Back to Journals » Drug Design, Development and Therapy » Volume 17
Network Pharmacology Study of Bioactive Components and Molecular Mechanisms of the Glycoside Fraction from Picrorhiza scrophulariiflora Against Experimental Colitis
Authors Wu P, Chang C, Zhu G, Zhai L, Zhang X, Huan Q, Gao Z, Deng H, Liang Y, Xiao H
Received 17 February 2023
Accepted for publication 29 April 2023
Published 23 May 2023 Volume 2023:17 Pages 1531—1546
DOI https://doi.org/10.2147/DDDT.S407339
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Peigen Wu,1– 3 Churui Chang,3 Guanglin Zhu,4 Lixiang Zhai,5 Xu Zhang,3 Qiuchan Huan,1,3 Zhengxian Gao,1,3 Huan Deng,2 Yue Liang,1 Haitao Xiao2,3
1Department of Pharmacy, Peking University Shenzhen Hospital, Shenzhen, People’s Republic of China; 2School of Pharmaceutical Sciences, Health Science Center, Shenzhen University, Shenzhen, People’s Republic of China; 3School of Pharmaceutical Sciences, Guizhou Medical University, University Town, Guizhou, People’s Republic of China; 4Traditional Chinese Medicine Hospital of Qijiang, Chongqing, People’s Republic of China; 5School of Chinese Medicine, Hong Kong Baptist University, Kowloon, Hong Kong Special Administrative Region, People’s Republic of China
Correspondence: Haitao Xiao, School of Pharmaceutical Sciences, Health Science Center, Shenzhen University, Shenzhen, 518060, People’s Republic of China, Email [email protected] Yue Liang, Department of Pharmacy, Peking University Shenzhen Hospital, Shenzhen, People’s Republic of China, Email [email protected]
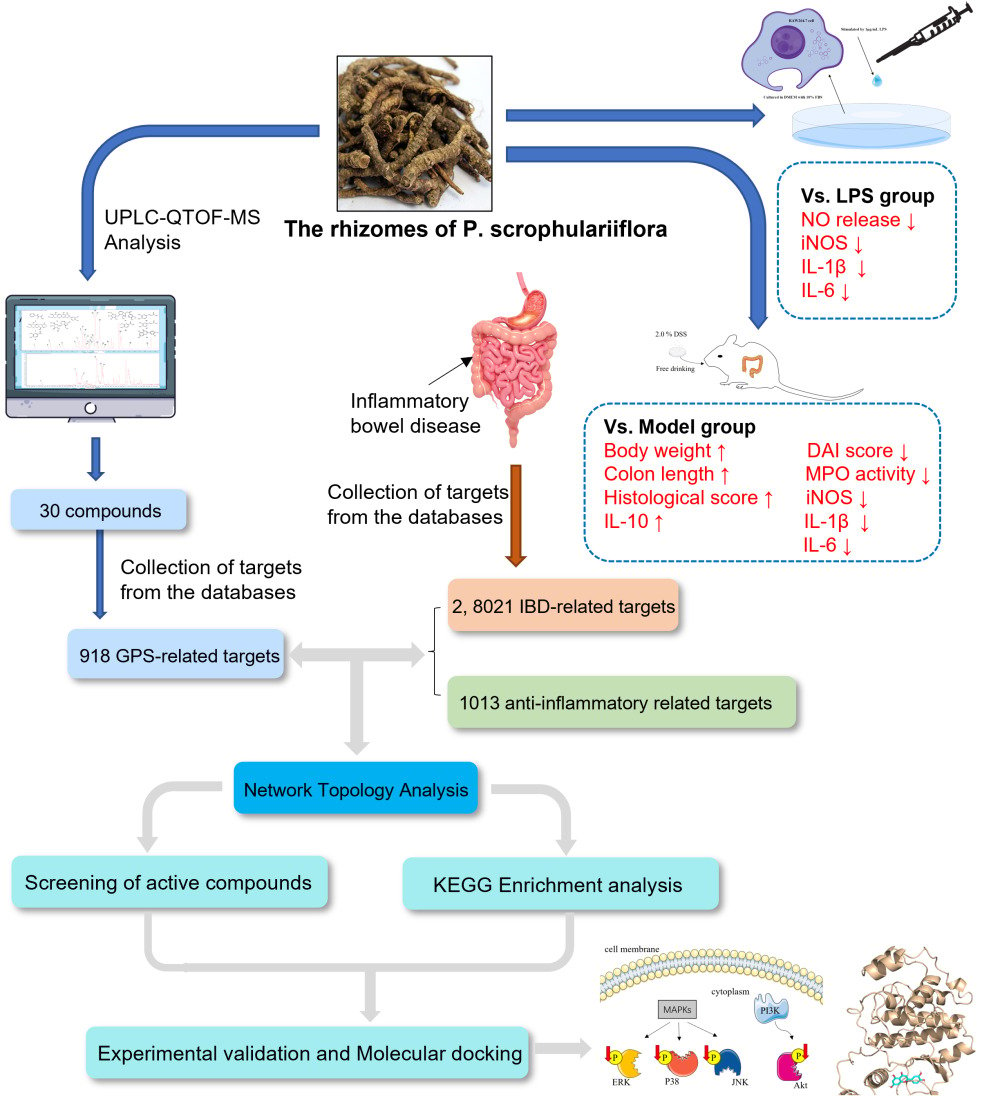
Purpose: To explore the potential mechanism of glycosidic fraction of Picrorhiza scrophulariiflora Pennell (GPS) extract for the treatment of colitis using UPLC-QTOF-MS analysis, network pharmacology and experimental research.
Methods: The active components of GPS extract were identified by UPLC-QTOF-MS analysis and extracted their targets from the databases, which was used for network pharmacology analysis. Kyoto Encyclopedia of genes and genomes (KEGG) pathway analysis was performed to discover potential therapeutic mechanisms, and the network pharmacology results were then validated by in vivo and in vitro experiments.
