Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 15
Obstacles to Early Diagnosis and Treatment of Pruritus in Patients with Chronic Kidney Disease: Current Perspectives
Authors Jha CM , Dastoor HD, Gopalakrishnan N, Holt SG
Received 2 July 2022
Accepted for publication 15 November 2022
Published 6 December 2022 Volume 2022:15 Pages 335—352
DOI https://doi.org/10.2147/IJNRD.S294147
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Chandra Mauli Jha,1 Hormaz Dara Dastoor,1 Natrajan Gopalakrishnan,2 Stephen Geoffrey Holt1,3
1SEHA Kidney Care, Abu Dhabi, United Arab Emirates; 2Institute of Nephrology, Madras Medical College, Chennai, India; 3Khalifa University, Abu Dhabi, United Arab Emirates
Correspondence: Chandra Mauli Jha, PO Box 61358; Al Bateen Post Office, Abu Dhabi, United Arab Emirates, Tel +971 50 1096 345 ; +971 2 55 80 482, Email [email protected]
Abstract: Chronic kidney disease-associated pruritus (CKD-aP) is a common condition amongst patients with advanced chronic kidney disease (CKD). Several studies have confirmed that more than four out of ten early-stage CKD patients suffer from this condition, while its prevalence among CKD patients on dialysis reaches up to seven out of ten. It is noted to be associated with other disabling symptoms and serious outcomes. It has significant impact on sleep, mood, daily activities, and quality of life of CKD patients, and increased mortality risk of patients on hemodialysis. The Dialysis Outcomes and Practice Patterns Study found 17% higher mortality among patients with moderate to extreme pruritus compared with patients with no or mild pruritus. Despite its high prevalence, ill-effect, and suffering associated with it, CKD-aP remains surprisingly under-reported on the patient’s part and under-recognized by the healthcare team. Even upon being noticed, it remains unattended and poorly treated. Its etiopathogenesis is complex and not fully understood. Many treatment options are available but good quality evidence about most of those is absent, and to date, only two medications are approved for use in this condition. While a validated guideline is very much required for the benefit of the patients and caretakers, further research on several aspects of this issue is required.
Keywords: chronic kidney disease, chronic kidney disease-associated pruritus, CKD-aP, pruritus, itching, uremia, uremic pruritus
Introduction
Chronic kidney disease (CKD) affects around 10% of the world’s population. Patients with CKD suffer from several chronic symptoms and amongst these pruritus is one of the more problematic which affects their health in many ways. This article aims to provide a brief narrative review of the clinical importance of this problem, its effect on health, the obstacles in early recognition, and barriers in the evidence-based management of patients with CKD suffering from this symptom.
Pruritus or itch is a sensation over the skin or adjoining mucosal surfaces arousing a desire to scratch. Such a sensation evokes an urge or desire to scratch with sensory, emotional, and motivational components.1 Whilst the itch–scratch–itch cycle can occasionally be pleasurable, it is mostly unpleasant and damaging, especially when chronic. Chronic pruritus is defined as an unpleasant sensation to the skin leading to the desire to scratch, which persists for more than six months.2
The evolution of the pruritic sensation is presumed, like pain, to be have arisen because it was protective in some way. People with congenital insensitivity to pain and itch rarely survive beyond their thirties.3–5 Itch is a common symptom of many dermatological and systemic diseases, notably including CKD. Pruritus in CKD has also previously been described as uremic pruritus to which there were objections, because that suggested causality while uremia was not always associated with pruritus, and it was suggested to be amended as “uremia associated pruritus”.6 However the accepted term now, after the acceptance of the concept and nomenclature of CKD, is chronic kidney disease-associated pruritus (CKD-aP). The present review will focus on understanding how important pruritus is in patients with CKD, what the difficulties are in its early diagnosis, and the challenges in its early treatment.
Materials and Method
PubMed, Science of Web, Cochrane database and American Society of Nephrology Kidney week abstracts were searched till 5th April 2022. Search terms were “Itching in Chronic Kidney Disease”, “Pruritus in Chronic Kidney Disease’, “itching in dialysis’, “pruritus in dialysis”, “Uremic pruritus”, and “CKD-aP”. The search was repeated on 15th June 2022 to supplement recent publications after 5th April 2022. EndNote software was used to organize the references. 4030 search results yielded 1163 nonduplicate references. Titles were screened for relevance, and 437 articles were selected to retrieve 121 full text and 316 abstracts. 112 of those were utilized in the synthesis of a narrative review of the subject (Figure 1).
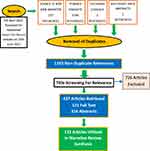 |
Figure 1 PRISMA Flow Diagram of Methodology. Notes: PRISMA Figure adapted from Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. Creative Commons.7 |
CKD-aP: Importance
Prevalence
In CKD patients, pruritus as an isolated symptom is uncommon whilst its presence in a cluster of two or more other symptoms together is high. Its prevalence in the different studies varies from 20% to 85%. In the earliest report, Young et al noted pruritus to be present in 85% of the small group of 36 patients on maintenance hemodialysis.8 However, more recent studies suggest a much lower prevalence.
A recent prospective, observational study by Wulczyn et al found that pruritus was present in 42% of 3685 participants from the Chronic Renal Insufficiency Cohort Study (CRIC) patients with CKD not on dialysis. The severity of the symptom at baseline was reported as moderate to extreme in approximately one third of stage 1 to 3a CKD patients, and in about half of stage 3b to 5 CKD patients. The severity of pruritus increased nonlinearly with the progression of renal impairment.9 A decrease in eGFR of 5 mL/min per 1.73 m2 was associated with a worsening of the symptom severity score by two points or less on a scale of zero to 100 points. Uremic symptom worsening score for any degree of decrease in eGFR was greater for lower initial eGFR.9
The largest study of CKD-aP prevalence in CKD patients not on dialysis was from the USA, Brazil, and France in the international, prospective CKD Outcomes and Practice Patterns Study (CKDOPPS) comprising 67% of a nephrology-clinic based cohort of 5658 adult patients of stage 3 to stage 5 CKD not on dialysis. It found that the overall prevalence of moderate pruritus was 24% (24% in Brazil, 29% in the USA, and 23% in France), and of severe to extreme pruritus 12% (11% in Brazil, 13% in the USA, and 10% in France). The prevalence of moderate-to-extreme pruritus was higher in the advanced stages of CKD with the adjusted rate being 19% higher in stage 5 compared to stage 3 disease.10
A study of RENINE/PROMs registry data of 2978 Dutch dialysis patients by van der Willik et al revealed that 50% of patients had itching which was described as “persistent itching” by 70% of those 50%.11
For dialysis patients, data from the Dialysis Outcomes and Practice Patterns Study (DOPPS 1996–2004) revealed that more than 70% of prevalent dialysis patients had some degree of pruritus. Moderate to extreme pruritus was experienced by 42% of prevalent HD patients during 2002/2003 of the study period.12 The incidence of moderate to severe degrees of pruritus in DOPPS II (2002–2004; 12 countries, 317 facilities, 10,265 patients) was 42% and in DOPPS I (1996–2001; 6 countries, 284 facilities, 10,810 patients) it was 45%.12 In the DOPPS Phases 4 to 6 (2009–2018; 23,264 hemodialysis patients from 21 countries) study, 67% patients had pruritus of some degree, leaving only 33% of patients pruritus free while moderate to extreme pruritus was experienced by 37%.13
A similar (44%) prevalence of moderate to extreme degree of pruritus was self-reported in Japan (JDOPPS) from 1999 to 2008. The overall prevalence was similar in all three study phases.14
In a study of 71,012 patients who had responded to the Kidney Disease Quality of Life-36 (KDQOL) survey amongst 73,124 dialysis patients at a large dialysis organization (LDO) in the USA during January 2009 to May 2012, 60% of respondents reported itching and 14.5% of the patients reported being “very much bothered” or “extremely bothered” by itchiness.15
The ITCH National Registry Study of hemodialysis patients from the USA suggested that 84% of patients suffered from pruritus, and symptoms had been present for more than one year in 59%.16
The pooled prevalence of CKD-aP from 11 different studies in China was 61% (95% CI, 52–69).17
A cross-sectional German Epidemiological Hemodialysis Itch Study (GEHIS) in hemodialysis patients in Germany found a point prevalence of 25.2% and a lifetime prevalence of 35.2% of chronic itch.18
Gatmiri et al reported the prevalence to be 58.6% among hemodialysis patients in Iran.19
Although there is no large-scale study on CKD-aP prevalence in peritoneal dialysis patients, reporting in small studies suggests the incidence similar to that in hemodialysis.20,21
Under-reporting
Interestingly, Rayner et al noted that among those patients who were nearly always or always bothered by itchy skin, 17% had not reported their symptoms to any healthcare provider.22 So it is likely that the true prevalence of the symptom of itch in CKD patients may be much higher.
