Back to Journals » Lung Cancer: Targets and Therapy » Volume 15
Overcoming Central β-Sheet #6 (Cβ6) ALK Mutation (L1256F), TP53 Mutations and Short Forms of EML4-ALK v3/b and v5a/b Splice Variants are the Unmet Need That a Re-Imagined 5th-Generation (5G) ALK TKI Must Deliver
Received 27 October 2023
Accepted for publication 1 February 2024
Published 27 February 2024 Volume 2024:15 Pages 19—27
DOI https://doi.org/10.2147/LCTT.S446878
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Fengying Wu
Alexandria TM Lee,1 Sai-Hong Ignatius Ou1,2
1University of California Irvine School of Medicine, Department of Medicine, Orange, CA, USA; 2Chao Family Comprehensive Cancer Center, Orange, CA, USA
Correspondence: Sai-Hong Ignatius Ou, Division of Hematology and Oncology, Department of Medicine, University of California Irvine School of Medicine, Chao Family Comprehensive Cancer Center, 200 South Manchester Ave, Suite 400, Orange, CA, 92868, USA, Tel +1 714-456-5153, Email [email protected]
Abstract: Despite the development and approval of seven anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitors (TKIs) spanning over three “generations” since the discovery of ALK fusion positive (ALK+) non-small cell lung cancer (NSCLC), there remains intrinsic and acquired resistances to these approved TKIs. Currently, a fourth-generation (4G) ALK TKI, NVL-655, is being developed to attack some of the unmet needs such as compound resistance mutations in cis. However, EML4-ALK variant 3 and TP53 mutations are intrinsic genomic alterations that negatively modulate efficacy of ALK TKIs. Potentially, in the shifting landscape where lorlatinib should be the first-line ALK TKI of choice based on the CROWN trial, the central β-sheet #6 (Cβ 6) mutation ALK L1256F will be the potential acquired resistance mutation to lorlatinib which may be resistant to current ALK TKIs. Here we opine on what additional capacities a putative fifth-generation (5G) ALK TKI will need to possess if it can be achieved in one single molecule. We propose randomized trial schemas targeting some of the intrinsic resistance mechanisms that will lead to approval of a prototypic fifth-generation (5G) ALK TKI and actually be beneficial to ALK+ NSCLC patients rather than just design a positive pivotal superiority trial for the sole purpose of drug approval.
Keywords: EML4-ALK variant 3a/b, TP53 mutation, ALK TKIs, fifth generation ALK TKI, ALK+ NSCLC, circulating tumor DNA, next-generation sequencing, Cβ 6 mutation
Introduction
Since the discovery of anaplastic lymphoma kinase fusions in NSCLC in 2007,1,2 tremendous progress has been achieved in the understanding and treatment of advanced ALK+ NSCLC. A total of seven ALK tyrosine kinase inhibitors (crizotinib, ceritinib, alectinib, brigatinib, ensartinib, lorlatinib, iruplinakib) have been approved for the first-line (1L) treatment of advanced ALK+ NSCLC in various regions of the world.3 Iruplinakib was approved on January 9, 2024 in China for the first-line treatment of advanced ALK+ NSCLC.
However, on-target and off-target resistance are major challenges to continual successful treatment of ALK+ NSCLC from sequential use of ALK TKIs.4
ALK+ NSCLC patients are generally diagnosed at the peak earning potential of their lives (median age in the early 50s). Already with only 2 generations of ALK TKIs available, sequencing of ALK TKIs have been shown to prolong overall survival (OS) to 7.5 years.5,6 Now with lorlatinib, a third-generation (3G) ALK TKI approved for 1L treatment of advanced ALK+ NSCLC where its median PFS is still not reached at median follow-up time of 36.7 months7 with potential of the eventual median PFS of > 60 months;3 together with the development of NVL-655, a 4th generation (4G) ALK TKI in clinical development,8,9 the OS of patients diagnosed with newly advanced ALK+ NSCLC in 2023 should exceed a decade. As many ALK+ NSCLC patients have become long-term survivors due to the introduction of successive generations of potential ALK TKIs, we have invariably created huge expectation among these patients to come up with even better treatment at the time of eventual progression, an invisible “TKI treadmill”. Thus anticipating future unmet needs in the treatment of advanced ALK+ NSCLC, a fifth-generation (5G) of ALK TKI will need to address some of the intrinsic resistance mechanisms that are becoming evident from all the randomized clinical trials.