Results: The results showed that GPS extract significantly alleviated the clinical signs of colitis, including body weight, disease activity index, colon shortening, and colon tissue damage, and inhibited the transcription and production of colonic IL-1β and IL-6 in DSS-induced colitis mice. In vitro, GPS extract also significantly suppressed nitric oxide (NO) production, iNOS expression, IL-1β and IL-6 transcription of LPS-activated RAW 264.7 cells. Network pharmacology integrated with experimental validation identified that GPS extract significantly suppressed Akt, p38, ERK, and JNK phosphorylation in vivo and in vitro, and luteolin, apocynin, caffeic acid, caffeic acid methyl ester, luteoloside, picroside II, aucubin, cinnamic acid, vanillic acid, and sweroside were the main components responsible for the anti-inflammatory effect of GPS. These findings demonstrate that the potential anti-inflammatory effect of GPS extract against colitis is achieved through suppressing PI3K/Akt and MAPK pathways, and that the abovementioned active components mainly exerted its anti-inflammatory effect.
Conclusion: The therapeutic effect of GPS extract on colitis is related to PI3K/Akt and MAPK pathways, which is a promising remedy for colitis therapy.
Keywords: Picrorhiza scrophulariiflora, colitis, bioactive compounds, molecular mechanism, network pharmacology
Graphical Abstract:
Introduction
As one of the modern refractory diseases listed by the World Health Organization, the occurrence of inflammatory bowel disease (IBD) seriously affects the quality of life of patients.1,2 To date, IBD is incurable, but its remission can be achieved and maintained with aminosalicylate, corticosteroids, immunosuppressants, and biologics.3,4 However, the abovementioned IBD treatments have obvious defects, such as high side effects, high cost, repeated recurrence, and even aggravation.5,6 Developing novel therapeutic strategies aimed at achieving durable clinical remission in the absence of serious adverse events has been a major topic in IBD research. Recently, many case studies have shown that traditional herbal medicine (THM) has great potential to induce IBD remission with low toxicity, suggesting that the development of new drugs from THM is a promising strategy for the treatment of IBD.7–9
Picrorhiza scrophulariiflora Pennell is a perennial herb of genus Picrorhiza (Scrophulariaceae), mainly distributed in southeastern Tibet and northwestern Yunnan province in China.10 The rhizome of P. scrophulariiflora is a well-known bitter herbal medicine in traditional Chinese medicine (TCM) to treat infantile malnutrition, dysentery, jaundice, and hemorrhoids, which has been officially listed in Chinese Pharmacopoeia since 1977.11 P. scrophulariiflora and its contained formulas are clinically used to treat colitis symptoms in China, and their clinical application has been documented in many ancient Chinese medical classics.12 In addition, Picrorhiza kurroa Royle ex Benth, the sister plant of P. scrophulariiflora, is a famous traditional medicine in Ayurvedic medicine, which is extensively used to treat various immunity-related diseases, such as asthma and arthritis, in India and Nepal.13 Previous studies have revealed that P. scrophulariiflora mainly contains flavonoids, triterpenoids, iridoid glycosides, phenylethanol glycosides, and phenolic glycosides, which are highly similar to the chemical compositions of P. kurroa.14 Pharmacological studies have revealed that P. scrophulariiflora has multiple biological properties, including hepatoprotection, neuroprotection, cardiovascular protection, immunomodulation, antiasthma, and oxidation,15,16 and that glycosides (glycosidic fraction) are the key components responsible for its efficacy.17 Based on all of above information, P. scrophulariiflora is a potentially effective regimen for the treatment of colitis. However, to date, no evidence-based study has been conducted to evaluate the efficacy of P. scrophulariiflora in colitis.
Therefore, we evaluated the protective effect of GPS, an effective fraction, from P. scrophulariiflora on dextran sulfate sodium (DSS)-induced colitis in C57BL/6 mice and LPS-stimulated RAW264.7 macrophages, and we investigated its anti-inflammatory bioactive components and mechanisms using network pharmacology.
Materials and Methods
Regents
DSS salt (colitis grade, molecular weight: 36,000–50,000 Daltons) was purchased from MP Biomedicals (Ohio, USA). The hematoxylin-eosin (H&E) staining solution was purchased from Sigma (St. Louis, MO, USA). Trizol reagent was obtained from Invitrogen (Carlsbad, USA), and Primescript™ RT reagent kits and SYBR® Premix Ex Taq™ II kits were acquired from Takara Bio Inc. (Kusatsu, Japan). Antibodies including p44/42 MAPK (Erk1/2), phospho-p44/42 MAPK (Thr202/Tyr204), p38 MAPK, phospho-p38 MAPK (Thr180/Tyr182), SAPK/JNK, phospho-SAPK/JNK (Thr183/Tyr185), Akt, phospho-Akt (Thr450), and beta-Actin were purchased from Cell Signaling Technology (MA, USA).
Preparation of Different Fractions of P. scrophulariiflora
The rhizomes of P. scrophulariiflora were collected from the Yunnan Province of China in August 2021. The samples were authenticated by Prof. Yongxian Cheng (Health Science Center, Shenzhen University), and voucher specimens (No. Ps20210901) were stored at the Institute for Inheritance-Based Innovation of Chinese Medicine, Shenzhen University. The air-dried and powdered P. scrophulariiflora rhizomes were extracted twice with 8-fold 70% ethanol under reflux for 2h each time. The extracting solutions were combined and concentrated under reduced pressure to obtain an ethanol extract of P. scrophulariiflora (EPS). Previous studies have reported that glycosides are the key components responsible for their biological properties, such as immunomodulation, hepatoprotection, and cardiovascular protection.11,13,18–21 Subsequently, glycoside and non-glycoside fractions of P. scrophulariiflora were prepared using a macroporous adsorption resin D-101 column (Solarbio, Cat#M0041) and eluted successively with water and ethanol (water, 40%, and 100% ethanol), as previously reported.22 The eluate of water, 40% ethanol, and 100% ethanol were concentrated and freeze-dried to yield the water fraction (WPS), 40% ethanol (glycoside fraction, GPS), and 100% ethanol fraction (non-glycoside fraction, NGPS) of P. scrophulariiflora, respectively.