Clinical Impact
CKD-aP cannot merely be considered only a symptom. It has been found to be associated with adverse health outcomes and higher morbidity and mortality. Reduced Health-Related Quality of life (HRQoL) in patients with CKD-aP has been noted in several studies of hemodialysis patients.12,13,16,18,20,23–28 DOPPS I & II noted a 17% higher mortality risk (p<0.0001) for patients with moderate to extreme pruritus compared with patients with mild or no pruritus. However, this higher mortality risk lost statistical significance after adjustment for sleep quality measures, suggesting that either combination of symptoms carried higher mortality risk or higher mortality risk associated with CKD-aP is because of its effect on quality of sleep.12 It remains a subject of further examination and research.
The DOPPS Phase 4–6 study showed that patients with severe pruritus had a higher rate of hospitalization and a larger number of deaths due to cardiovascular and infection-related causes compared with those with less severe symptoms. Patients extremely bothered by pruritus were also less likely to be employed, more likely to withdraw from dialysis, and more likely to miss hemodialysis sessions. There were also strong associations between pruritus severity and a number of other variables such as recovery time after a hemodialysis session, self-reported physical and mental quality of life, depressive symptoms, poor sleep quality, self-reported restless sleep, dizziness and faintness etc., all related in a monotonic mathematical fashion.13 Patients extremely bothered by pruritus had a hazard ratio of withdrawal from dialysis 1.50 (95% CI, 1.05–2.14), adjusted all-cause mortality 1.24 (95% CI, 1.08–1.41), cardiovascular mortality 1.29 (95% CI, 1.06–1.57), and infection-related mortality 1.44 (95% CI, 1.05–1.96) compared with those patients not bothered at all by the symptom of pruritus.13
Mathur et al found that the intensity of itching assessed with different instruments such as the visual analogue scale (VAS), numerical rating scale (NRS), itch measurement of sleep (itch-MOS), or Self-Assessed Disease Severity etc. were all associated with lower HR-QOL in every domain measured, including sleep, mood, emotional distress, social functioning, and work etc.16
Whilst the DOPPS data suggested that the effect of severe pruritus on the mortality may be mediated through the quality and nature of sleep, a study by Narita et al of 1773 adult Japanese hemodialysis patients found that severe CKD-aP was independently associated with an increased death rate.29
Amongst the sleep disturbances recognized to be associated with CKD-aP were difficulty in falling asleep, impaired sleep quality, nocturnal wakefulness, daytime somnolence, and restless sleep etc.13,20,23 In addition restless leg syndrome and chronic pain were more likely to be present in patients with CKD-aP.18,30,31
The finding of an association with increased chance of missing hemodialysis sessions noted in DOPPS 4 to 6 was also noted among Large Dialysis Organization (LDO) patients of USA together with an increase in the use of medications such as IV antibiotics, erythropoiesis stimulating agents, and IV iron.15
Thus, based on the burden of the suffering, the morbidity, the mortality, and the poor quality of life associated with the CKD-aP, it is important (importance summarized in Table 1) that itch symptom in CKD patients is looked for and addressed while caring for these patients at all stages of disease including patients on dialysis.
 |
Table 1 Importance of CKD-aP |
Obstacles to the Early Diagnosis
Experts have described the diagnosis of CKD-aP as challenging.32 There are several issues related to early diagnosis as CKD-aP may not merely be a symptom but rather a chronic systemic condition related to kidney disease, with or without any other comorbid conditions to confound the causes of itching.33 The presence of comorbidities in patients with CKD which in themselves may cause pruritus makes the evaluation of CKD-aP difficult. Such comorbidities may be dermatological, hepatobiliary, hematological, endocrine, neurological, or oncological etc. In addition, such patients with CKD frequently receive a large number of medications which can cause itching, posing diagnostic uncertainty.
Additionally, there are widely variable clinical presentations of CKD-aP with symptoms that may be paroxysmal lasting from a few minutes to few hours or regular and continuous and may cause minimal disruption to complete restlessness. While the skin may be normal, xerosis cutis and dryness of the skin is observed in the majority of the patients. The absence of any primary skin lesion is common and described to be the rule but excoriation and prurigo secondary to itching might be present. Pruritus is generalized and symmetrical in about ~50% of the patients or localized to the face, arm, back, fistula arm, or lower limbs in others.16,32,33 There may be chronic changes leading to crusts, papules, ulcerations, erosions, prurigo nodularis, or evidence of secondary infection such as impetigo etc.34
Recognition
The recognition of the problem is the first step toward treatment. Given the association with negative outcomes associated with CKD-aP, the symptom of pruritus should be considered important at any time in advanced CKD (stage 5 regardless of whether on dialysis or not) and considered diagnostic of CKD-aP unless an obvious alternate cause is present.
As noted above, symptom of pruritus is under-reported by CKD patients.22 A factor that may be relevant in under-reporting may be the patients’ lack of awareness of a link of pruritus with kidney disease, or their acceptance that itch is something they may have to live with. Other communication barriers such as the language and available length and timing of consultations, during which they may prioritize other health issues, may also be responsible factors.35 Healthcare teams may also not be actively seeking these symptoms. Rayner et al found that the majority of medical directors of dialysis facilities across the globe underestimated the prevalence of CKD-aP to merely one-third to one-fifth of the true prevalence.22 Zucker et al used the following criteria for the diagnosis of pruritus of CKD: 1) more than or equal to three episodes of itch during a period of 2 weeks, with the symptom appearing a few times a day, lasting at least few minutes, and troubling the patient; 2) the appearance of an itch in a regular pattern during a period of 6 months, but less frequently than listed above; and 3) absence of prolonged pruritus caused by an additional disease.36
An early diagnosis can be improved by the inclusion of pruritus-related information in pre-dialysis education of patients, the inclusion of systemic symptom assessment in routine kidney care, the use of a checklist, development of guidelines, and use of validated questionnaires in day-to-day clinical practice and quantification of the symptom.
Obstacles to the Treatment
Under-Recognition
Unrecognition is the first important obstacle in early management of CKD-aP. Around 17% of patients who were nearly always or always bothered by itch had not reported their symptoms to any healthcare provider. This was higher in the USA (33%), compared to the Gulf Cooperation Council countries (12%), and variable in Europe, where it varied from 21% in Sweden to 8% in Italy.22 This under-reporting on the part of the patients was supplemented by underestimation of the symptom burden by clinicians involved in their care. In a large fraction of facilities where one-fifth to half of the patient population had severe pruritus, only 1% of the medical directors were able to estimate the exact prevalence of the pruritus. Around two-thirds of medical directors estimated that <5% of their patients had severe pruritus while the true prevalence of severe pruritus was 13 to 26%. Additionally the patients were more likely to report the symptom of pruritus to a nephrologist and dialysis staff members than to a dermatologist or primary care physician.22
The use of a checklist and symptom scoring system can help in early recognition of pruritus. For example, Integrated Palliative care Outcome Scale (IPOS-renal) contains a question abound itching.37,38 When regularly applied to patients this may help patients report and identify issues.
