Back to Journals » Drug Design, Development and Therapy » Volume 18
Obacunone Alleviates Inflammatory Pain by Promoting M2 Microglial Polarization and by Activating Nrf2/HO-1 Signaling Pathway
Received 22 November 2023
Accepted for publication 11 April 2024
Published 18 April 2024 Volume 2024:18 Pages 1265—1275
DOI https://doi.org/10.2147/DDDT.S451281
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Fubei Nan, Qingxin Tian, Shuangdong Chen
Department of Anesthesiology, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China
Correspondence: Shuangdong Chen, Department of Anesthesiology, the First Affiliated Hospital of Wenzhou Medical University, Nanbaixiang Street, Ouhai District, Wenzhou, Zhejiang, 325035, People’s Republic of China, Tel +8613732058295, Email [email protected]
Background: Treating inflammatory pain (IP) continues to pose clinical challenge, because of the lack of effective pharmacological interventions. Microglial polarization serves as pivotal determinant in IP progress. Obacunone (OB), a low-molecular-weight compound with a diverse array of biological functions, having reported as an activator of nuclear factor E2-related factor 2 (Nrf2), exhibits anti-inflammatory property. However, it remains uncertain whether OB can alleviate IP by facilitating the transition of microglial polarization from the M1 to M2 state through modulating Nrf2/ heme oxygenase-1 (HO-1) pathway.
Methods: We induced an mice IP model by subcutaneously administering Complete Freund’s Adjuvant (CFA) into the hind paw. Paw withdrawal latency (PWL) in seconds (s) and paw withdrawal frequency (PWF) were employed to evaluate the establishment of the IP model, while a caliper was used to measure the maximal dorsoventral thickness of the mice paw. Nerve injury was assessed by Hematoxylin-Eosin (HE) Staining. Western blot and got conducted for detection of M1/M2 microglial polarization markers, Nrf2 and HO-1 in spinal cord tissues respectively.
Results: In comparison to the control cohort, PWF, M1 phenotype marker iNOS, CD86, paw thickness increased significantly within CFA cohort, while PWL, M2 phenotype marker Arg-1, interleukin-10 (IL-10) decreased in the CFA group. In comparison to model cohort, OB treatment decreased PWF, paw thickness, M1 phenotype marker iNOS, CD86 significantly, while PWL, M2 phenotype marker Arg-1, IL-10, Nrf2, HO-1 increased significantly. The morphological injuries of sciatic nerve in CFA mice were obviously improved by OB treatment. OB inhibited the release of M1-related IL-1β, CXCL1 but promoted M2-related TGF-β, IL-10 in serum in CFA mice. The intervention of the Nrf2 inhibitor ML385 mitigated analgesic effect of OB.
Conclusion: We demonstrate that OB is able to attenuate inflammatory pain via promoting microglia polarization from M1 to M2 and enhancing Nrf2/HO-1 signal. OB treatment may be a potential alternative agent in the treatment of IP.
Keywords: inflammatory pain, obacunone, Nrf2, HO-1
Introduction
Pain serves as a sentinel, alerting us to potential or actual tissue damage and triggering an adaptive protective response.1 When peripheral tissues are subjected to noxious stimuli, it leads to pain hypersensitivity through primary sensory neurons peripheral sensitization.2,3 CFA activates cellular immune responses and boosts the generation of immunoglobulins, thereby acting as an immune enhancer.4,5 CFA-induced responses above result in inflammation in tissue within injected area, as well as cytokines release.6
Microglia are macrophage-like cells in central nervous system (CNS), holding homeostasis within brain, as well as spinal cord. A growing body of evidence from previous research indicates that, microglia serve as pivotal determinant within pain pathogenesis.7–9 Evidence supported, microglia/macrophages exhibit high plasticity and adapt their phenotypes in response to diverse microenvironmental cues, including proinflammatory and anti-inflammatory signals.10 The pro-inflammatory M1-like phenotype, typified by specific biomarker expression, inclusive of nitric oxide synthase (iNOS) and CD86, tends to release harmful mediators. In contrast, the anti-inflammatory M2-like phenotype, defined by molecular signatures like CD206 and interleukin-10 (IL-10), produces beneficial mediators.11 Multiple studies have demonstrated that encouraging a shift in microglia from the M1 to the M2 state could hold promise as a therapy for inflammatory pain (IP) treatment.12–14