Intrinsic Biological Resistance to ALK TKIs in ALK+ NSCLC
It is important to note ALK+ NSCKC is not a monolithic molecular subgroup of NSCLC.10 The companion diagnostics approved early in the treatment of advanced ALK+ NSCLC are fluorescence-in-situ hybridization (FISH) and immunohistochemistry (IHC), which can only detect the presence of DNA breakpoint or aberrant ALK protein expression, respectively, but none of them can detect the fusion partner. FISH is highly operator dependent and IHC in the past was dependent on the reagents used which can affect the staining intensity.11 Now with an automated Ventana system the results of the IHC are dichotomous (Yes/No) and not by degrees of staining which will likely lead to rare false positive and false negative results.12
Indeed, FISH and IHC each potentially has a 10–15% false positive rate when measured against each other when analyzed from pivotal randomized phase 3 trials where PFS was also recorded13,14 (Table 1). From retrospective central laboratory analyses, both PROFILE1014 and ALEX phase 3 trials identified about ~15% of the patients enrolled from a single diagnostic test determined by central laboratory who were not positive by an alternative test (FISH followed by IHC [PROFILE1014]13 or IHC followed by FISH [ALEX]14) (Table 1). Indeed, the FISH/IHC “double positive” patients had numerically better (lower) hazard ratio (HR) than the overall trial population in both trials. Importantly, patients whose samples did not test positive by a second test by central laboratory did not benefit from crizotinib in PROFILE1014 or from alectinib in ALEX (Table 2).
 |
Table 1 Percentage of Double Positive Test (PROFILE1014 and ALEX) from Retrospective Analysis of Central Labs |
 |
Table 2 Comparison of Efficacy of Crizotinib (PROFEILE 1014) and Alectinib (ALEX) per Retrospective Analysis of Central Laboratory Analysis by FISH and IHC |
With the advent of next-generation sequencing (NGS), we know there are up to > 90 DNA fusion breakpoints in ALK+ NSCLC.15 The vast majority of the ALK fusions in ALK+ NSCLC are echinoderm microtubule-associated protein-like 4 (EML4)-ALK fusion but there are different breakpoints occurring in the EML4 gene. The two most common variants (variant 1 and variant 3) accounted for about 75% of the EML4-ALK fusion variants. Variant 1 is generated from the fusion of exons 1–13 of EML4 to exons 20–29 of ALK (E13:A20) while variant 3 is generated from fusion of exons 1–6 of EML4 to exons 20–29 of ALK (E6:A20).10 It has been demonstrated there is differential protein stability16 with the longer/larger variant 1 with more disorganized helical structure of EML4 leading to proteosome degradation and hence being more sensitive to ALK TKIs than variant 3 which has a shorter more compact structure and less likely to be directed to proteosomes for degradation.16 Indeed all six (data not avaialble for iruplinlakib) approved ALK TKIs had lower IC50 against EML4-ALK v1 than EML4-ALK v3.3,17 Analysis of circulating tumor DNA (ctDNA) from both first-line (1L)18–20 and second-line 2L21 randomized phase 3 trials of next-generation ALK TKIs (alectinib, brigatinib, lorlatinib) indicated EML4-ALK v3 had a shorter PFS than EML4-ALK v1 confirming many retrospective analysis.10
Additionally, EML4-ALK v3 has two splice variants generated by differential splicing by inclusion of a cryptic exon (exon EML4 6b) of 11 amino acids (EML4-ALK v3b).22 Hence commercial sequencing reports using DNA next-generation sequencing (NGS) report both variant 3a and 3b, EML4-ALK v3a/b. EML4-ALK v3a is more resistant to crizotinib than EML4-ALK v3b.23 Importantly, the ratio of v3a/3b is dynamic and the ratio of the v3a/v3b increased with treatment with crizotinib leading to more of the more resistant EML4-ALK v3a to crizotinib.22 Thus, targeting differential splicing of EML4-ALK v3a/b could potentially target one unmet need that could not be achieved by current generations of ALK TKIs.23
Furthermore, we now have increasing understanding that TP53 mutations (mt) conferred poorer response to ALK TKIs from retrospective analysis,16,24 and from the 1L and second-line (2L) randomized trials described above.19–21,25 The combination of EML4-ALK v3/TP53mt had the shortest PFS outcome.19–21 This is important since from the CROWN results, lorlatinib is likely to achieve a long median PFS of > 60 months3 but for patients with EML4-ALKv3/TP53mt. achieved only a median PFS of 20 months when treated with lorlatinib.20
Fourth-Generation (4G) ALK TKI in Development and Anticipating Future Unmet Needs
Currently only one fourth-generation (4G), NVL-655, is in clinical development.9 NVL-655 is primarily designed to overcome double ALK mutations in cis which are generated by sequential use of ALK TKIs.26 The available pre-clinical data did not suggest NVL-655 has selective activity against EML4-ALKv3 or TP53 mutations.9 Indeed, preliminary efficacy data showed that the ORR was 54% among ALK+ NSCLC patients with single or compound ALK mutations but only 22% among ALK+ NSCLC patients without any known ALK mutations.9 These early preliminary results indicate that current 4G ALK TKI as a pure ALK TKI is unlikely to overcome off-target resistances when on-target acquired resistance ALK mutations were not detected.