Ignored Symptom Cluster
The second obstacle to the treatment lies in our approach to addressing the pruritic symptom individually instead of recognizing it as a component of other related issues which could exert the synergistic effects upon each other resulting in the amplified suffering of the patient. More recently the occurrence of such related symptoms together referred to as a “symptom cluster”, causing synergistic effects, and where treatment of one of those symptoms may affect the others and the outcome, has been recognised39,40 (Table 2). Addressing identified modifiable risk factors associated with CKD-aP should also be the part of a strategy of the management of CKD-aP. Symptom clusters may have a common pathological mechanism, the management of which may bring broader involvement and effect.41 Understanding about the science of the symptom cluster at present is not evolved much, less so in the area of CKD. It requires a change in our outlook and more research in this area of CKD is needed.42
 |
Table 2 Symptom-Cluster of CKD-aP |
Pathogenesis
The third obstacle to treatment lies in our inadequate current understanding of the pathological mechanism of the CKD-aP which is based upon pieces of information derived from association and intervention. It lacks comprehensive nature. A brief view of it may illustrate the limitation of the different treatments of CKD-aP which limits effectiveness of our approach to treatment. The pathogenesis of CKD-aP is multifactorial, with roles of metabolic derangements in uremia, immune dysfunction, microinflammation, histological changes in the dermis, and alterations in receptors as well as dysregulation in peripheral and central nervous system, endogenous opioid systems, and the psyche etc. (Figure 2).
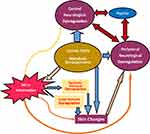 |
Figure 2 Pathogenic factors of CKD-aP and their interactions. |
Metabolic and Uremic
Uremic metabolic derangements likely lie at the center of the issue and an important reciprocal relationship between urea clearance (kt/v) and incidence of CKD-aP exists in dialysis patients.43,44 Wulczyn et al noted that in patients not on dialysis a decrease in eGFR of 5 mL/min per 1.73 m2 was associated with a worsening of the symptom severity score by two points or less on a scale of zero to 100 points. Uremic symptom worsening score for any degree of decrease in eGFR was greater for lower initial eGFR.9 Transplantation dramatically reduces the prevalence and intensity of CKD-aP to ~12% (versus 35% controls) with reversal of skin histopathological changes observed in CKD-aP.45 Different accumulated toxic metabolites or deranged levels of nontoxic metabolites may act as pruritogens or modify the itch perception mechanism. Hsu et al associated a higher serum aluminum levels with a higher prevalence of CKD-aP.46 Parathyroidectomy was noted to be an effective therapy in a group of patients with CKD-aP, although not always.47 An elevated calcium phosphate product and elevated parathyroid hormone level in the group of patients who benefitted from parathyroidectomy suggests that CKD mineral bone disease may be a factor related to CKD-aP.11,12 However the calcium phosphate product is an artificial construct and unlikely to be highly representative in the complex physiology of mineral trafficking and it is highly likely that the subtleties of mineral trafficking have been missed by the crude association of the calcium phosphate product. Nevertheless, there is more calcium in the basal skin layer in patients with CKD-aP and this is an area that needs further research.48,49
Hiroshige et al in their prospective study found that good dialysis and a good nutritional status reduced the prevalence and degree of pruritus in the patients with disabling symptoms but without significant disorders of calcium and phosphate metabolism. Significantly higher values of blood urea nitrogen (BUN) and plasma beta 2-microglobulin pre-dialysis were observed in those pruritic patients with lower dialysis dose estimated by Kt/Vurea and normalized protein catabolic rate (nPCR).50 Wu et al compared serum metabolic profiles of severely pruritic patients against mildly pruritic patients with uremia using ultra-performance liquid chromatoraphy–quadruple time-of-flight mass spectrometry (UPLC-QTOF MS) and identified nine candidate biomarkers which belonged to chemical groups of phospholipids, uremic toxins, and steroids.51 The role of accumulated mid and large molecules in the causation of CKD-aP is supported by a randomized, prospective, double-blind study comparing the effect of high permeability hemodialysis (HPHD) against conventional hemodialysis (CHD) on CKD-aP by Chen et al which showed that the degree of itching in the trial group (HPHD) was significantly lower than that in the control group (2.23±1.05 vs 5.45±1.91, P=0.009) as measured by visual analogue scale (VAS) of itching.52 Lim et al compared the effect of dialysis with a medium cut-off (MCO) dialyzer to that with high-flux dialyzers on quality of life outcomes in maintenance hemodialysis patients. Their finding was that dialysis with MCO dialyzers improved physical functioning and reduced the scores for morning pruritus distribution and the frequency of scratching during sleep.53
Microinflammation and Dysregulated Immune Function
There is also a probable role of microinflammation and dysregulated immune function in patients with CKD-aP. This dysfunction appears to be both at the systemic level and local dermatological levels. A few risk factors such as white blood cell (WBC) count >6700/µL, or the presence of hepatitis C or ascites associated with severe pruritus identified in the DOPPS study suggest the role of microinflammation.11 Additionally, significantly raised blood levels of cytokines may play a key role in CKD-aP including Interleukin-31 (IL-31), Interleukin-6 (IL-6), and Interleukin-2 (IL-2) as well as other evidences of immune activation or dysregulation such as raised Th1 lymphocytes or C-reactive protein (CRP).54,55
In comparison to patients without pruritus, itching patients have been noted to have significantly higher dermal mast cell counts which were mainly degranulated and diffusely spread, as well as an increased ratio of Th1:Th2 T cells with higher circulating level of histamine, serotonin, tryptase, and other interleukins.21,55–58 Whilst the antihistamines and serotonin receptor inhibitor ondansetron has not shown therapeutic benefit, the mast cell stabilizer cromolyn and leukotriene receptor antagonist Montelukast have shown benefit in the relief of CKD-aP. Tacrolimus which blocks calcineurin mediated IL-2 production from helper T cells has shown therapeutic benefit in a few studies.21,59–62
The seemingly unconnected observation that patients on hemodialysis receiving a statin appear to suffer less from pruritus is also suggested to be due to its ability to lower serum proinflammatory cytokines and CRP.63
Metabolic derangements of CKD with systemic microinflammation bring multiple skin changes such as dryness, xerosis, increased number of dermal mast cells, secretion of pruritogens from local dermal mast cells, deposition of calcium and magnesium micro-crystals, and the proliferation of nerve fibers etc., which are considered contributive to cause pruritus.
