinuation of medicines due to different factors, of which cost of the medications (33.1%) was the leading reason, followed by fear of adverse drug reactions (31.5%) (Table 5).
 |
Table 5 Level of Adherence of Respondents and Factors That Affect Adherence of Respondents for Their Medication (n=302) |
Associated Factors with Medication Discontinuation
Both binary and multinomial logistic regressions were performed to determine how patient, medication, and health care provider-related factors were associated with patients’ medication discontinuation. In the bivariate analysis, gender, age, educational status, residence, types of dosage forms, interaction with health care providers, frequency of administration, fear of adverse drug reactions, being unfamiliar with the administration of drugs, religion, cost of the medication, getting some improvement from illness, and number of drugs were the candidate variables for multiple logistic regression (p<0.2). In the final model, being male and living in a rural area, types of DF, frequency of administration, cost of the medication, getting some improvement from illness, and number of drugs were significantly associated with patients’ medication discontinuation (p<0.05).
Patients who were male were 2.21 times (AOR = 2.21, 95% CI: 1.29, 3.79) more likely than those who were female to have stopped taking their medications. In terms of place of residence, patients in rural areas were 1.98 times (AOR = 1.98, 95% CI: 1.03, 3.83) more likely to stop taking their medications than patients in urban areas. Furthermore, patients’ decision to stop taking their medication was impacted by DF types 4.59 times (AOR = 4.59, 95% CI: 1.28, 16.52). It had a 2.22-fold effect on medication discontinuation in terms of administration frequency (AOR = 2.22, 95% CI: 1.08, 4.57). Additionally, 3.09 times as many medication discontinuations were impacted by medication cost (AOR: 3.09, 95% CI: 1.69, 5.68). About 3.29 times (AOR: 3.29, 95% CI: 1.10, 9.87) was the medication discontinuation rate impacted by getting some improvement from illness. Medication discontinuations were impacted by the number of drugs 4.81 times (AOR: 3.29, 95% CI: 1.67, 13.85) (Table 6).
 |
Table 6 Associated Factors with Medication Discontinuation (n=302) |
Discussions
When deciding whether to continue or stop treatment, a patient’s personal preferences are a major factor.10 Manufacturers should take into account the practical concerns that varying patients may have regarding the final drug product’s dimensions, palatability, and appearance. Enhancing swallowability and visual identification are two main benefits that should be optimized. Medical and pharmacy professionals are essential in prescribing and dispensing these patient-centric drug products because they ensure that patients receive the best formulation.16
According to the results of this study, the oral route was the most preferable (71.2%, 95% CI: 65.9, 76.5) RoA. Because of its patient acceptability, affordability, ease of use, and manufacturing capabilities, oral DF is used at a high rate.6 According to a review of several studies, respondents preferred oral over other RoA.1 The topical route was the second preferred (12.3%, 95% CI: 8.6, 16.2) RoA by our respondents, which might be associated with its advantages for local treatment, preventing or reducing systemic side effects.6 This result was less than a study conducted in Germany, which showed that creams and ointments were preferred by more than 24% of the patients.8 Parenteral RoA was the third preferable route (11.3%) in our study; some patients were willing to take parenteral medications, which might be due to their need for rapid improvement from their disease symptoms.20 The cost of producing these dosage forms is raised by the requirement that the products be sterile. Compared to the oral method, parenteral also raises the risk of side effects.6,16
In the current study, tablets are the most preferable DF (42.5%, 95% CI: 36.9, 48.2), followed by capsule DF (19.9, 95% CI: 15.6, 24.3). This result was less than that of two studies: one conducted in western Saudi Arabia found that respondents preferred tablets (69.6%) and capsules (37.6%) as DF,17 while another conducted in Germany found that tablets were the most preferred DF (68.2%) in the study population as a whole.8 However, our finding was higher than an investigation carried out in the UK, Saudi Arabia, and Jordan, which showed that tablets and capsules were preferred by 12% and 11% of patients, respectively.21 Our results disagreed with an Indian study that found tablet DF was the most widely accepted form of oral liquid therapy, regardless of the patient’s age.22
This study finding showed that frequency of administration significantly affects medication preference (72.2%, 95% CI: 67.2, 77.2). This result is consistent with the study conducted by Witticke et al, which showed that tablets with a once-daily dosage frequency were the most preferred form, with a high prevalence in the ambulatory setting.20 In the Keil et al study, patients also preferred the once-daily dosage regimen.23 This finding was higher than a study done in Saudi Arabia that showed that tablets with weekly drug administration were the most preferable (37.7%, 95% CI: 32.1, 43.4).8 Less frequent administration was preferred over more frequent administration, as would be expected from the majority of studies looking at dose frequency.1,8