Another less well-known mutation, ALK L1256F, located in the central β-sheet number 6 (Cβ6), that has been demonstrated pre-clinically to confer resistance to lorlatinib26 and likely NVL-655 but could be overcome by alectinib pre-clinically.26 So far no (Cβ6) ALK L1256F mutation has been reported from patient cases in the literature. The PFS2 data from the CROWN study indicated there is effective post-lorlatinib treatment although the breakdown of the efficacy (chemotherapy, alectinib, other ALK TKI) is required to identify the most effective treatment.24 Given the adoption of 1L lorlatinib is likely to increase over time especially when the median PFS of lorlatinib from CROWN finally matures, the potential emergence of ALK L1256F as the initiating mutation underlying a separate set of compound resistance mutations would create a new category of novel compound ALK L1256F resistant mutations creating a new unmet need.
Qualities That a Fifth-Generation (5G) ALK TKI Must Possess to Overcome Unmet Needs
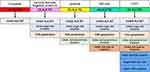
The evolution of the expected or ideal “functional capacities” of successive generations of ALK TKI are depicted in Figure 1. The potential additional capacities of a prototypic 5G ALK TKI are also depicted. As described above, the ability to overcome TP53 mutations, “reverse” the differential splicing ratio of EML4-ALK v3a/b, and to overcome Cβ6 mutation will be desirable properties of a 5G ALK TKI. Whether these additional properties can be structurally conferred in one single molecule is unknown. Another approach will be to overcome this evolving unmet need by a multi-targeted TKI or combination therapy approach. Targeting RNA splicing by targeting kinases involved in RNA splicing has been successful in a subset of muscular dystrophy and is being investigated in multiple cancer types.23,27
On-target resistance mutations are one major pathway conferring resistance to current ALK TKIs approved or in clinical trial. There are many off-target resistances that involve histologic transformation or activation of bypass signaling pathways.4 Alterations in the MET gene especially MET amplification, is a common off-target resistance mechanism.28 Combination therapy with a MET TKI such as capmatinib or tepotinib has been successfully combined with next generation ALK TKIs.29 While we do not expect 5G ALK TKI to be able to overcome MET amplification the ability to combine with MET TKIs would still be important.
If Such 5G ALK TKIs Can Be Designed in the Future, What is the Development Pathway of the Compounds (Molecularly Based Design/Stratification versus Clinical-Base Design/Stratifications)?
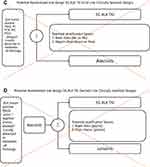
We have previously proposed the pivotal phase 3 clinical designs of a 4G ALK TKI in the 1L, 2L, and 3L setting.8 We expanded this thought exercise to a prototypic 5G ALK TKI on the assumption that lorlatinib should be the 1L treatment of choice for advanced ALK+ NSCLC given the likely eventual mature PFS of lorlatinib from CROWN will be > 60 months and our perspective is to advance the discourse on treatment of advanced ALK+ NSCLC for the next decade and beyond by extending beyond the > 60 months PFS 1L lorlatinib will achieve. We proposed two randomized trials molecularly based schemas: one in the 1L setting (Figure 2A) and one in the 2L setting (Figure 2B). Given our projection that the mature PFS of lorlatinib will be > 60 months,3 a pivotal head-to-head 1L against lorlatinib will be seemingly impractical. However, TP53 mutations have been demonstrated to significantly negatively modulate median PFS treated with alectinib,18,25,30 brigatinib,19 and even with lorlatinib.20 Hence 1L treatment of TP53+/ALK+ NSCLC patients remained unsatisfactory and an unmet need. Therefore, a frontline trial of a 5G ALK TKI versus alectinib, brigatinib, or lorlatinib among TP53+/ALK+ NSCLC patients is scientifically sound, and easier to achieve superior outcome for the investigational ALK TKI given the control arm PFS of only 16–20 months. Lastly, TP53 mutations represent ~40% of advanced ALK+ NSCLC patients26 thus maintaining the commercial viability of developing a 5G ALK TKI in the first-line setting.