Dysregulation of Nervous System
Neural itch pathways at the central and peripheral levels are becoming better understood.64–67 There are mechano-sensitive and mechano-insensitive C-fibers that respond differently to histamine or other pruritogens such as cowhage.68 Microneurography of human skin nerves has demonstrated that itch induced by different agents activates different neuronal populations in the peripheral nervous system (PNS).69 Additionally, most CKD patients have altered skin innervation with abnormal neuron-specific enolase (NSE)-immunoreactive nerve terminals and fibers sprouting throughout the layers of the epidermis in patients on maintenance hemodialysis.70,71 A peripheral neuropathic mechanism is suggested by therapeutic relief by gabapentin and pregabalin which have proven beneficial effects in the treatment of neuropathic pain.72–74 There is also emerging evidence around a dysregulated endogenous opioid system being causative or contributory to CKD-aP.75 There are three endogenous opioid receptors, μ-opioid receptor (MOR), κ-opioid receptor (KOR), and δ-opioid receptor (DOR), whose corresponding endogenous ligands are endorphins, dynorphins, and enkephalins respectively, which are present in the central nervous system as well on peripheral nerve fibers. Evidence suggests that the KOR system suppresses itch whilst the MOR system stimulates itch.76 MOR overactivity, KOR underactivity, or antagonism would act synergistically to promote itch. The finding of an increased ratio of MOR agonist β-endorphin to KOR agonist Dynorphin A in hemodialysis patients with CKD-aP is suggestive of such a mechanism.77
Treatment Options and Limitations
There is a general dearth of high-quality evidence around treatment for CKD-aP together with the absence of a systematic approach to therapy. Although there is plenty of anecdotal literature on treatment options which have been used in several small trials or case series, very few of those have adequate power or quality, and there are no international guidelines or validated algorithms available to use those effectively. Expert opinion-based algorithmic approaches have been suggested by several authors in review articles and a stepwise approach is suggested by Patel et al.32,78–80 The treatments advocated can be broadly classified into topical, systemic, physical, and empirical (Table 3). Simonsen et al have performed a systematic review of these treatments but found that the majority of those studies lacked quality due to flawed technology, small sample sizes, or heterogenicity.81 At present there are only two drugs approved for the treatment of CKD-aP: selective peripheral KOR agonist Nalfurafine approved in Japan and selective peripheral KOR agonist Difelikefalin approved by the FDA, USA.82–85
 |
Table 3 Various Treatments Used in CKD-aP |
Clinical Evaluation
A regular periodical evaluation plan for CKD-aP should be available in CKD and dialysis clinics with elements including history, examination, investigation, evaluation of quality of life (QOL), sleep assessment, severity scoring of pruritus, and assessment of modifiable risk factors (Table 4), and the presence of the symptom cluster (Table 2). A validated symptom scoring system should be used to guide the management more objectively. A salient clinical evaluation should focus upon:
- The history to gather information about the location, severity, duration, character, frequency, and exacerbating and relieving factors of the pruritus.
- A system review to note presence of systemic diseases, review of the drug history, and smoking status should form a central part of evaluation.
- Every patient with advanced CKD should be evaluated for sleep, its quality, and for depression and anxiety.
- Examination should note excoriation marks, xerosis (dryness) of skin, prurigo nodularis, lichenification etc., which as being a sign of CKD-aP might be an alert for a primary dermatological condition for which a note should be made of.
- Evaluation of if a primary dermatological condition – a comorbidity – could be a possible cause of itching rather than CKD, including low threshold for referral to dermatologist should be practiced. However, the treatment for CKD-aP should not be delayed pending evaluation.
- Signs of heart failure and ascites which are recognized risk factors (Table 4).
- Blood tests to establish CKD stage, CRP, hemoglobin, white cell count, calcium, phosphate, albumin, PTH, hepatitis serology, aluminum level, ferritin level etc.
 |
Table 4 Risk Factors and Hazard Ratio of Moderate to Extreme CKD-aP |
Severity Assessment
Quantification of symptoms and QOL brings objectivity in the management. Currently used assessment tools can be divided into: 1) scales measuring the severity of the itching only; 2) multidimensional pruritus scale which measures several components of pruritus; and 3) multidimensional scales which measure the severity of itching and its impact upon the quality of life.
There are four severity measuring scales: 1) the most commonly used visual analog scale (VAS); 2) the numeric rating scale (NRS); 3) the verbal rating scale (VRS); and 4) the Kidney Disease Quality of Life-Short Form (KDQOL-SF).86 VAS uses a horizontal or vertical line in which the extreme left or lowest point represents no itching and the extreme right or top the worst. NRS is similar to VAS where the severity is measured on a numerical scale from zero to ten. The verbal rating scale (VRS) has four severities of itching – no, low, moderate, or severe.87 One question in the KDQOL-SF survey assesses the itching severity.88 Multidimensional itching scale measures several components of the pruritus. There are two such scales, 5-D itching scale and the itching severity scale (ISS).89,90 The 5-D itching scale evaluates the five domains of itching, the degree, duration, direction, disability, and distribution. The itching severity scale (ISS) measures the duration, frequency, pattern, intensity, treatment, symptoms, sensation, and effect of itching on QOL and is a valid and reliable measure of itching.90 The dermatology QOL index (DLQI) and the Skindex measure the impact of skin disease on QOL. For day-to-day clinical use, the simple severity measuring scales VAS or NRS are excellent useful tools.
Treatments
Whilst CKD is a global problem, the various treatments used for CKD-aP may not be available everywhere. In view of this it would be helpful to simplify the management plan in a stepwise manner using the most effective and least invasive treatments first. We shall briefly review a few treatment options that are generally safe and effective followed by how to use those in a real management plan (Table 3 and Figure 3).
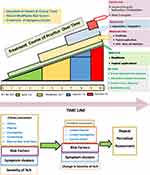 |
Figure 3 Clinical management of CKD-aP. |
Risk Factors
Several studies have identified risk factors associated with higher prevalence and serious outcome of CKD-aP (Table 4). Addressing dialysis adequacy and/or increasing dialysis time is often a good empirical first step yet there is no evidence that intensifying dialysis beyond recommended Kt/V values may have any beneficial effect. One randomized trial examined the effect of dialysis dose upon CKD-aP and noted benefit of mean kt/v 1.28 compared to mean kt/v of 1.09.50 However another nonrandomized study has noted increased risk of CKD-aP with higher dose of dialysis (kt/v 1.82).63
Attention to CKD mineral bone diseases (CKD-MBD) to control the parathyroid hormone level and calcium levels constitutes a very wide target. Serum phosphate control is frequently difficult to achieve without increasing time on dialysis. Correction of iron deficiency and anemia is also important. Stopping smoking is clearly advisable on several grounds. High flux hemodialysis (HFHD), hemodiafiltration (HDF), and high permeability hemodialysis have been linked with improved symptom of pruritus.50,91 The use of magnesium-free dialysate has not brought beneficial effect.92 Although the low ferritin is noted to be a risk factor of CKD-aP, we do not know if iron administration would benefit CKD-aP.
Gut binding of various uremic toxins with unpleasant activated charcoal and or cholestyramine may require more evidence to be recommended but has some logic and can be tried in patients with poor symptom control.93,94
Topical Treatment and Emollients
Xerosis is noted in about 85% of patients with CKD-aP which is believed to add to the intensity of itch. Emollients often improve symptoms and have few downsides. It should be considered first-line treatment of xerosis.95 There is no evidence as to whether preemptive use of emollients in patients with advanced CKD without itch or xerosis could be effective. Topical preparation containing structured natural lipids and endocannabinoids have been reported to be effective in controlling pruritus and xerosis in maintenance hemodialysis patients.96 A multicenter double-blind, randomized controlled trial of 100 dialysis patients with moderate to severe uremic xerosis randomized to glycerol 15% and paraffin 10% in an oil-in-water emulsion versus an oil-in-water emulsion alone, showed benefit to the former preparation.97 Evidence regarding benefit of topical application of Tacrolimus is contradictory in different studies. Kuypers et al found topical Tacrolimus application effective in reducing symptom of pruritus.62 However, in another study it was noted ineffective compared to placebo.98
Antihistamines and Immunomodulation
Antihistamines are noted to be the most commonly prescribed medicines.22,34 DOPPS data showed that prescription or nonprescription (over the counter) oral antihistamines were used on 57% of first-line therapies by Medical Directors of dialysis units.22 Interestingly and paradoxically, antihistamines are the least evaluated in the treatment of CKD-aP. A few small studies suggest antihistamines ineffective or least effective in CKD-aP. A survey study by Gilchrest et al of 237 patients undergoing maintenance hemodialysis noted that topical emollients and orally administered antipruritic agents provided relief in only 33 (18%) and 31 (17%) patients respectively, thus specifically suggesting that antihistamines were not more effective than emollients.34 Weisshaar et al tested 11 patients on hemodialysis against ten healthy controls to evaluate the effect of the 5-HT3 receptor antagonists tropisetron 5 mg and ondansetron 8 mg and the antihistamine cetirizine 10 mg. Their finding was that while these medicines were able to prevent itching in normal individuals, that effect was lost in patients of CKD-aP.99 Desloratadine 5 mg thrice weekly was compared with gabapentin 300 mg thrice weekly by Marquez et al in a prospective, open-label, cross-over clinical trial in 22 patients on chronic hemodialysis with sustained pruritus over a period of at least 60 days, and found Desloratadine to be effective and more tolerable.100 In the absence of a proper evaluation of antihistamines in CKD-aP, several reviewers on this topic have simply repeatedly mentioned that antihistamines were not effective.32,78–80,101,102 However, if effective, it might suggest the cause of itching a primary dermatological condition or an etiology other than CKD-ap. It has also been suggested that any benefit of antihistamines in ameliorating CKD-aP is possibly due to their sedative effects.