In this study, respondents’ medication preference was affected not only by types of DF but also by formulation physical characteristics such as shape, color, and size. Some of our respondents experienced difficulty taking medications orally, which was comparable with other studies. Patients appear to be aware of the inconveniences connected to solid form characteristics, such as swallowing difficulties due to size and shape.10 About 25.8% (95% CI: 20.9, 31.1) of the respondents’ DF color affects their preference. White was the most preferable color (13.9%, 5% CI: 9.9, 17.9). The shape of the medication affected about 33.1% (95% CI: 28.1, 38.1) of respondents’ DF preferences. About 21.5% (95% CI: 16.6, 26.5) of them preferred round formulations. More than half of the respondents (63.9%, 95% CI: 58.3–69.2) DF preference was affected by the size of the medication. Small-sized formulations were the most preferred. Our study’s results, along with those of earlier research, indicated that round shapes are most preferred for swallowing convenience. Furthermore, this study demonstrated that white was DF’s preferred color. Numerous studies have indicated that one of the most prevalent reasons people prefer a white color is the perception that a white formulation contains no additional additives.2 Our results regarding preferred shape and color matched those of a study done in the UK, Saudi Arabia, and Jordan, which indicated that small, round shapes were preferred, as were pink or white as colors.21 However, our finding was contrary to a study finding in the UK, which demonstrated that small, round tablets have a negative effect at every stage of life and are least accepted by older people and their careers.4
The majority of current study respondents (59.9%) had a history of medication discontinuation due to different factors, which was consistent with research from earlier studies carried out in Gondar town, which revealed that over 60% of the patients had poor adherence and had trouble remembering their medications.6 Being male and living in an urban area, types of DF, high frequency of administration, high cost of the medication, getting some improvement from illness, and high number of drugs were significantly associated with patients’ medication discontinuation (p<0.05). The likelihood of medication discontinuation was 2.21 times (AOR:2.21, 95% CI: 1.29, 3.79) higher in male patients. Regarding residence, patients living in rural areas had 1.98 times (AOR:1.98, 95% CI: 1.03, 3.83) higher chance of medication discontinuation than patients living in urban areas. Additionally, types of DF affected patients’ medication discontinuation 4.59 times (AOR:4.59, 95% CI: 1.28, 16.52). Many factors related to drug therapy in older adults make medication adherence difficult, primarily DF characteristics.4,16 Our findings imply that tailoring medication schedules and DF to personal preferences could be an effective approach to increase adherence.8
In this study, the frequency of administration affected medication discontinuation 2.22 times (AOR:2.22, 95% CI: 1.08, 4.57). Patients who used less frequently dosed medications had higher adherence rates across all studies, and in 75% (15 of 20) of the studies, these differences were statistically significant (P<0.05).24 Getting some improvement from illness affected medication discontinuation 3.29 times (AOR: 3.29, 95% CI: 1.10, 9.87). Improved communication and access to medication information seem to be two of the most important things for patients. Appropriate information should be given to the patient to not discontinue, although the disease is improving.25 A higher number of medications affected medication discontinuations 4.81 times (AOR: 3.29, 95% CI: 1.67, 13.85). The practice of polypharmacy was one factor found to affect nonadherence.26 Lower adherence was found to be significantly correlated with more medications taken (p = 0.001).27 The main strategies used for improving adherence were regimen simplifications.12
In the current study, the cost of the medication affected medication discontinuation 3.09 times (AOR:3.09, 95% CI: 1.69, 5.68). One of the major factors that affected the adherence of the respondents was the cost of the medication. A study conducted in Canada showed that costs lead people to not adhere to their prescription medications, particularly people with low incomes, people with illnesses, or people who do not have insurance.24 Higher costs have a negative impact on adherence.28 The population-based estimates of the overall prevalence of non-adherence related to cost range from 5.1% to 10.2%.29
The type of DF had its own impact on non-adherence to medication accounts, since different respondents had different attitudes and preferences towards different DF. Identifying the factors that contribute to patient adherence is essential to developing and recommending prompt solutions. In addition to helping with identification, the use of appealing, two-colored preparations and intriguing shapes also helps with indication memorability and DF timing. Memorability and the visual appeal of medication are also affected by palatability, even though it is helpful in improving swallowability. Preferences regarding formulation are also influenced by factors such as the patient, medication, environment, and illness. Therefore, developing an age-appropriate DF for special age groups necessitates a comprehensive, patient-centered strategy to increase acceptance and adherence.4 Patients’ acceptance of oral medications can be predicted in part by the colour, shape, and size of the drug formulation. It can be quantified not only in monetary terms but also potentially provide valuable guidance to pharmaceutical companies developing new products or to legislators eager to increase patient compliance through improved prescription or dispensing practices.10 Making prescriptions for pharmaceutical formulations based on patient preferences may improve treatment compliance, which benefits disease management.30 By choosing the best DF and formulation composition based on the characteristics of the intended patient population, the drug product’s design provides the opportunity to satisfy the needs and preferences of patients.23
Limitation of the Study
Finding a sufficient number of comparable studies, both domestically and internationally, to compare the outcomes was challenging. Another drawback was that the study could not be broadly applied both nationally and regionally because it was limited to Gondar town and had a small sample size.