Figure 2 Continued.
Stratifications for the 1L trial would potentially include TP53 mutations as determined from tumor versus plasma (with or without tumor). The ability to detect ctDNA such asTP53 mutations from the plasma is highly correlated to tumor burden.20,25,31 Advanced ALK+ NSCLC with undetectable ctDNA (e.g. TP53 mutations) achieved the best PFS among patients in alectinib-25,30 or lorlatinib-treatment group.31 Thus, if TP53 mutation is only detected from tumor genotyping versus plasma genotyping this may have prognostic significance.
Second stratification factor will be EML4-ALK v3 versus non-EML4-ALK v3 given the double genotype of EML4-ALKv3+/TP53mt+ has the worst PFS from CROWN and supported from a real-world study utilizing the Guardant plasma genotyping database.20,30 This stratification also allows isolation of the individual group contribution of TP53 mutations versus the contribution of EML4-ALK variants which will also allow enrollment more ALK+ NSCLC compared with just TP53+ or EML4-ALK v3 patients (and potentially expand the indication of 5G ALK TKI) as TP53 mutations are relatively common among high tumor burden advanced ALK+ NSCLC (~40–45%).20 A broader stratification could be the “long” form of EML4-ALK (v1 + v2) vs the short form of EML4-ALK (v3a/b + v5a/b), thus is another option but the placement of EML4-ALK variants outside these 4 groups will have to be determined specifically in the protocol.
For the 2L randomized trial molecularly stratified design, we proposed post-lorlatinib where a prototypic 5G ALK TKI will be tested against chemotherapy or alectinib. There is no ALK TKI approved by the FDA for post 1L lorlatinib progression treatment. The impressive PFS2 reported in CROWN were aggregate of alectinib, other non-alectinib ALK TKIs and chemotherapy in equal proportion of patients who received these subsequent treatments.32 Given that Cβ6 ALK F1256F mutation could be overcome by alectinib,26 either standard platinum-based chemotherapy or alectinib should be acceptable by health authorities with the comparator arm under further analysis from CROWN (Figure 2B). Given the likelihood of PFS with either alectinib or chemotherapy will be short, ALK+ NSCLC patients regardless of TP53 mutation status should be enrolled with TP53 mutations as a stratification factor.
The purpose of this perspective is to propose trial design schemas to actually benefit patients as the indication from the trial design will be the indication strictly followed by the vast majorities of health authorities globally in contract to the relatively lax reimbursement practice in the US allowing “off-label”, “earlier-line”, or “later line” use.29 We avoid a clinically based design that soly for the purpose of drug approval, but the primary endpoint will not benefit patients or advance the treatment of ALK+ NSCLC with intrinsic resistance disease. For example, a head-to-head clinically based 1L trial of a prototypic 5G ALK TKI against alectinib will not benefit patients since first, lorlatinib will very likely within 2–3 years’ time demonstrate PFS that will likely double the PFS achieved by alectinib in ALEX . And with the adoption of lorlatinib as 1L treatment, the role of a 5G ALK lies as second-line treatment post-lorlatinib or ideally as 1L trearment of specific sub-population such as TP53+ and/or EML4-v3+ ALK+ NSCLC that is relatively refractory to 1L lorlatinib. Thus, the additive sequencing a 5G ALK TKI after alectinib is unlikely to duplicate the PFS achieved by 1L lorlatinib alone. Second and more importantly, the design avoids addressing the TP53 mutations that led to only ~20 months for 1L lorlatinib (Figure 2C).