UPLC-QTOF-MS Analysis of the GPS Extract
We identified the components in the GPS extract using the UPLC-QTOF-MS method, as previously reported, but with minor changes.1 The mobile phase was (A) 0.1% formic acid in water and (B) 0.1% formic acid acetonitrile. The optimized linear gradient was as follows: flow rate 0.40 mL/min, 0–6 min, 5–75% B; 6–9 min, 75–100% B; 9–12 min, 100% B; 12–12.10 min, 100–5% B; and 12.10–15 min, 5%B.
Cell Culture and Viability Assay
RAW 264.7 cells were obtained from ATCC (Manassas, VA, United States) and cultured routinely, as in our previous studies.23,24 Cell viability was detected with a CCK-8 kit according to the manufacturer’s instructions.
NO Release Assay
In total, 5×104 cells/well were seeded in 48-well plates overnight and treated with GPS extract (50 to 150 µg/mL) for 1 h, and then challenged with 1 µg/mL LPS for 24 h. Nitric oxide (NO) inhibition (%) was determined by a NO release assay using Griess reagent according to the manufacturer’s instructions.
Colitis Induction and Evaluation
Male C57BL/6 mice (6–8 weeks old, 20–22 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The animal studies were approved by the Ethics Committee and performed according to Institutional Guidelines and Animal Ordinance (Health Science Center, Shenzhen University, No. 2018020). Before the experiment, the animals were acclimated to the feeding environment for a week.
Colitis was copied in mice with 2% DSS drinking water, as in our previous study,2 and then colitis mice were randomly divided into DSS model, cyclosporin A (CSA), and two GPS extract treated groups. At the same time, the vehicle control group without DSS was set up. During the experiment, the doses of GPS extract were set at 200 and 400 mg/Kg, respectively, according to the preliminary experiment, and the mice were intragastric for 7 consecutive days. In parallel, the dose of CSA was set at 25 mg/Kg as in a previous study.25 The vehicle control and DSS model groups were given the same volume of vehicle. Body weight, fecal consistency, and fecal occult blood were recorded daily. The disease activity index (DAI) was determined based on the above three items, as previously described.26–28 At the end of the experiment, all mice were euthanized, and the colonic segment was excised. After measuring the length of the colon, colon tissues were collected for further experiments.
Histological Examination
Colonic tissues were fixed in 4% paraformaldehyde, embedded in paraffin, stained with hematoxylin and eosin (H&E), and histopathological damage was evaluated blinded and scored as described previously.23
Real-Time PCR Analysis
The total RNA of RAW264.7/colon tissue was extracted using Trizol reagent, following the manufacturer’s instructions. Reverse transcriptase was used to synthesize the cDNA. Amplification was performed using the SYBR Green master, and the amount was quantitatively monitored in real time. The transcription of the target gene was normalized by β-actin and data were analyzed using the 2−ΔΔCT method. The primer sequences are shown in Table S1.
Western Blot, ELISA Analysis, and Myeloperoxidase (MPO) Determination
Proteins from RAW264.7 cells/colon tissues were extracted with ice cold RIPA buffer containing protease and phosphatase inhibitors, and analyzed by immunoblotting as previously reported.23,24 The primary antibodies used were Akt, p-Akt, p38, p-p38, ERK, p-ERK, JNK, p-JNK, and β-actin. The intensity of the bands was measured using ImageJ. In parallel, total protein was extracted from colon tissue for cytokine assays, as described in our previous studies.23,24 Then, the levels of IL-6 and IL-1β in colonic homogenates and supernatants of LPS-treated RAW 264.7 cells were measured using ELISA kits following the manufacturer’s protocol. The levels of MPO in supernatants extracted from colon tissues were also determined according to the manufacturer’s protocols.
Network Pharmacology Analysis
The construction of the GPS prediction targets database was based on LC-MS-MS identification results (Table S2) and network databases, including Pubchem (https://pubchem.ncbi.nlm.nih.gov/), SwissTargetPrediction (http://swisstargetprediction.ch/), and TargetNet (http://targetnet.scbdd.com/). And the construction of IBD and anti-inflammatory related predicted targets database was based on network database including GEO (https://www.ncbi.nlm.nih.gov/gds), OMIM (https://omim.org/), GeneCards (https://www.genecards.org/), DisGeNET (https://www.disgenet.org/), CTD (http://ctdbase.org/) database with “inflammatory bowel disease” and “anti-inflammatory” as keywords, respectively. The selection of validated human species-associated target genes in the STRING (https://cn.string-db.org/) database and the drug-, disease-, and anti-inflammation-related genes were intersected using a Venn diagram. Then, the intersection targets were imported into the STRING database for an analysis of the protein–protein interactions of the predicted targets and visualized using Cytoscape 3.8.2. Network topology analysis and screening were performed to derive the hub genes using Cytoscape’s analysis tool. Subsequently, in the R software, “clusterProfiler”, “org.Hs.eg.db”, “enrichplot”, “pathview”, “ggnewscale”, and “DOSE” were imported, and the q-value was set to 0.05. Then, we targeted hub genes with the filtered core intersection, ran the script, and drew a KEGG enrichment analysis bubble plot.