The mast cell stabilizer Cromolyn as topical 4% cream and systemic administration has been noted to be effective while nicotinamide was not found effective.60,102,103 Systemic use of cromolyn should be considered in patients with high WBC count and/or elevated CRP without any evident source of infection. UVB phototherapy believed to act on mast cells locally has been found to be effective in few studies.103–105 Its side effect of sun burn and tanning are recognized while long-term safety is undetermined.
Neuroanalgesics
Gabapentin and pregabalin are two effective drugs found to be effective in the control of symptoms of CKD-aP. Their effectiveness has been confirmed in several studies and meta-analyses. However the numerous side effects such as somnolence, dizziness, dry mouth, and weight gain are a significant disincentive to their use.106–109
Drugs Acting Upon Opioid Receptors
MOR overactivity increases pruritus but MOR antagonist naloxone and naltrexone were found ineffective in some studies.110,111 However, the peripheral KOR receptor agonist Nalfurafine licensed in Japan for CKD-aP in the dose of 2.5–5 µg daily has been found effective in the control of the symptoms.84,85,111 The peripheral KOR receptor agonist Difelikefalin approved for CKD-aP by the FDA administered IV in a dose of 0.5 µg/kg of body weight three times in a week has been shown to improve pruritus and quality of sleep, and its efficacy was maintained over a year.83,112,113
Clinical Treatment Plan
In the absence of validated guidelines and the different algorithms suggested by different experts, we recommend the following treatment plan in the light of the available evidence (Figure 3):
- Education and awareness of patients and caretakers.
- Identification and treatment of risk factors.
- Optimization of dialysis adequacy.
- Prioritization of kidney transplantation where possible.
- Continuous periodical evaluation as described above in the section “Clinical Evaluation”.
- Use of emollients and topical treatments.
- Systemic use of Gabapentin or Pregabalin if the symptom is more than moderate.
- Peripheral KOR agonist for severe and extreme pruritus.
Hemodialysis Patients: A Special Group
The patients receiving hemodialysis constitute a special group as they have higher incidence and prevalence of CKD-aP of more severe degree. These patients have frequent interaction with the medical team which provides enhanced opportunities for early detection, frequent assessment, and better management as well as research opportunities. In spite of the fact that CKD-aP is associated with higher mortality, its management rarely ranks highly in “quality of care” assessment whilst the anemia management, bone mineral management, and dialysis adequacy are often accounted. Thus, it would be important for the dialysis unit to develop and adopt a CKD-aP policy. Suggested measures would be:
- Separate periodical nursing evaluation and physician’s evaluation of CKD-aP should be in a regular management plan. A local flow chart should be developed and maintained.
- Antihistamines should be avoided because of paucity of evidence for benefit in this condition and higher risks of sedative effect in a uremic state.
- Dialysis adequacy should be ensured.
- High flux hemodialysis (HFHD), hemodiafiltration (HDF), high permeability hemodialysis, and use of MCO dialyzers should be considered within budgetary limits, if not being used.
- Smoking cessation should be promoted.
- Other symptom cluster issues should be addressed.
- Transplant should be prioritized in severe cases.
- Research should be directed toward this patient group in order to understand and treat this disabling symptom.
Conclusion
CKD-aP is a common symptom in advanced stages of CKD which is associated with considerable suffering, decreased HRQoL, serious outcomes, and increased mortality necessitating its early diagnosis and management. Paradoxically in clinical practice it commonly goes unidentified and untreated because of various factors on the part of patients as well as the clinicians and knowledge gap. Its evaluation and management is complex and challenging. International Guidelines and a validated treatment algorithm are needed. Several aspects of this problem remain as knowledge gaps and should be a priority area of research (Table 5). The condition CKD-aP should be considered for a separate diagnostic code from the International Classification of Diseases (ICD). In the absence of international guidelines, institutional policies should be developed to increase awareness, early recognition, and management tailored to the locally available resources.
 |
Table 5 Areas of Research |
Acknowledgments
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
- Chandra Mauli Jha: The author has received travel and educational grant assistance from Fresenius, Amgen, and Roche in the past. There are no conflicts of interest in this work.
- Hormaz Dara Dastoor: The author has received assistance from Fresenius, Amgen, and Roche in the past. The author reports no conflicts of interest in this work.
- Natrajan Gopalakrishnan: The author reports no conflicts of interest in this work.
- Stephen Geoffrey Holt: The author reports no competing conflict of interest in this work.
References
1. Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7(7):535–547. doi:10.1038/nrn1950
2. Ständer S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the international forum for the study of Itch. Acta Derm Venereol. 2007;87(4):291–294. doi:10.2340/00015555-0305
3. Ross SE. Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr Opin Neurobiol. 2011;21(6):880–887. doi:10.1016/j.conb.2011.10.012
4. Cox JJ, Reimann F, Nicholas AK, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444(7121):894–898. doi:10.1038/nature05413
5. Sandroni P, Martin DP, Bruce BK, Rome JD. Congenital idiopathic inability to perceive pain: a new syndrome of insensitivity to pain and itch with preserved small fibers. Pain. 2006;122(1–2):210–215. doi:10.1016/j.pain.2006.01.033
6. Saxena AK. ”Uremic pruritus”: a misnomer. Hemodial Int. 2005;9(4):416–417. doi:10.1111/j.1542-4758.2005.01161.x
7. Page MJ et al . (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ, n71. doi: 10.1136/bmj.n71
8. Young AW, Sweeney EW, David DS, et al. Dermatologic evaluation of pruritus in patients on hemodialysis. N Y State J Med. 1973;73(22):2670–2674.
9. Wulczyn KE, Zhao SH, Rhee EP, Kalim S, Shafi T. Trajectories of uremic symptom severity and kidney function in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2022;17:496–506. doi:10.2215/CJN.13010921
10. Sukul N, Speyer E, Tu C, et al. Pruritus and patient reported outcomes in non-dialysis CKD. Clin J Am Soc Nephrol. 2019;14(5):673–681. doi:10.2215/CJN.09600818
11. van der Willik EM, Lengton R, Hemmelder MH, et al. Itching in dialysis patients: impact on health-related quality of life and interactions with sleep problems and psychological symptoms - results from the RENINE/PROMs registry. Nephrol Dial Transplant. 2022;37:1731–1741. doi:10.1093/ndt/gfac022
12. Pisoni RL, Wikström B, Elder SJ, et al. Pruritus in hemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2006;21(12):3495–3505. doi:10.1093/ndt/gfl461
13. Sukul N, Karaboyas A, Csomor PA, et al. Self-reported pruritus and clinical, dialysis-related, and patient-reported outcomes in hemodialysis patients. Kidney Medicine. 2021;3(1):42–53.e1. doi:10.1016/j.xkme.2020.08.011
14. Kimata NFD, Saito A, Akizawa T, et al. Pruritus in hemodialysis patients: results from the Japanese Dialysis Outcomes and Practice Patterns Study (JDOPPS). Hemodial Int. 2014;18(3):657–667. doi:10.1111/hdi.12158
15. Ramakrishnan K, Bond TC, Claxton A, et al. Clinical characteristics and outcomes of end-stage renal disease patients with self-reported pruritus symptoms. Int J Nephrol Renovasc Dis. 2013;7:1–12. doi:10.2147/IJNRD.S52985
16. Mathur VS, Lindberg J, Germain M, et al. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(8):1410–1419. doi:10.2215/cjn.00100110
17. Hu X, Sang Y, Yang M, Chen X, Tang W. Prevalence of chronic kidney disease-associated pruritus among adult dialysis patients. Medicine. 2018;97(21):e10633. doi:10.1097/md.0000000000010633
18. Weiss M, Mettang T, Tschulena U, et al. Health-related quality of life in haemodialysis patients suffering from chronic itch: results from GEHIS (German Epidemiology Haemodialysis Itch Study). Qual Life Res. 2016;25(12):3097–3106. doi:10.1007/s11136-016-1340-4
19. Gatmiri SM, Mahdavi-Mazdeh M, Lessan-Pezeshki M, Abbasi M. Uremic pruritus and serum phosphorus level. Acta Med Iran. 2013;51:477–481.