Nuclear factor E2-related factor 2 (Nrf2) has been commonly recognized as the key transcription factor that regulates inflammation and oxidative stress in pain.15 Nrf2 resides in the cytoplasm controlled within normal conditions, where it is retained by associating with Kelch-like ECH-associated protein 1 (Keap-1). However, in response to harmful insults, it dissociates from Keap-1, subsequently translocating to the nucleus, prompting the transcription of enzymes being antioxidant and anti-inflammatory, including heme oxygenase-1 (HO-1), thus modulating the inflammatory process.16 Previous studies have affirmed anti-inflammatory role of Nrf2/HO-1 pathway in pathological conditions, such as myocardial ischemia-reperfusion injury, diabetic nephropath.17,18 Similarly, previous studies have corroborated that compounds like sulforaphane and sinomenine promote microglial polarization from the M1 to the M2 phenotype by activating Nrf2/HO-1 pathway, ultimately yielding neuroprotective effects.19,20
When it comes to treating inflammatory pain, common practices include the use of NSAIDs and opioid medications. However, these methods come with notable drawbacks, including variable effectiveness, the potential for developing drug tolerance, unwanted adverse effects, and significant costs. Therefore, it’s worth considering the use of natural compounds derived from plants for pain management.21 Obacunone (OB) is a natural limonoid compound extracted from plants like phellodendri and dictamnus-dasycarpus, possessing diverse biological properties, including anti-inflammatory, antioxidative, and anti-tumor activities.22,23 It has been established that OB can enhance Nrf2 activity and protect against oxidative stress-related lung fibrosis.23 However, whether OB pretreatment can foster M2 microglial polarization and exert analgesic effects through the Nrf2/HO-1 pathway remains enigmatic. Aim of the study is to explore the pain-relieving capabilities of OB in the context of inflammatory pain and to clarify the molecular processes.
Materials and Methods
Animals
Male C57BL/6 weighing 18–22 g mice got procured from Zhejiang Vital River Experimental Animal Technology Co., Ltd for this study. Mice got housed in a controlled environment within 21–23°C. Food and water was accessible anytime during the whole study. All animal experiments were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Laboratory Animal Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University (ID: WYYY-IACUC-AEC-2024-028).
Induction of IP with CFA and Experimental Design
The IP model was induced following the administration of CFA, as described in previous studies.6 Briefly, a subcutaneous injection of CFA 25 µL got performed in the left hind paw, establishing the IP model. To study the effect of OB on inflammatory pain, mice were distributed into three distinct groups at random: a CFA + OB (10 mg/kg) group, a CFA group, a control group. To study the crucial involvement of Nrf2 in the analgesic effects of OB in CFA mice, mice were separated at random into four groups: a CFA + OB (10 mg/kg) group, a CFA group, a control group, and CFA + OB (10 mg/kg) + ML385(30 mg/kg) group, following the establishment of the IP model, OB (10 mg/kg) was administered via intraperitoneal injections to the mice once a day for three consecutive days, starting the treatment at 1 day after CFA injection. Mice in the CFA + OB + ML385 group, ML385(30 mg/kg) was intraperitoneal given by injection 2 hour before OB administration. Behavioral tests were conducted prior to CFA injection (naive baseline) and on the 1st, 3rd, and 5th days following the CFA administration. Then, all animal were scarified for further experiments after behaviors tests. CFA was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). OB was obtained from YUANYE Bio-Technology Co., Ltd. (Shanghai, China), and ML385 was obtained from MedChem Express (New Jersey, USA). The doses of two drugs were based on prior studies.24
Behavioral Testing
Behavioral testing got completed according to established procedures.6 We measured the animals’ paw withdrawal reactions to both mechanical and thermal provocations. This was done utilizing a von Frey monofilament with a force of 0.16 g from North Coast Medical Inc, CA, USA, for mechanical sensitivity and a Model 336 analgesia meter from IITC Inc, Life Science Instruments, CA, USA, for temperature sensitivity. To evaluate pain severity, we recorded paw withdrawal frequency (PWF) (calculated as the percentage of withdrawals during 10 trials), and paw withdrawal latency (PWL) in seconds.
Paw Thickness Measurement
Here, mice were gently placed in a piece of cloth and, after settling, a caliper was used to measure the maximal dorsoventral thickness of the paw.3 Each paw was measured three times and an average was calculated.