Molecular Docking
AutoDock Vina- 1.2.0 was used for all molecular docking. The crystallin structures of ERK (PDB: 6GDM), JNK (PDB: 1UKI), P38 (PDB: 3GCP), and Akt (PDB: 4GV1) from the PDB (https://www1.rcsb.org/) database were used as docking targets. The validated ERK inhibitor SCH772984, JNK inhibitor SP600125, P38 inhibitor SB203580, and Akt inhibitor AZD5363 were used as reference ligands. Prior to docking, the classical MM2 force field was used to optimize the structure of the small molecules. These models determine the free energy of binding between the ligand and the receptor, with lower binding free energies indicating stronger ligand receptor interactions. It is generally accepted that binding energies between ligands and receptors that are less than −4.25 kcal/mol, −5.0 kcal/mol, or −7.0 kcal/mol, indicate a certain, good, or strong binding activity, respectively.29 Finally, PyMOL-2.5.2 was used to visualize the molecular docking results.
Statistical Analysis
Statistical analysis was executed using GraphPad Prism 8.0 with a one-way analysis of variance (ANOVA) and Duncan’s multiple range tests. KEGG and GO enrichment analysis was processed using the R package with clusterProfiler. P-values < 0.05 were considered statistically significant.
Results
GPS Exhibits Potent Anti-Inflammatory Activity on LPS-Induced RAW264.7 Cells
After determining the safe concentrations of EPS, WPS, GPS, and NGPS fractions used in RAW 264.7 cells, their anti-inflammatory activities were preliminarily screened by NO release assay under LPS stimulation. As shown in Figure S1, the inhibitory activity of the GPS fraction against NO release was significantly higher than that of the WPS and NGPS fractions, and verified that the GPS fraction was mainly responsible for the anti-inflammatory efficacy of P. scrophulariiflora. Subsequently, we further investigated the effects of GPS on other inflammatory markers in LPS-stimulated RAW 264.7 cells. As shown in Figure 1, LPS treatment significantly increased NO release, transcription and expression of iNOS in RAW264.7 cells, but these increases were significantly reversed after GPS treatment (Figures 1A–C). In addition, LPS significantly increased the transcription and production of inflammatory cytokines IL-1β and IL-6, but these were also suppressed by GPS treatment in a dose-dependent manner (Figures 1D and E). Collectively, these data indicate that GPS has a potent inhibitory effect against LPS-induced inflammation in vitro.
GPS Exhibits Potent Anti-Inflammatory Activity Against DSS-Induced Colitis in Mice
The in vivo anti-inflammatory effects of GPS were determined in DSS-induced colitis mice. As shown in Figures 2A and B, mice treated with GPS or CSA showed a marked improvement in clinical symptoms of colitis, presenting lower body weight loss and DAI scores compared to the mice from the DSS model. Compared with the control group, mice treated with DSS showed shortened colons, which is a key indicator of colonic inflammation. As expected, this was significantly reversed by the treatment with both low- and high-dose GPS (Figure 2C). DSS treatment also resulted in extensive colonic tissue damage, including ulceration, inflammatory cell infiltration, and surface epithelial destruction, with a high histological score. However, mice treated with low and high doses of GPS exhibited less colonic damage and lower histological scores (Figures 2D and E). In addition, colonic MPO activity, a marker of neutrophil infiltration, was greatly increased in the DSS model group, whereas the MPO activities in the GPS-treated groups were also significantly suppressed (Figure 2F). Finally, we detected the transcription and production of inflammatory mediators in the colon tissues of colitis mice. As shown in Figure 3, compared to the mice in the control group, the transcription of IL-1β, IL-6 and iNOS, as well as the production of IL-1β and IL-6, were significantly elevated, whereas the transcription of IL-10 was dramatically decreased in the colon tissues of colitis mice. However, these inflammatory response changes were also significantly reversed by both GPS and CSA treatment. Taken together, these data indicate that GPS has potent anti-inflammatory properties against experimental colitis.
Network Pharmacology Predicts Potential Bioactive Components and Anti-Inflammatory Mechanisms of GPS Against Colitis
To explore the potential bioactive components and anti-inflammatory mechanisms of GPS against colitis, a network pharmacology analysis was performed. First, compounds contained in GPS were analyzed using a UPLC-ESI-MS/MS system, and 29 peaks representing individual chemical constituents were identified by comparing their retention time (tR), m/z, and ultraviolet (UV) absorption characteristics (lambda max) with relevant references (Figures 4, S2 and Table S2). Subsequently, compound-, IBD-, and anti-inflammatory-related targets were screened according to the guidelines of previous studies.30–33 In brief, 918 GPS-related targets were obtained from database Pubchem, SwissTargetPrediction and TargetNet based on the chemical components of GPS identified in Table S2; 8021 IBD-related targets were obtained from database OMIM, GeneCards, DisGeNET, and differential genes extracted from GSE9452 chip; and 1013 anti-inflammatory-related targets were obtained from database OMIM, GeneCards, and CTD, respectively. As shown in Figure 5A, 361 crossover targets were screened and identified as potential targets of GPS for anti-inflammatory activities to treat colitis. Then, a PPI network was constructed to systematically summarize the interactions of the 361 crossover GPS targets related to IBD therapy. As shown in Figure 5B, 77 predicted hub genes were obtained, of which the top 15 were MAPK3, RELA, STAT3, MAPK1, JUN, SRC, TNF, MAPK14, EP300, IL6, FOS, HSP90AA1, Akt1, and TP53, which are considered crucial targets of GPS against colitis. To further screen the pathways corresponding to GPS targets, a KEGG analysis was performed, and the results showed that PI3K/Akt and MAPK signaling pathways were closely related to these GPS-target genes (Figure 5C). In addition, a compound-target network was constructed to predict the anti-inflammatory bioactive components of GPS against colitis. The results showed that luteolin, apocynin, caffeic acid, caffeic acid methyl ester, luteoloside, picroside II, aucubin, cinnamic acid, vanillic acid, and sweroside might be candidate bioactive substances for the treatment of IBD (Figure 5D).