Conclusion
The oral route was more preferable than other RoA, and tablet DF was the preferred DF. The shape of the medication affected respondents’ DF preference, which showed that the majority of them preferred round formulations. Some respondents also had difficulties taking medicines orally, which was associated with a lack of appropriate flavor in the formulations, large sizes of the formulations, and types of DF. The majority of the respondents had a history of discontinuing their medication for different reasons. Being male, living in rural areas, the unavailability of types of preferred DF, the high frequency of administration, the higher cost of the medication, getting some improvement from illness, and the high number of drugs were significantly associated with medication discontinuations. Pharmacists, regulatory bodies, and pharmaceutical companies can all benefit from these findings as they work to improve patient-centered formulations. Further research should be conducted with a large sample size and a multicenter study to determine the effect of DF differences on patients’ adherence and preference for medication.
Abbreviations
CI, Confidence Interval; ETB, Ethiopian Birr; DF, Dosage Forms; RoA, Route of administration; SPSS, Satirical Packaging for the Social Science.
Data Sharing Statement
The corresponding author may obtain access to the data upon a justifiable request.
Ethical Concerns
The University of Gondar’s School of Pharmacy’s Ethical Review Committee granted ethical approval (approval number SOP 188/15). Informed consent was obtained from each participant and the study’s objective was fully explained to them to ensure the respondents’ participation went smoothly and effectively. Participants’ names were not recorded, and their information will never be given to third parties. The study methodology also complied with the Declaration of Helsinki.
Acknowledgments
The study’s participants, facilitators, and data collectors are all appreciated by the authors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Funding is not available to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stewart KD, Johnston JA, Matza LS., et al. Preference for pharmaceutical formulation and treatment process attributes. Patient Preference Adherence. 2016;10:1385–1399. doi:10.2147/PPA.S101821
2. Almukainzi M. Assessment of oral solid dosage forms administration manner and acceptability. Pharmacia. 2021;68(2):393–400. doi:10.3897/pharmacia.68.e65604
3. Kabeya K, Satoh H, Hori S, Sawada Y. Experimental study on patient preferences regarding the shape and size of medical tablets and capsules using three-dimensionally printed plastic model formulations. Patient Prefer Adher. 2021;15:863–870. doi:10.2147/PPA.S306582
4. Shariff ZB, Kirby DJ, Missaghi S, Rajabi-Siahboomi A, Maidment ID. Patient-centric medicine design: key characteristics of oral solid dosage forms that improve adherence and acceptance in older people. Pharmaceutics. 2020;12(10):1–15. doi:10.3390/pharmaceutics12100905
5. Nyol S, Gupta M. Immediate drug release dosage form: a review. J Drug Delivery Ther. 2013;3(2):155–161.
6. Nyamweya N, Tirop L. Assessment of human pharmaceutical products registered in Kenya by route of administration and type of dosage form. East Cent Afr J Pharm Sci. 2012;15(2):38–46.
7. Johnson MJ. The medication adherence model: a guide for assessing medication taking. Res Theory Nurs Pract. 2002;16(3):179–192. doi:10.1891/rtnp.16.3.179.53008
8. Witticke D, Seidling HM, Klimm HD, Haefeli WE. Do we prescribe what patients prefer? Pilot study to assess patient preferences for medication regimen characteristics. Patient Prefer Adher. 2012;6:679–684. doi:10.2147/PPA.S35950
9. Molitorisová M, Kotlářová J, Snopková M, Waczulíková I. Support of medication adherence by community pharmacists in Czech and Slovak Republics: a questionnaire survey study. Eur Pharm J. 2018;65(1):15–23. doi:10.1515/afpuc-2017-0006
10. Kurczewska-Michalak M, Kardas P, Czajkowski M. Patients’ preferences and willingness to pay for solid forms of oral medications—results of the discrete choice experiment in Polish outpatients. Pharmaceutics. 2020;12(3):1–9. doi:10.3390/pharmaceutics12030236
11. Hailat M, Al-Shdefat RI, Muflih SM, et al. Public knowledge about dosage forms, routes of drug administration and medication proper storage conditions in Riyadh District, Saudi Arabia. Pharm Health Serv Res. 2020;11(3):205–213. doi:10.1111/jphs.12359
12. Elnaem MH, Irwan NA, Abubakar U, Syed Sulaiman SA, Elrggal ME, Cheema E. Impact of medication regimen simplification on medication adherence and clinical outcomes in patients with long-term medical conditions. Patient Prefer Adher. 2020;14:2135–2145. doi:10.2147/PPA.S268499