Hematoxylin-Eosin (HE) Staining
For histological analysis, we excised the sciatic nerve from the mice, dehydrated it, and then embedded it in paraffin. The nerve tissue was then sectioned into thin slices using a rotary microtome and stained with hematoxylin and eosin dyes. These tissue sections were examined under a microscope to observe nerve growth in the various groups of mice.
ELISA
Adhering to the guidelines provided by the manufacturers, we quantified the serum concentrations of IL-1β, CXCL1, TGF-β, and IL-10 using ELISA kits.
Western Blot
Following the experimental procedures, mice were deeply anesthetized and sacrificed. Spinal dorsal horn (L3-L5) tissues were promptly dissected, and RIPA lysis buffer (Beyotime, China) along with protease inhibitors got supplemented. Total protein was extracted using the lysis buffer. After centrifugation at 12,000 g and 4°C for 30 min, subsequently harvesting supernatant. We detected protein concentration utilizing an enhanced BCA protein assay kit (Proteintech, Wuhan, China). Equivalent amounts of protein got loaded onto 10‑15% percent SDS-PAGE gel for electrophoresis gel for different protein detections, subsequently transferred onto PVDF membranes (Millipore, MA). Submerging PVDF membranes with 5% milk for 2h at room temperature, they subsequently got exposed to primary antibodies at 4°C for a whole night. After having the membranes washed, they were sent for incubation with appropriate secondary antibodies, and subjected to detection by ECL Western blotting detection system. The following primary antibodies were used: anti-Nrf2 (1:2000, Proteintech), anti-HO-1 (1:2000, Hua-bio), anti-iNOS (1:1000, Affinity), anti-CD86 (1:1000, Affinity), anti-IL-10 (1: 2000, ABclonal), anti-Arg-1 (1:1000, Affinity) and anti-β-actin (1:2000, Proteintech).
Statistical Analysis
The results are expressed as the mean ± standard deviation (SD). Statistical assessments encompassed one-way analysis of variance for parameters such as paw thickness, Western blot, ELISA data results. For the data derived from behavioral tests, a two-way repeated-measures analysis of variance was employed. All statistical analyses were carried out using Prism 5 software, with statistical significance established at a threshold of P < 0.05.
Results
OB Influence on CFA-Induced Mechanical Allodynia
We first investigated OB influence on CFA-Induced mechanical allodynia. See in Figure 1, PWF was elevated in CFA cohort in comparison to the control group, indicated mechanical allodynia occurs, the administration of OB caused a significant inhibition of PWF as compare to CFA cohort. Subsequently, the administration of OB relieved the mechanical pain sensitivity on the ipsilateral hindlimb within CFA mice.
OB Influence on CFA-Induced Thermal Hyperalgesia
We also explored OB influence on thermal hyperalgesia caused by CFA. See in Figure 2, PWL was decreased in CFA cohort in comparison to the control group, indicated thermal hyperalgesia occurs, the administration of OB caused a significant elevation of PWL as compare to CFA cohort. Subsequently, the administration of OB relieved the thermal hyperalgesia on the ipsilateral hindlimb within CFA mice.
Effect of OB on Paw Thickness
To elucidate the relationship between OB’s anti-inflammatory properties and the inflammatory response, we measured paw thickness. As displayed by Figure 3, the CFA cohort exhibited increased paw thickness in comparison to the control, administration of OB after CFA injection significantly reduced paw thickness at day 5 as compare to CFA cohort (P < 0.05).
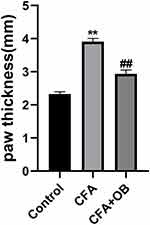 |
Figure 3 Effects of OB on paw thickness. The data obtained are represented as the means ± SD. **P < 0.01 when compared to the control group. ##P < 0.01 when compared to CFA group, n = 6 per group. |
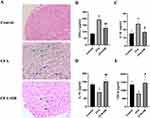
Effect of OB on Histopathological Damages and Inflammatory Indicators in CFA Mice
To assess OB’s capability to safeguard against inflammatory pain, an examination of the sciatic nerve’s tissue structure was conducted using light microscopy. Figure 4 illustrates that in the HE-stained cross-sections, the control group showcased densely packed myelinated nerve fibers with a uniform structure. In contrast, the CFA model group displayed disrupted nerve fibers with varying diameters, and the fibers showed clear signs of myelin loss and axonal contraction. However, the structural damage to the sciatic nerve caused by CFA was markedly mitigated following treatment with OB. The effects exerted by OB on the expression of various inflammatory indicators in serum, such as M1-related IL-1β, CXCL1 and M2-related TGF-β, IL-10, were studied. Results revealed that OB inhibited the release of M1-related IL-1β, CXCL1 but promoted M2-related TGF-β, IL-10 in serum in CFA mice.