A 2L clinically stratified design pitting a 5G ALK TKI against lorlatinib post-alectinib again does not address the short 5.5 months PFS achieved by lorlatinib post-alectinib in their phase 1/2 trial that led to the initial approval lorlatinib post alectinib/ceritinib.33 Furthermore, lorlatinib should be used in 1L setting8 and sequencing a prototypic 5G ALK TKI is still unlikely to reach a cumulative PFS of > 60 months8 (Figure 2D). Approval of a 5G ALK TKI in the 2L setting does not benefit ALK+ NSCLC in most of the regions of the world since a second-line blanket indication may be the ideal situation to use a 5G ALK TKI as off-label use beyond the trial indication outside US is prohibited.
Third, CNS metastasis remains a significant comorbidity in advanced ALK+ NSCLC35 and it is a given that all next-generation ALK TKIs should be optimized to have potent CNS activity. Another important factor is if the protocol allows 1 regimen of platinum-based chemotherapy (as not all regions of the world are reimbursing first-line use of next-generation ALK TKI). Thus allowing 1 prior chemotherapy regimen will allow more patients to be eligible given some of the genotyping results, especially TP53 mutation status, may not be available when treatment began. One challenge of the design of these trials will be to satisfy the FDA requirement for development of simultaneous companion diagnostic NGS tests for TP53 mutation and EML4-ALK variants from both tumor tissue and plasma, which may be too expensive a requirement and “a bridge too far” for many small biotech companies.36 Thus, companies that develop a 5G ALK TKI must be well capitalized to meet this challenge.
Conditions Necessary for the Successful Deployment of 5G ALK TKI
All the above-mentioned resistances and unmet needs since the first-generation ALK TKI, crizotinib, require general adoption of NGS. As discussed above, two (FISH and IHC) of the four (tumor and plasma NGS by Foundation Medicine Inc.) FDA approved companion diagnostic tests for detection of ALK fusions cannot identify the actual fusion or the particular EML4-ALK variant nor the TP53 mutations status. We understand that IHC is a fast and cheap method to detect ALK fusion especially in resource-constrained regions of the world, but to continue further advancement of the treatment of ALK+ NSCLC patients, it is important to go beyond the “tip of the iceberg” by identifying the ALK variants and the TP53 mutation status at the time of diagnosis. As we strive to fully incorporate NGS in our practice of precision medicine, the inevitable need to overcome TP53 mutations and EML4-ALK variant 3 (and 5) from these NGS reports never goes away.34
Disclosure
Dr Sai-Hong Ignatius Ou reports honoraria from AnHeart Therapeutics, BMS, Claris Life Science, Pfizer, JNJ/Janssen, Daiichi Sankyo, Eli Lilly, OncLive, and DAVA Oncology LLP; has received research funding to his institution from BluePrint Medicines, Daiichi Sankyo, ERASCA Theper- atucis, Janssen/JNJ, Merus, Mirati Thepereutics, Merck, Nuvalent, Pfizer, Roche, Revolution Medicine, Sanofi, and Takeda; is a scientific advisory board member of AnHeart Therapeutics and Elevation Oncology; having stock ownership in Turning Point Therapeutics, Elevation Oncology, MBrace Therapeutics, BlossomHill Therapeutics, Lilly, Nuvalent, and Theseus Therapeutics, outside the submitted work. Dr. Lee reports no conflicts of interest in this work.
References
1. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. doi:10.1038/nature05945
2. Rikova R, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:190–203. doi:10.1016/j.cell.2007.11.025
3. Ou SI, Lee ATM, Nagasaka M. From preclinical efficacy to 2022 (36.7 months median follow -up) updated CROWN trial, lorlatinib is the preferred 1st-line treatment of advanced ALK+ NSCLC. Crit Rev Oncol Hematol. 2023;187:104019. doi:10.1016/j.critrevonc.2023.104019
4. Schneider JL, Lin JJ, Shaw AT. ALK-positive lung cancer: a moving target. Nat Cancer. 2023;4(3):330–343. doi:10.1038/s43018-023-00515-0
5. Duruisseaux M, Besse B, Cadranel J, et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget. 2017;8(13):21903–21917. doi:10.18632/oncotarget.15746
6. Pacheco JM, Gao D, Smith D, et al. Natural history and factors associated with overall survival in stage IV ALK-rearranged non-small cell lung cancer. J Thorac Oncol. 2019;14(4):691–700. doi:10.1016/j.jtho.2018.12.014
7. Solomon BJ, Bauer TM, Mok TSK, et al. Efficacy and safety of first line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomized, open-label CROWN study. Lancet Respir Med. 2023;11(4):354–366. doi:10.1016/S2213-2600(22)00437-4
8. Ou SI, Nagasaka M, Brazel D, et al. Will the clinical development of 4th-generation “double mutant active” ALK TKIs (TPX-0131 and NVL-655) change the future treatment paradigm of ALK+ NSCLC? Transl Oncol. 2021;14(11):10. doi:10.1016/j.tranon.2021.101191
9. Lin JJ, Johnson M, Felip E, et al. Safety and preliminary activity of the selective ALK inhibitor NVL-655 in patients with ALK fusion-positive solid tumors. Presented at the 35th AACR-NCI-EORTC symposium on October 13, 2023. Cancer Res. 2023;2023:1.