13. Tebbi CK. Treatment compliance in childhood and adolescence. Cancer. 1993;71:S10.
14. Delamater AM. Improving patient adherence. Clin Diabetes. 2006;24(2):71–77. doi:10.2337/diaclin.24.2.71
15. Cutler RL, Fernandez-Llimos F, Frommer M, Benrimoj C, Garcia-Cardenas V. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ open. 2018;8(1):1–13. doi:10.1136/bmjopen-2017-016982
16. Shariff ZB, Dahmash DT, Kirby DJ, Missaghi S, Rajabi-Siahboomi A, Maidment ID. Does the formulation of oral solid dosage forms affect acceptance and adherence in older patients? A mixed methods systematic review. J Am Med Dir Assoc. 2020;21(8):1015–1023. doi:10.1016/j.jamda.2020.01.108
17. Alhaddad MS, Abdallah QM, Alshakhsheer SM, Alosaimi SB, Althmali AR, Alahmari SA. General public knowledge, preferred dosage forms, and beliefs toward medicines in western Saudi Arabia. Saudi Med J. 2014;35(6):578–584.
18. Bhosle M, Benner JS, DeKoven M, Shelton J. Difficult to swallow: patient preferences for alternative valproate pharmaceutical formulations. Patient Prefer Adher. 2009;3:161–171. doi:10.2147/PPA.S5691
19. Reginster JY. Adherence, patient preference and dosing frequency: understanding the relationship. BONE. 2005;36:S2–S6.
20. Hamad Y, Dodda S, Frank A, et al. Perspectives of patients on outpatient parenteral antimicrobial therapy: experiences and adherence. In: InOpen Forum Infectious Diseases. Vol. 7. US: Oxford University Press; 2020:1–7.
21. Alyami H, Dahmash E, Alyami F, et al. Dosage form preference consultation study in children and young adults: paving the way for patient-centred and patient-informed dosage form development. Eur J Hosp Pharm. 2017;24(6):332–337. doi:10.1136/ejhpharm-2016-001023
22. Pokharkar V, Sajith M, Vallet T, et al. Acceptability of different oral dosage forms in paediatric patients in hospital setting. Arch Dis Child. 2022;107(9):796–801. doi:10.1136/archdischild-2021-322604
23. Menditto E, Orlando V, De Rosa G, et al. Patient centric pharmaceutical drug product design—The impact on medication adherence. Pharmaceutics. 2020;12(1):1–23. doi:10.3390/pharmaceutics12010044
24. Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC, Care AJ. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15(6):e22–e23.
25. Kvarnström K, Westerholm A, Airaksinen M, Liira H. Factors contributing to medication adherence in patients with a chronic condition: a scoping review of qualitative research. Pharmaceutics. 2021;13(7):1–41. doi:10.3390/pharmaceutics13071100
26. Atinga RA, Yarney L, Gavu NM, Barengo NC. Factors influencing long-term medication non-adherence among diabetes and hypertensive patients in Ghana: a qualitative investigation. PLoS One. 2018;13(3):1–15. doi:10.1371/journal.pone.0193995
27. Basheti IA, El Hait SS, Qunaibi EA, Aburuz S, Bulatova N. Associations between patient factors and medication adherence: a Jordanian experience. Pharm Pract. 2016;14(1):1–7.
28. Gast A, Mathes T. Medication adherence influencing factors—an (updated) overview of systematic reviews. Syst. 2019;8:1–7.44.
29. Holbrook AM, Wang M, Lee M, et al. Cost-related medication nonadherence in Canada: a systematic review of prevalence, predictors, and clinical impact. Syst. 2021;10(1):1–3.
30. MacKenzie-Smith L, Marchi P, Thorne H, Timeus S, Young R, Le Calvé P. Patient preference and physician perceptions of patient preference for oral pharmaceutical formulations: results from a real-life survey. Inflamm Intest Dis. 2018;3(1):43–51. doi:10.1159/000493346
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.