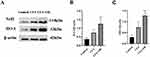
Effect of OB on Microglial Polarization
To investigate if preemptive administration of OB mediates its pain-relieving effects by fostering a shift in microglial cells towards the M2 phenotype, Western blot analysis was employed to measure the expression levels of markers indicative of M1 and M2 microglia within the spinal cord. As depicted in Figure 5, markers characteristic of M1 microglia (CD86, iNOS) showed a marked elevation post-CFA injection. Conversely, indicators for M2 microglia (IL-10, Arg-1) were notably reduced in the CFA group when juxtaposed with the control group. Nonetheless, relative to the CFA-treated animals, administration of OB appreciably reduced the levels of iNOS and CD86, while it increased the levels of IL-10 and Arg-1. Therefore, the above-mentioned results suggest that OB may alleviate pain by steering microglial polarization towards the phenotype of M2.
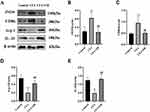
Effect of OB on Nrf-2, and HO-1 Expression in Spinal Cord Tissues
Nrf2/HO-1 pathway serves as pivotal determinant involved in microglia polarization modulation, as well as subsequent mitigation of the inflammatory response.25 Preceding outcomes undeniably underscored the therapeutic potential of OB pretreatment against CFA-induced inflammation pain. However, it was imperative to ascertain its connection with Nrf2/HO-1 pathway. Outcomes of Western Blot, as depicted in Figure 6, illuminated a significant increase in Nrf2 and HO-1 levels within spinal cord tissues of the CFA cohort in comparison to the control cohort. Notably, OB treatment further augmented Nrf2 and HO-1 expression when compared to the CFA cohort.
Crucial Involvement of Nrf2 in the Analgesic Effects of OB in CFA Mice
To delve deeper into the mechanistic insights regarding the protective influences of OB against IP mediated by Nrf2 activation, mice got pretreated by Nrf2 inhibitor (ML385, 30 mg/kg) for a duration of 2 hours, in accordance with previous research standards,24,26 subsequently, the mice were administered OB to assess alterations in pain behavior. As demonstrated in Figure 7, it became apparent that ML385 markedly suppressed analgesic impact by OB on the ipsilateral hindlimb within CFA mice.
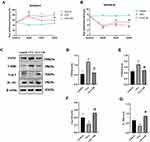
OB Influence on CFA-Induced Chronic Pain
As suggested by Figure 8, CFA results in nociceptive hyperalgesia in mice, which indicated that the mouse model of chronic pain had been constructed effectively. The values of mechanical threshold in CFA + OB mice were higher after OB treatment on days 26–28. The OB treatment also increased the latency to fall in CFA-induced mice on days 26 to 28 respectively in Figure 8. As a result, OB alleviated pain behavior in the CFA mice.
As revealed by Western blotting, the CFA group had higher spinal expression levels of CD86 and iNOS than the control, OB treatment reduced the CD86 and iNOS levels. CFA group had lower spinal expression levels of IL-10 and Arg-1 than control group, the levels of IL-10, Arg-1 were increased respectively by OB administration as depicted in Figure 8.
Discussion
Previous investigation got meticulously designed for the exploration of potential analgesic properties of OB in a CFA-induced IP model, along with the underlying mechanistic pathways. Our findings suggest that OB treatment facilitates a shift in microglia polarization from M1 to M2 phenotype and upregulating the Nrf2/HO-1 pathway. These outcomes collectively offer a promising therapeutic approach for alleviating IP.