10. Zhang SS, Nagasaka M, Zhu VW, et al. Going beneath the tip of the iceberg. Identifying and understanding EML4-ALK variants and TP53 mutations to optimize treatment of ALK fusion positive (ALK+) NSCLC. Lung Cancer. 2021;158:126–136. doi:10.1016/j.lungcan.2021.06.012
11. Ou SH, Bartlett CH, Mino-Kenudson M, et al. Crizotinib for the treatment of ALK-rearranged non-small cell lung cancer: a success story to usher in the second decade of molecular targeted therapy in oncology. Oncologist. 2012;17(11):1351–1375. doi:10.1634/theoncologist.2012-0311
12. McLeer-Florin A, Duruisseaux M, Pinsolle J, et al. ALK fusion variants detection by targeted RNA-next generation sequencing and clinical responses to crizotinib in ALK-positive non-small cell lung cancer. Lung Cancer. 2018;116:15–24. doi:10.1016/j.lungcan.2017.12.004
13. Thorne-Nuzzo T, Willimas C, Catallini C, et al. A sensitive ALK immunohistochemistry companion diagnostic test identifies patients eligible for treatment with crizotinib. J Thorac Oncol. 2017;12(5):804–813. doi:10.1016/j.jtho.2017.01.020
14. Mok T, Peters S, Camidge DR, et al. Outcomes according to ALK status determined by central immunohistochemistry or fluorescence in situ hybridization in patients with ALK-Positive NSCLC enrolled in the phase 3 ALEX study. J Thorac Oncol. 2021;16(2):259–268. doi:10.1016/j.jtho.2020.10.007
15. Ou SI, Nagasaka M, Nagasaka M. A catalog of 5’ fusion partners in ALK-positive NSCLC circa 2020. JTO Clin Res Rep. 2020;1(1):100015. doi:10.1016/j.jtocrr.2020.100015
16. Heuckmann JM, Balke-Want H, Malchers F, et al. Differential protein stability and ALK inhibitor sensitivity of EML4-ALK fusion variants. Clin Cancer Res. 2012;18(17):4682–4690. doi:10.1158/1078-0432.CCR-11-3260
17. Horn L, Whisenant JG, Wakelee H, et al. Monitoring therapeutic response and resistance: analysis of circulating tumor DNA in patients with ALK+ lung cancer. J Thorac Oncol. 2019;14(11):1901–1911. doi:10.1016/j.jtho.2019.08.003
18. Camidge DR, Dziadziuszko R, Peters S, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J Thorac Oncol. 2019;14(7):1233–1243. doi:10.1016/j.jtho.2019.03.007
19. Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK inhibitor-naive advanced ALK-positive NSCLC: final results of phase 3 ALTA-1L Trial. J Thorac Oncol. 2021;16(12):2091–2108. doi:10.1016/j.jtho.2021.07.035
20. Bearz A, Martini JF, Jassem J, et al. Efficacy of lorlatinib in treatment-naive patients with ALK-positive advanced non-small cell lung cancer in relation to EML4:ALK variant type and ALK mutations. J Thorac Oncol. 2023;18:1581–1593. doi:10.1016/j.jtho.2023.07.023
21. Yang JC, Liu G, Lu S, et al. Brigatinib versus alectinib in ALK-positive NSCLC after disease progression on crizotinib: results of phase 3 ALTA-3 trial. J Thorac Oncol. 2023;S1556–0864(23):730.