Verification of the Anti-Inflammatory Mechanism of GPS in vivo and in vitro
To verify the regulatory effect of GPS on PI3k/Akt and MAPK signaling pathways, we examined the protein expression of p-Akt, p-p38, p-ERK, and p-JNK in LPS-induced RAW 264.7 cells and in the colon tissues of DSS-induced colitis mice. As shown in Figure 6, GPS treatment significantly suppressed inflammation-induced upregulation of p-Akt, p-p38, p-ERK, and p-JNK in LPS-induced RAW 264.7 cells and colon tissues of DSS-induced colitis mice, verifying that GPS was effective in regulating PI3k/Akt and MAPK signaling pathways in vivo and in vitro.
Validation of Anti-Inflammatory Activities of Main Bioactive Components of GPS and Molecular Docking
To further verify the anti-inflammatory activities of the predicted main bioactive components from GPS, their activities were performed on LPS-induced RAW264.7 macrophage cells. As shown in Figure 7A, all of these predicted main bioactive components of GPS exhibited significant inhibitory effects on LPS-induced NO release from LPS-induced RAW264.7 macrophage cells, verifying that these components are anti-inflammatory functional substances of GPS. As the compound-target network revealed that these 10 predicted bioactive components of GPS mainly targeted to the genes MAPK1, MAPK3, MAPK8, MAPK14, and Akt1, we docked these bioactive molecules with MAPK1 and MAPK3 encoding protein ERK, MAPK8 encoding protein JNK, MAPK14 encoding protein p38, and Akt1 encoding protein Akt, respectively. The binding energies were calculated to evaluate the affinity of the components to the protein targets. As shown in Table 1, these predicted bioactive components could bind well to the target proteins Akt, P38, ERK, and JNK, which were better than 5-ASA, a first-line anti-inflammatory drug used to treat IBD in clinics. Among these, luteolin, luteoloside, and picroside II had the lowest binding energies in the molecular docking of all target proteins, respectively. Notably, luteolin, luteoloside, and picroside II showed the lowest binding energies when bound to all target proteins, and their binding energies to JNK, p38, and Akt were comparable to those of JNK, p38, and Akt inhibitors SP600125, SB203580, and AZD5363, respectively. Concurrently, 3D docking diagrams were generated to identify the binding sites of luteolin, luteoloside, and picroside II to all target proteins. As shown in Table S3 and Figure 7B, luteolin, luteoloside, and picroside II interact with target proteins mainly through hydrogen bonding, hydrophobic interaction, and Pi-Pi stacking. Of note, the optimal conformations of luteolin, luteoloside, and picroside II with all target proteins were the “IN” conformations, suggesting that luteolin, luteoloside, and picroside II may occupy the active conformations of those kinases and form a competitive relationship with ATP, which prevents ATP from further phosphorylating those kinases and inhibiting their activities.
 |
Table 1 Docking Results of ERK, JNK, p38 and Akt with References Ligands and 10 Bioactive Components of GPS |
Discussion
Even if multiple drug regimens are already available, incomplete cures and recurrence of IBD remain major problems, making it necessary to develop new safe and effective strategies to treat IBD. THMs are typically preferred as a treatment since they are low in toxicity and their effectiveness has been well tested in long-term use.34 P. scrophulariiflora is a good candidate because this herb was recorded to treat colitis symptoms in TCM, and its sister plant of P. kurroa, an Ayurvedic medicine highly chemically similar to P. scrophulariiflora, has shown an effective therapeutic effect on immunity-related diseases.10 In this study, we investigated the anti-inflammatory effect of the glycoside fraction of P. scrophulariiflora (GPS) against DSS-induced colitis in mice. Our results showed that oral administration of GPS significantly attenuated the severity of DSS-induced colitis in mice, as indicated by reduced DAI scores, colon shortening, histological damage, and colonic MPO activity. The in vitro assays also showed that GPS significantly suppressed LPS-induced IL-1β, IL-6, and iNOS transcription and the IL-1β, IL-6, and NO production from macrophage RAW264.7 cells.
As is known, inflammation resulting from an abnormal immune system response is one of the most prominent pathological features and pathogenesis of IBD. Macrophages are the main sentinels used to phagocytose and eliminate foreigners that invade colonic tissue. Activated macrophages by pathogens or inflammatory irritants secrete excessive proinflammatory cytokines to initiate the innate immune response and the consequential adaptive immune response that leads to inflammation.35,36 Both clinical and animal studies have found that accumulated macrophages are observed in the inflamed gut.24,37 In addition, the removal of macrophages or the suppression of their immune response preventing the development of colitis has been well verified.38–40 In the present study, our data showed that GPS significantly reduced the clinical signs and pathological manifestations of experimental colitis and suppressed the expression and production of inflammatory cytokines IL-6 and IL-1β in the colon tissues. It is well known that IL-6 and IL-1β are pro-inflammatory cytokines released by activated macrophages. However, our results from LPS-induced RAW264.7 macrophage cells showed that GPS could also significantly suppress NO release, iNOS, IL-6, and IL-1β transcription, as well as IL-6 and IL-1β production induced by LPS in RAW264.7 macrophage cells. Taken together, these data indicate that GPS has a potent anti-inflammatory effect against colonic inflammation, which is closely associated with inhibition of macrophage activation.