OB, a naturally occurring bioactive compound predominantly found in citrus fruits, has been recognized for its robust anti-inflammatory and anti-tumor attribute.22 However, its applicability in IP management has hitherto remained uncharted. In the scope of this study, our results indicate that OB effectively mitigates mechanical allodynia and thermal hyperalgesia in comparison to the CFA-induced cohort. Furthermore, OB administration notably hinders the development of CFA-induced paw thickness relative to the CFA group. In our study, OB was linked to a decrease in M1-associated 1L-1β, CXCL1 that could aggravate IP by increasing inflammation, while increased the levels of M2 markers like TGF-β and IL-10 that could relieve IP. HE results showed that myelinated nerve fibers were irregularly spaced within the CFA-treated group, with myelin sheaths of inconsistent thickness and evident vacuolation in the nerve fibers, indicating reduced myelination. However, these degenerative changes appeared to be lessened in groups that received OB treatment. Collectively, these results underscore the ability of OB to inhibit CFA-induced inflammatory pain.
It is well established that IP is a pathological condition closely linked to inflammation, characterized by an imbalance between pro-inflammatory and anti-inflammatory status. Microglia, serving as CNS innate immune cells, serve as pivotal determinant within regulating inflammatory responses, owing to their ability to adopt either the M1 or M2 phenotype.11,12 Achieving a harmonious equilibrium in microglia polarization between M1 and M2 states is of paramount importance in averting uncontrolled and prolonged neuroinflammation, particularly in the context of treating neurodegenerative disorders.27 Consequently, the quest for effective strategies capable of driving microglia toward the M2 phenotype holds great potential in mitigating neuroinflammation. Several approaches have been identified that exert analgesic effects by modulating microglial polarization, including interventions such as dexmedetomidine, electroacupuncture, sinomenine, and others.20,28,29 In this study, M1 markers levels, iNOS, and CD86, were analyzed within the lumbar spinal dorsal horn. Their expression was significantly elevated in the CFA mice group, but notably decreased following OB treatment. In contrast, the M2 markers, Arg-1 and IL-10, exhibited reduced expression within the lumbar spinal dorsal horn of CFA mice but were augmented after OB treatment. We also continue the evaluation for four weeks after CFA injection and measure the polarity of macrophages. As revealed by Western blotting, the data showed that treatment with OB significantly counteracted the influence CFA had on microglial M1 polarization, as demonstrated by a decrease in the expression of M1 markers (iNOS, and CD86), and an increase in the expression of M2 markers (Arg-1 and IL-10). Therefore, it is evident that OB pretreatment ameliorates IP not only by suppressing M1 microglia polarization but also by promoting M2 polarization.
Further exploration into the molecular mechanisms underlying the conversion of microglia polarization to the M2 phenotype by OB treatment unveiled the pivotal role of Nrf2. Nrf2, a central transcription factor, possesses the capacity to regulate downstream target genes, exerting dual effects in terms of anti-inflammation and antioxidant functions.15 Among its target genes, HO-1 has drawn particular interest from researchers due to its cytoprotective capabilities.16 Previous studies have demonstrated that Nrf2/HO-1 pathway activation yields favorable outcomes, significantly reducing inflammatory responses in various pathological processes, including cardiovascular diseases, renal ischemia-reperfusion injury, and cancer.17,30,31 Moreover, other studies have confirmed that substances like dexmedetomidine and camptothecin promote microglial polarization, thereby inhibiting neuroinflammation, through Nrf2/HO-1 pathway activation.32,33 In summary, the Nrf2/HO-1 pathway plays an indispensable role within regulating microglia polarization. To ascertain whether OB’s ability to relieve IP is linked to the Nrf2/HO-1 pathway, we analyzed Nrf2 and HO-1 protein levels following OB treatment. Spinal Nrf2 and HO-1 levels were substantially elevated following OB treatment in CFA mice. The data indicated Nrf2/HO-1 pathway activation after OB treatment, aligning with prior research.24 To validate if OB’s protective influences on IP are reliant on Nrf2 activation, the modelled mice got processed with an Nrf2 inhibitor (ML385), and subsequently treated with OB. The analgesic effect of OB against CFA was notably reversed in the presence of the Nrf2 inhibitor ML385. These results robustly corroborate the idea that spinal Nrf2/HO-1 axis activation is integral to the analgesic effects of OB in CFA-induced inflammatory pain.