22. Song Z, Lian S, Mak S, et al. Deep RNA sequencing revealed fusion junctional heterogeneity may predict crizotinib treatment efficacy in ALK-Rearranged NSCLC. J Thorac Oncol. 2022;17(2):264–276. doi:10.1016/j.jtho.2021.09.016
23. Nagasaka M, Ou SI. Targeting alternative splicing as adjunctive treatment in EML4-ALK v3a/b+ NSCLC: knowing our Socratic paradox and learning from spinal muscular atrophy. J Thorac Oncol. 2022;17(2):182–185. doi:10.1016/j.jtho.2021.11.010
24. Zhu VW, Nagasaka M, Madison R, et al. A novel sequentially evolved EML4-ALK Variant 3 G1202R/S1206Y double mutation in cis confers resistance to lorlatinib: a brief report and literature review. JTO Clin Res Rep. 2020;2(1):100116. doi:10.1016/j.jtocrr.2020.100116
25. Dziadziuszko R, Peters S, Mok T, et al. Circulating Cell-free DNA as a prognostic biomarker in patients with advanced ALK+ non-small cell lung cancer in the global phase III ALEX trial. Clin Cancer Res. 2022;28(9):1800–1808. doi:10.1158/1078-0432.CCR-21-2840
26. Okada K, Araki M, Sakashita T, et al. Prediction of ALK mutations mediating ALK-TKIs resistance and drug re-purposing to overcome the resistance. EBio Med. 2019;41:105–119. doi:10.1016/j.ebiom.2019.01.019
27. Bashari A, Siegfried Z, Karni R. Targeting splicing factors for cancer therapy. RNA. 2023;29(4):506–515. doi:10.1261/rna.079585.123
28. Dagogo-Jack I, Yoda S, Lennerz JK, et al. MET alterations are a recurring and actionable resistance mechanism in ALK-positive lung cancer. Clin Cancer Res. 2020;26(11):2535–2545. doi:10.1158/1078-0432.CCR-19-3906
29. Dagogo-Jack I, Kiedrowski LA, Heist RS, et al. Efficacy and Tolerability of ALK/MET combinations in patients with ALK-rearranged lung cancer with acquired MET amplification: a retrospective analysis. JTO Clin Res Rep. 2023;4(8):100534. doi:10.1016/j.jtocrr.2023.100534
30. Parikh K, Dimou A, Leventakos K, et al. Impact of EML4-ALK fusion variant and co-occurring TP53 mutation on treatment duration of first-line next-generation ALK TKIs in ALK fusion+ NSCLC. J Clin Oncol. 2023;41(16_suppl):9029. doi:10.1200/JCO.2023.41.16_suppl.9029
31. Soo RA, Martini JF, van der Wekken AJ, et al. Early circulating tumor DNA dynamics and efficacy of lorlatinib in patients with treatment-naive, advanced, ALK-positive NSCLC. J Thorac Oncol. 2023;18(11):1568–1580. doi:10.1016/j.jtho.2023.05.021
32. Solomon BJ, Bauer TM, Felip E, et al. Progression-free survival with subsequent anticancer therapies from a phase 3 trial of lorlatinib in treatment naive patients with ALK+ advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2022;40(16_suppl):9069. doi:10.1200/JCO.2022.40.16_suppl.9069
33. Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global Phase 2 study. Lancet Oncol. 2018;19(12):1654–1667. doi:10.1016/S1470-2045(18)30649-1
34. Lee JB, SI O. Plasma genotyping from the CROWN, ALTA-1L, and ALEX trials: can we speak with one voice on what to test, how to test, when to test, and for what purpose? J Thorac Oncol. 2023;18(11):1434–1442. doi:10.1016/j.jtho.2023.09.003
35. Drilon A, Lin JJ, Filleron T, et al. Frequency of brain metastases and multikinase inhibitor outcomes in patients with RET-rearranged lung cancers. J Thorac Oncol. 2018;13(10):1595–1601. doi:10.1016/j.jtho.2018.07.004
36. Ou SH, Soo RA, Kubo A, et al. Will the requirement by the US FDA to simultaneously co-develop companion diagnostics (CDx) delay the approval of receptor tyrosine kinase inhibitors for RTK-rearranged (ROS1-, RET-, AXL-, PDGFR-α-, NTRK1-) non-small cell lung cancer globally? Front Oncol. 2014;4:58. doi:10.3389/fonc.2014.00058
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.