Further, we explored the action mechanism of GPS against colitis and the active components of GPS in its anti-inflammatory effects using the network pharmacology method. It is well known that network pharmacology is a new approach based on the theory of system biology to analyze the network of biological systems and select specific signal nodes to elaborate the principle and law of the interaction between organism and drug.41,42 Currently, network pharmacology is widely used to predict the main active ingredients and potential targets of medicines with multicomponents and multitargets, and it is becoming a cutting-edge research field in TCMs.43,44 In this study, a protein–protein interaction network was first constructed to systematically summarize the effect targets of anti-inflammatory properties against colonic inflammation, and 77 hub genes were found. Subsequently, the KEGG analysis showed that these hub genes were mainly enriched in PI3K/Akt and MAPK signaling pathways. Previous studies reported that MAPK and PI3K/Akt were two key signaling pathways for macrophage activation, and protein expression of p-ERK, p-p38, p-JNK, and p-Akt was upregulated in the pathological process of colonic inflammation.45–47 Inhibition of PI3K/Akt or MAPK signal with inhibitor could suppress macrophage activation and alleviate colitis.48–51 Based on the predicted results of network pharmacology, we experimentally confirmed that GPS could significantly downregulate the protein expression of p-Akt, p-p38, p-ERK, and p-JNK in LPS-induced RAW264.7 macrophage cells and colon tissues of DSS-treated colitis mice. In parallel, a compound-target network showcasing the correlation between bioactive compounds and GPS-related hub genes was also constructed to identify the anti-inflammatory components from GPS, and the top 10 candidate compounds in this network, namely luteolin, apocynin, caffeic acid, caffeic acid methyl ester, luteoloside, picroside II, aucubin, cinnamic acid, vanillic acid, and sweroside, were identified as the main components responsible for the anti-inflammatory effects of GPS. As expected, our NO release assay confirmed that these compounds all exhibited significant inhibitory activities on NO release from LPS-induced RAW264.7 macrophage cells. In vivo, previous studies have also verified that luteolin,52 apocynin,53 caffeic acid,54 picroside II,55 and vanillic acid56 have potent anti-inflammatory effects against experimental colitis in multiple animal models. Molecular docking showed that except for the binding conformations of apocynin, cinnamic acid and vanillic acid to ERK, and vanillic acid to JNK, other compounds had good affinities with the crystal structures of target proteins Akt, P38, ERK, and JNK, which were better than or approximately equal to 5-ASA (a first-line anti-inflammatory drug used to treat IBD) to interact with those target proteins. Collectively, these findings clearly demonstrated that GPS could attenuate DSS-induced colonic inflammation by reducing macrophage activation through inhibition of PI3K/Akt and MAPK signaling pathways, and compounds such as luteolin, apocynin, caffeic acid, caffeic acid methyl ester, luteoloside, picroside II, aucubin, cinnamic acid, vanillic acid, and sweroside were the main components responsible for its anti-inflammatory effects.
Conclusion
In conclusion, our study demonstrated that GPS had potent anti-inflammatory effects against DSS-induced colitis in mice, which is attributed to the main components of GPS compounds, such as luteolin, apocynin, caffeic acid, caffeic acid methyl ester, luteoloside, picroside II, aucubin, cinnamic acid, vanillic acid, and sweroside, to reduce macrophage activation through inhibiting PI3K/Akt and MAPKs signaling pathways, suggesting GPS could serve as the potential agent in the treatment of colitis.
Ethics Statement
This study is involving human data from public databases Pubchem, SwissTargetPrediction, TargetNet, GEO, OMIM, GeneCards, DisGeNET and CTD. Due to involving human databases belong to public databases and users can download relevant data for free for research and publish relevant articles, the Ethics committee of Shenzhen University confirms that this study would have received a waiver of ethical approval (ethical No: PN-202300005). All animal experiments were also approved by the Ethics Committee of Shenzhen University and performed in accordance with the Institutional Guidelines and Animal Ordinance (permit No. 202110144).
Acknowledgments
This work was kindly funded by SZU Top Ranking Project (86000000210) and Foundations of Shenzhen Science and Technology Innovation Committee (JCYJ20210324093810026).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sun X, Guo C, Zhao F, et al. Vasoactive intestinal peptide stabilizes intestinal immune homeostasis through maintaining interleukin-10 expression in regulatory B cells. Research Support, Non-U.S. Gov’t. Theranostics. 2019;9(10):2800–2811. doi:10.7150/thno.34414