Conclusion
OB treatment notably attenuated mechanical allodynia and thermal hyperalgesia severity in the context of CFA-induced mice. These ameliorative effects of OB can be attributed to its facilitation of microglial polarization, transitioning from the M1 to the M2 phenotype, and activating Nrf2/HO-1 pathway. These empirical observations lend considerable support to the feasibility of OB as a prospective therapeutic agent in the realm of IP management, showcasing promise for future clinical applications.
Funding
This work was supported by a grant from the Natural Science Foundation of Zhejiang Province (LQ21H290006).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kidd BL, Urban LA. Mechanisms of inflammatory pain. Br J Anaesth. 2001;87(1):3–11. doi:10.1093/bja/87.1.3
2. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi:10.1016/j.cell.2009.09.028
3. Boettger MK, Uceyler N, Zelenka M, et al. Differences in inflammatory pain in nNOS-, iNOS- and eNOS-deficient mice. Eur J Pain. 2007;11(7):810–818. doi:10.1016/j.ejpain.2006.12.008
4. Geboes L, De Klerck B, Van Balen M, et al. Freund’s complete adjuvant induces arthritis in mice lacking a functional interferon-gamma receptor by triggering tumor necrosis factor alpha-driven osteoclastogenesis. Arthritis Rheum. 2007;56(8):2595–2607. doi:10.1002/art.22791
5. Ahmed O, Fahim H, Mahmoud A, Ahmed E. Bee venom and hesperidin effectively mitigate complete Freund’s adjuvant-induced arthritis via immunomodulation and enhancement of antioxidant defense system. Arch Rheumatol. 2018;33(2):198–212. doi:10.5606/ArchRheumatol.2018.6519
6. Han K, Zhang A, Mo Y, et al. Islet-cell autoantigen 69 mediates the antihyperalgesic effects of electroacupuncture on inflammatory pain by regulating spinal glutamate receptor subunit 2 phosphorylation through protein interacting with C-kinase 1 in mice. Pain. 2019;160(3):712–723. doi:10.1097/j.pain.0000000000001450
7. Chen O, Luo X, Ji RR . Macrophages and microglia in inflammation and neuroinflammation underlying different pain states. Med Rev (2021). 2023;3(5):381 407. doi:10.1515/mr-2023-0034
8. Calvo M, Bennett DL. The mechanisms of microgliosis and pain following peripheral nerve injury. Exp Neurol. 2012;234(2):271–282. doi:10.1016/j.expneurol.2011.08.018
9. Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron. 2018;100(6):1292–1311. doi:10.1016/j.neuron.2018.11.009
10. Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron. 2017;95(6):1246–1265. doi:10.1016/j.neuron.2017.07.010
11. Pisanu A, Lecca D, Mulas G, et al. Dynamic changes in pro- and anti-inflammatory cytokines in microglia after PPAR-γ agonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson’s disease. Neurobiol Dis. 2014;71:280–291. doi:10.1016/j.nbd.2014.08.011
12. Piotrowska A, Kwiatkowski K, Rojewska E, Makuch W, Mika J. Maraviroc reduces neuropathic pain through polarization of microglia and astroglia — evidence from in vivo and in vitro studies. Neuropharmacology. 2016;108:207–219. doi:10.1016/j.neuropharm.2016.04.024
13. Gui X, Wang H, Wu L, et al. Botulinum toxin type A promotes microglial M2 polarization and suppresses chronic constriction injury-induced neuropathic pain through the P2X7 receptor. Cell Biosci. 2020;10(1):45. doi:10.1186/s13578-020-00405-3
14. Jiang Y, Wang J, Li H, Xia L. IL-35 promotes microglial M2 polarization in a rat model of diabetic neuropathic pain. Arch Biochem Biophys. 2020;685:108330. doi:10.1016/j.abb.2020.108330
15. Vasavda C, Xu R, Liew J, et al. Identification of the NRF2 transcriptional network as a therapeutic target for trigeminal neuropathic pain. Sci Adv. 2022;8(31):eabo5633. doi:10.1126/sciadv.abo5633
16. Farina M, Vieira LE, Buttari B, Profumo E, Saso L. The Nrf2 pathway in ischemic stroke: a review. Molecules. 2021;26(16):5001. doi:10.3390/molecules26165001
17. Shen Y, Liu X, Shi J, Wu X. Involvement of Nrf2 in myocardial ischemia and reperfusion injury. Int J Biol Macromol. 2019;125:496–502.