20. Tessari G, Dalle Vedove C, Loschiavo C, et al. The impact of pruritus on the quality of life of patients undergoing dialysis: a single centre cohort study. J Nephrol. 2009;22(2):241–248.
21. Mettang T, Fritz P, Weber J, Machleidt C, Hübel E, Kuhlmann U. Uremic pruritus in patients on hemodialysis or continuous ambulatory peritoneal dialysis (CAPD). The role of plasma histamine and skin mast cells. Clin Nephrol. 1990;34(3):136–141.
22. Rayner HC, Larkina M, Wang M, et al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol. 2017;12(12):2000–2007. doi:10.2215/CJN.03280317
23. Weiss M, Mettang T, Tschulena U, Passlick-Deetjen J, Weisshaar E. Prevalence of chronic itch and associated factors in haemodialysis patients: a representative cross-sectional study. Acta Derm Venereol. 2015;95(7):816–821. doi:10.2340/00015555-2087
24. Hayani K, Weiss M, Weisshaar E. Clinical findings and provision of care in haemodialysis patients with chronic itch: new results from the German epidemiological haemodialysis itch study. Acta Derm Venereol. 2016;96(3):361–366. doi:10.2340/00015555-2280
25. Ibrahim MK, Elshahid AR, El Baz TZ, Elazab RM, Elhoseiny SA, Elsaie ML. Impact of uraemic pruritus on quality of life among end stage renal disease patients on dialysis. J Clin Diagn Res. 2016;10(3):WC01–WC5. doi:10.7860/JCDR/2016/16273.7488
26. Lopes GB, Nogueira FC, de Souza MR, et al. Assessment of the psychological burden associated with pruritus in hemodialysis patients using the kidney disease quality of life short form. Qual Life Res. 2012;21(4):603–612. doi:10.1007/s11136-011-9964-x
27. Plewig N, Ofenloch R, Mettang T, Weisshaar E. The course of chronic itch in hemodialysis patients: results of a 4-year follow-up study of GEHIS (German Epidemiological Hemodialysis Itch Study). J Eur Acad Dermatol Venereol. 2019;33(7):1429–1435. doi:10.1111/jdv.15483
28. Satti MZ, Arshad D, Javed H, et al. Uremic pruritus: prevalence and impact on quality of life and depressive symptoms in hemodialysis patients. Cureus. 2019;11(7):e5178. doi:10.7759/cureus.5178
29. Narita I, Alchi B, Omori K, et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006;69(9):1626–1632. doi:10.1038/sj.ki.5000251
30. Arzhan S, Roumelioti ME, Unruh ML. Itch and ache on dialysis: new approaches to manage uremic pruritus and restless legs. Blood Purif. 2020;49(1–2):222–227. doi:10.1159/000504081
31. Scherer JS, Combs SA, Brennan F. Sleep disorders, restless legs syndrome, and uremic pruritus: diagnosis and treatment of common symptoms in dialysis patients. Am J Kidney Dis. 2017;69(1):117–128. doi:10.1053/j.ajkd.2016.07.031
32. Mettang T, Kremer AE. Uremic pruritus. Kidney Int. 2015;87(4):685–691. doi:10.1038/ki.2013.454
33. Verduzco HA, Shirazian S. CKD-associated pruritus: new insights into diagnosis, pathogenesis, and management. Kidney Int Rep. 2020;5(9):1387–1402. doi:10.1016/j.ekir.2020.04.027
34. Gilchrest BA, Stern RS, Steinman TI, Brown RS, Arndt KA, Anderson WW. Clinical features of pruritus among patients undergoing maintenance hemodialysis. Arch Dermatol. 1982;118(3):154–156. doi:10.1001/archderm.1982.01650150016012
35. Aresi G, Rayner HC, Hassan L, et al. Reasons for underreporting of uremic pruritus in people with chronic kidney disease: a qualitative study. J Pain Symptom Manage. 2019;58(4):578–586.e2. doi:10.1016/j.jpainsymman.2019.06.010
36. Zucker I, Yosipovitch G, David M, Gafter U, Boner G. Prevalence and characterization of uremic pruritus in patients undergoing hemodialysis: uremic pruritus is still a major problem for patients with end-stage renal disease. J Am Acad Dermatol. 2003;49(5):842–846. doi:10.1016/s0190-9622(03)02478-2
37. Raj R, Ahuja K, Frandsen M, Murtagh FEM, Jose M. Validation of the IPOS-renal symptom survey in advanced kidney disease: a cross-sectional study. J Pain Symptom Manage. 2018;56(2):281–287. doi:10.1016/j.jpainsymman.2018.04.006
38. Palliative Care Outcome Scale. IPOS-Renal for kidney disease patients. Available from: https://pos-pal.org/maix/ipos-renal.php.