2. Chang YY, Huan QC, Peng J, et al. P2Y1R Ligation Suppresses Th17 Cell Differentiation and Alleviates Colonic Inflammation in an AMPK-Dependent Manner. Research Support, Non-U.S. Gov’t. Front Immunol. 2022;13:820524. doi:10.3389/fimmu.2022.820524
3. Ma X, Di Q, Li X, et al. Munronoid I Ameliorates DSS-Induced Mouse Colitis by Inhibiting NLRP3 Inflammasome Activation and Pyroptosis Via Modulation of NLRP3. Research Support, Non-U.S. Gov’t. Front Immunol. 2022;13:853194. doi:10.3389/fimmu.2022.853194
4. Chang Y, Zhai L, Peng J, Wu H, Bian Z, Xiao H. Phytochemicals as regulators of Th17/Treg balance in inflammatory bowel diseases. Biomed Pharmacother. 2021;141:111931. doi:10.1016/j.biopha.2021.111931
5. Limketkai BN, Iheozor-Ejiofor Z, Gjuladin-Hellon T, et al. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Meta-Analysis Systematic Review. Cochrane Database Syst Rev. 2019;2:CD012839. doi:10.1002/14651858.CD012839.pub2
6. Zhai L, Peng J, Zhuang M, et al. Therapeutic effects and mechanisms of Zhen-Wu-Bu-Qi Decoction on dextran sulfate sodium-induced chronic colitis in mice assessed by multi-omics approaches. Phytomedicine. 2022;99:154001. doi:10.1016/j.phymed.2022.154001
7. Torres J, Ellul P, Langhorst J, et al. European Crohn’s and Colitis Organisation Topical Review on Complementary Medicine and Psychotherapy in Inflammatory Bowel Disease. Review. J Crohns Colitis. 2019;13(6):673–685e. doi:10.1093/ecco-jcc/jjz051
8. Yang L, Luo H, Tan D, et al. A recent update on the use of Chinese medicine in the treatment of inflammatory bowel disease. Review. Phytomedicine. 2021;92:153709. doi:10.1016/j.phymed.2021.153709
9. Yuan S, Wang Q, Li J, et al. Inflammatory bowel disease: an overview of Chinese herbal medicine formula-based treatment. Review. Chin Med. 2022;17(1):74. doi:10.1186/s13020-022-00633-4
10. Sharma T, Sharma U, Kumar S. Iridoid glycosides from Picrorhiza genus endemic to the Himalayan region: phytochemistry, biosynthesis, pharmacological potential and biotechnological intercessions to boost production. Review. Crit Rev Biotechnol. 2022;1–16. doi:10.1080/07388551.2022.2117681
11. Zeng S, Wang D, Cao Y, et al. Immunopotentiation of Caffeoyl Glycoside from Picrorhiza scrophulariiflora on activation and cytokines secretion of immunocyte in vitro. Research Support, Non-U.S. Gov’t. Int Immunopharmacol. 2008;8(12):1707–1712. doi:10.1016/j.intimp.2008.07.016
12. Mehta S, Sharma AK, Singh RK. Advances in Ethnobotany, Synthetic Phytochemistry and Pharmacology of Endangered Herb Picrorhiza kurroa (Kutki): a Comprehensive Review (2010-2020). Review. Mini Rev Med Chem. 2021;21(19):2976–2995. doi:10.2174/1389557521666210401090028
13. Zahiruddin S, Khan W, Nehra R, et al. Pharmacokinetics and comparative metabolic profiling of iridoid enriched fraction of Picrorhiza kurroa - An Ayurvedic Herb. Comparative Study. J Ethnopharmacol. 2017;197:157–164. doi:10.1016/j.jep.2016.07.072
14. Ma LZ, Kang LP, Nan TG, Zhan ZL, Guo LP. [Advances in research on chemical composition of Picrorhiza scrophulariiflora and P. kurroa and their biological activities]. Review. Zhongguo Zhong Yao Za Zhi. 2021;46(23):6114–6129. doi:10.19540/j.cnki.cjcmm.20210708.601
15. Li T, Zheng R, Xu L, et al. Picroside II alleviates liver injury induced by alpha-naphthylisothiocyanate through AMPK-FXR pathway. Research Support, Non-U.S. Gov’t. Toxicol Appl Pharmacol. 2020;408:115248. doi:10.1016/j.taap.2020.115248
16. Smit HF, Kroes BH, van den Berg AJ, et al. Immunomodulatory and anti-inflammatory activity of Picrorhiza scrophulariiflora. Research Support, Non-U.S. Gov’t. J Ethnopharmacol. 2000;73(1–2):101–109. doi:10.1016/s0378-8741(00)00268-3
17. Xu X, Wang WT, Zhao ZY, et al. Effects of total iridoid glycosides of Picrorhiza scrophulariiflora against non-alcoholic steatohepatitis rats induced by high-fat and high-sugar diet through regulation of lipid metabolism. Chin Herb Med. 2020;12(1):67–72. doi:10.1016/j.chmed.2019.12.005
18. Sharma S, Sharma P, Kulurkar P, Singh D, Kumar D, Patial V. Iridoid glycosides fraction from Picrorhiza kurroa attenuates cyclophosphamide-induced renal toxicity and peripheral neuropathy via PPAR-gamma mediated inhibition of inflammation and apoptosis. Phytomedicine. 2017;36:108–117. doi:10.1016/j.phymed.2017.09.018
19. Sinha K, Kumar S, Rawat B, et al. Kutkin, iridoid glycosides enriched fraction of Picrorrhiza kurroa promotes insulin sensitivity and enhances glucose uptake by activating PI3K/Akt signaling in 3T3-L1 adipocytes. Phytomedicine. 2022;103:154204. doi:10.1016/j.phymed.2022.154204
20. Guo ZJ, Hou FF, Liu SX, et al. Picrorhiza scrophulariiflora improves accelerated atherosclerosis through inhibition of redox-sensitive inflammation. Research Support, Non-U.S. Gov’t. Int J Cardiol. 2009;136(3):315–324. doi:10.1016/j.ijcard.2008.12.102
21. Morikawa T, Nakanishi Y, Inoue N, et al. Acylated iridoid glycosides with hyaluronidase inhibitory activity from the rhizomes of Picrorhiza kurroa Royle ex Benth. Phytochemistry. 2020;169:112185. doi:10.1016/j.phytochem.2019.112185
22. Zhang N, Wei DZ, Liu JW, Xin XJ. inventors; Methods for enrichment of iridoid glycosides in Picrocotyl Rhizoma. China Patent Application. 2022;1:76.