18. Landis RC, Quimby KR, Greenidge AR. M1/M2 macrophages in diabetic nephropathy: nrf2/HO-1 as therapeutic targets. Curr Pharm Des. 2018;24(20):2241–2249. doi:10.2174/1381612824666180716163845
19. Subedi L, Lee JH, Yumnam S, Ji E, Kim SY. Anti-Inflammatory effect of sulforaphane on LPS-activated microglia potentially through JNK/AP-1/NF-κB Inhibition and Nrf2/HO-1 activation. Cells. 2019;8(2):194. doi:10.3390/cells8020194
20. Bi F, Zhang Y, Liu W, Xie K. Sinomenine activation of Nrf2 signaling prevents inflammation and cerebral injury in a mouse model of ischemic stroke. Exp Ther Med. 2021;21(6):647. doi:10.3892/etm.2021.10079
21. Mobasheri A, Spring-Charles A, Gamaleri FC, McSwan J, Garg M, Sethi VS. Evidence-Based Opinions from Multidisciplinary Experts on Use of Naturopathic Herbal Remedies in Pain Management. J Pain Res. 2022;17:599–608. doi:10.2147/JPR.S432090
22. Gao Y, Hou R, Liu F, et al. Obacunone causes sustained expression of MKP-1 thus inactivating p38 MAPK to suppress pro-inflammatory mediators through intracellular MIF. J Cell Biochem. 2018;119(1):837–849. doi:10.1002/jcb.26248
23. Xu S, Chen W, Xie Q, Xu Y. Obacunone activates the Nrf2-dependent antioxidant responses. Protein Cell. 2016;7(9):684–688. doi:10.1007/s13238-016-0297-y
24. Li J, Deng SH, Li J, et al. Obacunone alleviates ferroptosis during lipopolysaccharide-induced acute lung injury by upregulating Nrf2-dependent antioxidant responses. Cell Mol Biol Lett. 2022;27(1):29. doi:10.1186/s11658-022-00318-8
25. Tao W, Hu Y, Chen Z, Dai Y, Hu Y, Qi M. Magnolol attenuates depressive-like behaviors by polarizing microglia towards the M2 phenotype through the regulation of Nrf2/HO-1/NLRP3 signaling pathway. Phytomedicine. 2021;91:153692. doi:10.1016/j.phymed.2021.153692
26. Singh A, Venkannagari S, Oh KH, et al. Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem Biol. 2016;11(11):3214–3225. doi:10.1021/acschembio.6b00651
27. Guo S, Wang H, Yin Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front Aging Neurosci. 2022;14:815347. doi:10.3389/fnagi.2022.815347
28. Wu Q, Zheng Y, Yu J, et al. Electroacupuncture alleviates neuropathic pain caused by SNL by promoting M2 microglia polarization through PD-L1. Int Immunopharmacol. 2023;123:110764. doi:10.1016/j.intimp.2023.110764
29. Qiu Z, Lu P, Wang K, et al. Dexmedetomidine Inhibits Neuroinflammation by Altering Microglial M1/M2 Polarization Through MAPK/ERK Pathway. Neurochem Res. 2020;45(2):345–353. doi:10.1007/s11064-019-02922-1
30. Zhang Y, Liu M, Zhang Y, et al. Urolithin A alleviates acute kidney injury induced by renal ischemia reperfusion through the p62-Keap1-Nrf2 signaling pathway. Phytother Res. 2022;36(2):984–995. doi:10.1002/ptr.7370
31. Rojo de la Vega M, Chapman E, Zhang DD. NRF2 and the Hallmarks of Cancer. Cancer Cell. 2018;34(1):21–43. doi:10.1016/j.ccell.2018.03.022
32. Wang N, Nie H, Zhang Y, et al. Dexmedetomidine exerts cerebral protective effects against cerebral ischemic injury by promoting the polarization of M2 microglia via the Nrf2/HO-1/NLRP3 pathway. Inflamm Res. 2022;71(1):93–106. doi:10.1007/s00011-021-01515-5
33. He D, Fu S, Zhou A, et al. Camptothecin Regulates Microglia Polarization and Exerts Neuroprotective Effects via Activating AKT/Nrf2/HO-1 and Inhibiting NF-κB Pathways In Vivo and In Vitro. Front Immunol. 2021;12:619761. doi:10.3389/fimmu.2021.619761
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.