39. Kim HJ, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs. 2005;28(4):270–284. doi:10.1097/00002820-200507000-00005
40. Miaskowski C, Barsevick A, Berger A, et al. Advancing symptom science through symptom cluster research: expert panel proceedings and recommendations. J Natl Cancer Inst. 2017;109(4):djw253. doi:10.1093/jnci/djw253
41. Kim HJ, Barsevick AM, Fang CY, Miaskowski C. Common biological pathways underlying the psychoneurological symptom cluster in cancer patients. Cancer Nurs. 2012;35(6):E1–E20. doi:10.1097/NCC.0b013e318233a81
42. Lockwood MB, Lash JP, Pauls H, et al. Physical symptom cluster subgroups in chronic kidney disease. Nurs Res. 2020;69(2):100–108. doi:10.1097/NNR.0000000000000408
43. Wu HY, Huang JW, Tsai WC, et al. Prognostic importance and determinants of uremic pruritus in patients receiving peritoneal dialysis: a prospective cohort study. PLoS One. 2018;13(9):e0203474. doi:10.1371/journal.pone.0203474
44. Krajewski PK, Krajewska M, Szepietowski JC. Pruritus in renal transplant recipients: current state of knowledge. Adv Clin Exp Med. 2020;29(6):769–772. doi:10.17219/acem/122174
45. Schricker S, Weisshaar E, Kupfer J, Mettang T. Prevalence of pruritus in a single cohort of long-term kidney transplant recipients. Acta Derm Venereol. 2020;100(4):adv00066. doi:10.2340/00015555-3421
46. Hsu CW, Weng CH, Chan MJ, Lin-Tan DT, Yen TH, Huang WH. association between serum aluminum level and uremic pruritus in hemodialysis patients. Sci Rep. 2018;8(1):17251. doi:10.1038/s41598-018-35217-6
47. Hu T, Wang B, Liao X, Wang S. Clinical features and risk factors of pruritus in patients with chronic renal failure. Exp Ther Med. 2019;18:964–971. doi:10.3892/etm.2019.7588
48. O’Neill WC. The fallacy of the calcium-phosphorus product. Kidney Int. 2007;72:792–796. doi:10.1038/sj.ki.5002412
49. Momose A, Kudo S, Sato M, et al. Calcium ions are abnormally distributed in the skin of haemodialysis patients with uraemic pruritus. Nephrol Dial Transplant. 2004;19:2061–2066. doi:10.1093/ndt/gfh287
50. Hiroshige K, Kabashima N, Takasugi M, Kuroiwa A. Optimal dialysis improves uremic pruritus. Am J Kidney Dis. 1995;25(3):413–419. doi:10.1016/0272-6386(95)90102-7
51. Wu Q, Zhang H, Ding JR, et al. UPLC-QTOF MS-based serum metabolomic profiling analysis reveals the molecular perturbations underlying uremic pruritus. Biomed Res Int. 2018;2018:4351674. doi:10.1155/2018/4351674
52. Chen ZJ, Cao G, Tang WX, et al. A randomized controlled trial of high-permeability haemodialysis against conventional haemodialysis in the treatment of uraemic pruritus. Clin Exp Dermatol. 2009;34(6):679–683. doi:10.1111/j.1365-2230.2008.03075.x
53. Lim JH, Park Y, Yook JM, et al. Randomized controlled trial of medium cut-off versus high-flux dialyzers on quality of life outcomes in maintenance hemodialysis patients. Sci Rep. 2020;10(1):7780. doi:10.1038/s41598-020-64622-z
54. Oweis AO, Al-Qarqaz F, Bodoor K, et al. Elevated interleukin 31 serum levels in hemodialysis patients are associated with uremic pruritus. Cytokine. 2021;138:155369. doi:10.1016/j.cyto.2020.155369
55. Kimmel M, Alscher DM, Dunst R, et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant. 2006;21(3):749–755. doi:10.1093/ndt/gfi204
56. Leong SO, Tan CC, Lye WC, Lee EJ, Chan HL. Dermal mast cell density and pruritus in end-stage renal failure. Ann Acad Med Singap. 1994;23(3):327–329.
57. Dimković N, Djukanović L, Radmilović A, Bojić P, Juloski T. Uremic pruritus and skin mast cells. Nephron. 1992;61(1):5–9. doi:10.1159/000186826
58. Dugas-Breit S, Schöpf P, Dugas M, Schiffl H, Ruëff F, Przybilla B. Baseline serum levels of mast cell tryptase are raised in hemodialysis patients and associated with severity of pruritus. J Dtsch Dermatol Ges. 2005;3(5):343–347. doi:10.1111/j.1610-0387.2005.05706.x
59. Ashmore SD, Jones CH, Newstead CG, Daly MJ, Chrystyn H. Ondansetron therapy for uremic pruritus in hemodialysis patients. Am J Kidney Dis. 2000;35(5):827–831. doi:10.1016/s0272-6386(00)70251-4
60. Vessal G, Sagheb MM, Shilian S, Jafari P, Samani SM. Effect of oral cromolyn sodium on CKD-associated pruritus and serum tryptase level: a double-blind placebo-controlled study. Nephrol Dial Transplant. 2010;25(5):1541–1547. doi:10.1093/ndt/gfp628
61. Mahmudpour M, Roozbeh J, Raiss Jalali GA, Pakfetrat M, Ezzat Zadegan S, Sagheb MM. Therapeutic effect of montelukast for treatment of uremic pruritus in hemodialysis patients. Iran J Kidney Dis. 2017;11(1):50–55.
62. Kuypers DR, Claes K, Evenepoel P, Maes B, Vanrenterghem Y. A prospective proof of concept study of the efficacy of tacrolimus ointment on uraemic pruritus (UP) in patients on chronic dialysis therapy. Nephrol Dial Transplant. 2004;19(7):1895–1901. doi:10.1093/ndt/gfh202
63. Duque MI, Thevarajah S, Chan YH, Tuttle AB, Freedman BI, Yosipovitch G. Uremic pruritus is associated with higher kt/V and serum calcium concentration. Clin Nephrol. 2006;66(3):184–191. doi:10.5414/cnp66184
64. Reszke R, Szepietowski JC. End-stage renal disease chronic itch and its management. Dermatol Clin. 2018;36(3):277–292. doi:10.1016/j.det.2018.02.007
65. Zakrzewska-Pniewska B, Jedras M. Is pruritus in chronic uremic patients related to peripheral somatic and autonomic neuropathy? Study by R-R interval variation test (RRIV) and by sympathetic skin response (SSR). Neurophysiol Clin. 2001;31(3):181–193. doi:10.1016/s0987-7053(01)00257-x
66. Martin CE, Clotet-Freixas S, Farragher JF, Hundemer GL. Have we just scratched the surface? A narrative review of uremic pruritus in 2020. Can J Kidney Health Dis. 2020;7:2054358120954024. doi:10.1177/2054358120954024
67. Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17(20):8003–8008. doi:10.1523/JNEUROSCI.17-20-08003.1997
68. Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100(4):2062–2069. doi:10.1152/jn.90482.2008
69. Handwerker HO. Microneurography of pruritus. Neurosci Lett. 2010;470(3):193–196. doi:10.1016/j.neulet.2009.06.092
70. Fantini F, Baraldi A, Sevignani C, Spattini A, Pincelli C, Giannetti A. Cutaneous innervation in chronic renal failure patients. An immunohistochemical study. Acta Derm Venereol. 1992;72(2):102–105.
71. Johansson O, Hilliges M, Ståhle-Bäckdahl M. Intraepidermal neuron-specific enolase (NSE)-immunoreactive nerve fibres: evidence for sprouting in uremic patients on maintenance hemodialysis. Neurosci Lett. 1989;99(3):281–286. doi:10.1016/0304-3940(89)90460-6
72. Razeghi E, Eskandari D, Ganji MR, Meysamie AP, Togha M, Khashayar P. Gabapentin and uremic pruritus in hemodialysis patients. Ren Fail. 2009;31(2):85–90. doi:10.1080/08860220802595476
73. Gunal AI, Ozalp G, Yoldas TK, Gunal SY, Kirciman E, Celiker H. Gabapentin therapy for pruritus in haemodialysis patients: a randomized, placebo-controlled, double-blind trial. Nephrol Dial Transplant. 2004;19(12):3137–3139. doi:10.1093/ndt/gfh496
74. Shavit L, Grenader T, Lifschitz M, Slotki I. Use of pregabalin in the management of chronic uremic pruritus. J Pain Symptom Manage. 2013;45(4):776–781. doi:10.1016/j.jpainsymman.2012.03.001
75. Kremer AE, Feramisco J, Reeh PW, Beuers U, Oude Elferink RP. Receptors, cells and circuits involved in pruritus of systemic disorders. Biochim Biophys Acta. 2014;1842(7):869–892. doi:10.1016/j.bbadis.2014.02.007
76. Cevikbas F, Lerner EA. Physiology and pathophysiology of itch. Physiol Rev. 2020;100(3):945–982. doi:10.1152/physrev.00017.2019
77. Kumagai H, Saruta T, Matsukawa S, et al. Prospects for a novel kappa-opioid receptor agonist, TRK-820, in uremic pruritus. In: Yosipovitch G, Greaves MW, Fleischer JA, McGlone F, editors. Itch, Basic Mechanisms and Therapy. New York, NY: Dekker; 2004:279–286.
78. Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35(4):383–391. doi:10.1016/j.semnephrol.2015.06.009
79. Lugon JR. Uremic pruritus: a review. Hemodial Int. 2005;9(2):180–188. doi:10.1111/j.1492-7535.2005.01130.x
80. Patel TS, Freedman BI, Yosipovitch G. An update on pruritus associated with CKD. Am J Kidney Dis. 2007;50(1):11–20. doi:10.1053/j.ajkd.2007.03.010
81. Simonsen E, Komenda P, Lerner B, et al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis. 2017;70(5):638–655. doi:10.1053/j.ajkd.2017.05.018
82. Kozono H, Yoshitani H, Nakano R. Post-marketing surveillance study of the safety and efficacy of nalfurafine hydrochloride (Remitch® capsules 2.5 μg) in 3762 hemodialysis patients with intractable pruritus. Int J Nephrol Renovasc Dis. 2018;11:9–24. doi:10.2147/IJNRD.S145720
83. Fishbane S, Jamal A, Munera C, Wen W, Menzaghi F. KALM-1 Trial Investigators. A Phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med. 2020;382(3):222–232. doi:10.1056/NEJMoa1912770
84. Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a Phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant. 2010;25(4):1251–1257. doi:10.1093/ndt/gfp588
85. Wikström B, Gellert R, Ladefoged SD, et al. Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol. 2005;16(12):3742–3747. doi:10.1681/ASN.2005020152
86. Pereira MP, Ständer S. Assessment of severity and burden of pruritus. Allergol Int. 2017;66(1):3–7. doi:10.1016/j.alit.2016.08.009
87. Phan NQ, Blome C, Fritz F, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol. 2012;92(5):502–507. doi:10.2340/00015555-1246
88. Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3(5):329–338. doi:10.1007/BF00451725
89. Elman S, Hynan LS, Gabriel V, Mayo MJ. The 5-D itch scale: a new measure of pruritus. Br J Dermatol. 2010;162(3):587–593. doi:10.1111/j.1365-2133.2009.09586.x
90. Majeski CJ, Johnson JA, Davison SN, Lauzon CJ. Itch severity scale: a self-report instrument for the measurement of pruritus severity. Br J Dermatol. 2007;156(4):667–673. doi:10.1111/j.1365-2133.2006.07736.x
91. Masi CM, Cohen EP. Dialysis efficacy and itching in renal failure. Nephron. 1992;62:257–261. doi:10.1159/000187055
92. Carmichael AJ, Dickinson F, McHugh MI, Martin AM, Farrow M. Magnesium free dialysis for uraemic pruritus. BMJ. 1988;297(6663):1584–1585. doi:10.1136/bmj.297.6663.1584
93. Pederson JA, Matter BJ, Czerwinski AW, Llach F. Relief of idiopathic generalized pruritus in dialysis patients treated with activated oral charcoal. Ann Intern Med. 1980;93(3):446–448. doi:10.7326/0003-4819-93-3-446
94. Silverberg DS, Iaina A, Reisin E, Rotzak R, Eliahou HE. Cholestyramine in uraemic pruritus. Br Med J. 1977;1(6063):752–753. doi:10.1136/bmj.1.6063.752
95. Twycross R, Greaves MW, Handwerker H, et al. Itch: scratching more than the surface. QJM. 2003;96(1):7–26. doi:10.1093/qjmed/hcg002
96. Szepietowski JC, Szepietowski T, Reich A. Efficacy and tolerance of the cream containing structured physiological lipids with endocannabinoids in the treatment of uremic pruritus: a preliminary study. Acta Dermatovenerol Croat. 2005;13(2):97–103.
97. Balaskas E, Szepietowski JC, Bessis D, et al. Randomized, double-blind study with glycerol and paraffin in uremic xerosis. Clin J Am Soc Nephrol. 2011;6(4):748–752. doi:10.2215/CJN.05490610
98. Duque MI, Yosipovitch G, Fleischer AB, Willard J, Freedman BI. Lack of efficacy of tacrolimus ointment 0.1% for treatment of hemodialysis-related pruritus: a randomized, double-blind, vehicle-controlled study. J Am Acad Dermatol. 2005;52(3 Pt 1):519–521. doi:10.1016/j.jaad.2004.08.050
99. Weisshaar E, Dunker N, Röhl FW, Gollnick H. Antipruritic effects of two different 5-HT3 receptor antagonists and an antihistamine in haemodialysis patients. Exp Dermatol. 2004;13:298–304. doi:10.1111/j.0906-6705.2004.00184.x
100. Marquez D, Ramonda C, Lauxmann JE, et al. Uremic pruritus in hemodialysis patients: treatment with desloratidine versus gabapentin. J Bras Nefrol. 2012;34(2):148–152. doi:10.1590/s0101-28002012000200007
101. Brennan FP, Josland E, Kelly JJ. Chronic pruritus: histamine is not always the answer. J Pain Symptom Manage. 2015;50(4):566–570. doi:10.1016/j.jpainsymman.2015.04.009
102. Schwartz IF, Iaina A. Uraemic pruritus. Nephrol Dial Transplant. 1999;14:834–839. doi:10.1093/ndt/14.4.834
103. Feily A, Dormanesh B, Ghorbani AR, et al. Efficacy of topical cromolyn sodium 4% on pruritus in uremic nephrogenic patients: a randomized double-blind study in 60 patients. Int J Clin Pharmacol Ther. 2012;50(7):510–513. doi:10.5414/cp201629
104. Omidian M, Khazanee A, Yaghoobi R, et al. Therapeutic effect of oral nicotinamide on refractory uremic pruritus: a randomized, double-blind study. Saudi J Kidney Dis Transpl. 2013;24(5):995–999. doi:10.4103/1319-2442.118070
105. Gilchrest BA, Rowe JW, Brown RS, Steinman TI, Arndt KA. Relief of uremic pruritus with ultraviolet phototherapy. N Engl J Med. 1977;297(3):136–138. doi:10.1056/NEJM197707212970304
106. Nofal E, Farag F, Nofal A, Eldesouky F, Alkot R, Abdelkhalik Z. Gabapentin: A promising therapy for uremic pruritus in hemodialysis patients: a randomized-controlled trial and review of literature. J Dermatolog Treat. 2016;27(6):515–519. doi:10.3109/09546634.2016.1161161
107. Foroutan N, Etminan A, Nikvarz N, Shojai Shahrokh Abadi M. Comparison of pregabalin with doxepin in the management of uremic pruritus: a randomized single blind clinical trial. Hemodial Int. 2017;21(1):63–71. doi:10.1111/hdi.12455
108. Yue J, Jiao S, Xiao Y, Ren W, Zhao T, Meng J. Comparison of pregabalin with ondansetron in treatment of uraemic pruritus in dialysis patients: a prospective, randomized, double-blind study. Int Urol Nephrol. 2015;47(1):161–167. doi:10.1007/s11255-014-0795-x
109. Eusebio-Alpapara KMV, Castillo RL, Dofitas BL. Gabapentin for uremic pruritus: a systematic review of randomized controlled trials. Int J Dermatol. 2020;59(4):412–422. doi:10.1111/ijd.14708
110. Pauli-Magnus C, Mikus G, Alscher DM, et al. Naltrexone does not relieve uremic pruritus: results of a randomized, double-blind, placebo-controlled crossover study. J Am Soc Nephrol. 2000;11(3):514–519. doi:10.1681/ASN.V113514
111. Legroux-Crespel E, Clèdes J, Misery L. A comparative study on the effects of naltrexone and loratadine on uremic pruritus. Dermatology. 2004;208(4):326–330. doi:10.1159/000077841
112. Weiner DE, Walpen S, Schaufler T, et al. Itch reduction with difelikefalin correlates with improved sleep quality in hemodialysis patients with pruritus. Oral Present ASN Kidney Week. 2021;2021:FR–OR 26.
113. Fishbane S, Wen W, Munera C, et al. Efficacy of difelikefalin in patients with moderate-to-severe chronic kidney disease–associated pruritus: pooled subgroup analysis of KALM-1 and KALM-2. Am J Kidney Dis. 2021;77:593–594. doi:10.1053/j.ajkd.2021.02.092
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