23. Zhai L, Huang T, Xiao HT, et al. Berberine Suppresses Colonic Inflammation in Dextran Sulfate Sodium-Induced Murine Colitis Through Inhibition of Cytosolic Phospholipase A2 Activity. Front Pharmacol. 2020;11:576496. doi:10.3389/fphar.2020.576496
24. Du SY, Huang HF, Li XQ, et al. Anti-inflammatory properties of uvaol on DSS-induced colitis and LPS-stimulated macrophages. Chin Med. 2020;15:43. doi:10.1186/s13020-020-00322-0
25. Sann H, Erichsen J, Hessmann M, Pahl A, Hoffmeyer A. Efficacy of drugs used in the treatment of IBD and combinations thereof in acute DSS-induced colitis in mice. Research Support, Non-U.S. Gov’t. Life Sci. 2013;92(12):708–718. doi:10.1016/j.lfs.2013.01.028
26. Wu MY, Liu L, Wang EJ, et al. PI3KC3 complex subunit NRBF2 is required for apoptotic cell clearance to restrict intestinal inflammation. Research Support, Non-U.S. Gov’t. Autophagy. 2021;17(5):1096–1111. doi:10.1080/15548627.2020.1741332
27. Cong J, Wu D, Dai H, et al. Interleukin-37 exacerbates experimental colitis in an intestinal microbiome-dependent fashion. Research Support, Non-U.S. Gov’t. Theranostics. 2022;12(11):5204–5219. doi:10.7150/thno.69616
28. Mei Y, Wang Z, Zhang Y, et al. FA-97, a New Synthetic Caffeic Acid Phenethyl Ester Derivative, Ameliorates DSS-Induced Colitis Against Oxidative Stress by Activating Nrf2/HO-1 Pathway. Research Support, Non-U.S. Gov’t. Front Immunol. 2019;10:2969. doi:10.3389/fimmu.2019.02969
29. Liu J, Tong X, Peng W, et al. Network Pharmacology Prediction and Molecular Docking-Based Strategy to Discover the Potential Pharmacological Mechanism of Huai Hua San Against Ulcerative Colitis. Drug Des Devel Ther. 2021;15:3255–3276. doi:10.2147/DDDT.S319786
30. Li X, Ma J, Guo L, et al. Identification of Bioactive Compounds and Potential Mechanisms of Kuntai Capsule in the Treatment of Polycystic Ovary Syndrome by Integrating Network Pharmacology and Bioinformatics. Oxid Med Cell Longev. 2022;2022:3145938. doi:10.1155/2022/3145938
31. Tian D, Gao Q, Lin J, et al. Uncovering the mechanism of the Shenzhi Jiannao formula against vascular dementia using a combined network pharmacology approach and molecular biology. Phytomedicine. 2021;90:153637. doi:10.1016/j.phymed.2021.153637
32. Zhou W, Chen Z, Lu A, Liu Z. Systems Pharmacology-Based Strategy to Explore the Pharmacological Mechanisms of Citrus Peel (Chenpi) for Treating Complicated Diseases. Am J Chin Med. 2021;49(2):391–411. doi:10.1142/S0192415X2150018X
33. Liu CS, Xia T, Luo ZY, et al. Network pharmacology and pharmacokinetics integrated strategy to investigate the pharmacological mechanism of Xianglian pill on ulcerative colitis. Phytomedicine. 2021;82:153458. doi:10.1016/j.phymed.2020.153458
34. Holleran G, Scaldaferri F, Gasbarrini A, Curro D. Herbal medicinal products for inflammatory bowel disease: a focus on those assessed in double-blind randomised controlled trials. Review. Phytother Res. 2020;34(1):77–93. doi:10.1002/ptr.6517
35. Pan X, Zhu Q, Pan LL, Sun J. Macrophage immunometabolism in inflammatory bowel diseases: from pathogenesis to therapy. Review. Pharmacol Ther. 2022;238:108176. doi:10.1016/j.pharmthera.2022.108176
36. Na YR, Stakenborg M, Seok SH, Matteoli G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Research Support, Non-U.S. Gov’t Review. Nat Rev Gastroenterol Hepatol. 2019;16(9):531–543. doi:10.1038/s41575-019-0172-4
37. Xiao HT, Peng J, Hu DD, et al. Qing-dai powder promotes recovery of colitis by inhibiting inflammatory responses of colonic macrophages in dextran sulfate sodium-treated mice. Chin Med. 2015;10:29. doi:10.1186/s13020-015-0061-x
38. Ai L, Ren Y, Zhu M, et al. Synbindin restrains proinflammatory macrophage activation against microbiota and mucosal inflammation during colitis. Research Support, Non-U.S. Gov’t. Gut. 2021;70(12):2261–2272. doi:10.1136/gutjnl-2020-321094
39. Wang X, Ji Y, Feng P, et al. The m6A Reader IGF2BP2 Regulates Macrophage Phenotypic Activation and Inflammatory Diseases by Stabilizing TSC1 and PPARgamma. Research Support, Non-U.S. Gov’t. Adv Sci. 2021;8(13):2100209. doi:10.1002/advs.202100209
40. Ghia JE, Galeazzi F, Ford DC, Hogaboam CM, Vallance BA, Collins S. Role of M-CSF-dependent macrophages in colitis is driven by the nature of the inflammatory stimulus. Research Support, Non-U.S. Gov’t. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G770–7. doi:10.1152/ajpgi.00453.2007
41. Kibble M, Saarinen N, Tang J, Wennerberg K, Makela S, Aittokallio T. Network pharmacology applications to map the unexplored target space and therapeutic potential of natural products. Research Support, Non-U.S. Gov’t Review. Nat Prod Rep. 2015;32(8):1249–1266. doi:10.1039/c5np00005j
42. Poornima P, Kumar JD, Zhao Q, Blunder M, Efferth T. Network pharmacology of cancer: from understanding of complex interactomes to the design of multi-target specific therapeutics from nature. Review. Pharmacol Res. 2016;111:290–302. doi:10.1016/j.phrs.2016.06.018
43. Xu H, Zhang Y, Wang P, et al. A comprehensive review of integrative pharmacology-based investigation: a paradigm shift in traditional Chinese medicine. Review. Acta Pharm Sin B. 2021;11(6):1379–1399. doi:10.1016/j.apsb.2021.03.024
44. Yang HY, Liu ML, Luo P, Yao XS, Zhou H. Network pharmacology provides a systematic approach to understanding the treatment of ischemic heart diseases with traditional Chinese medicine. Review. Phytomedicine. 2022;104:154268. doi:10.1016/j.phymed.2022.154268
45. Huang XL, Xu J, Zhang XH, et al. PI3K/Akt signaling pathway is involved in the pathogenesis of ulcerative colitis. Research Support, Non-U.S. Gov’t. Inflamm Res. 2011;60(8):727–734. doi:10.1007/s00011-011-0325-6
46. Zhang X, Zuo L, Geng Z, et al. Vindoline ameliorates intestinal barrier damage in Crohn’s disease mice through MAPK signaling pathway. Research Support, Non-U.S. Gov’t. FASEB J. 2022;36(11):e22589. doi:10.1096/fj.202200234RR
47. Zhao X, Kang B, Lu C, et al. Evaluation of p38 MAPK pathway as a molecular signature in ulcerative colitis. Comparative Study Research Support, Non-U.S. Gov’t. J Proteome Res. 2011;10(5):2216–2225. doi:10.1021/pr100969w
48. Bai D, Zhao Y, Zhu Q, et al. LZ205, a newly synthesized flavonoid compound, exerts anti-inflammatory effect by inhibiting M1 macrophage polarization through regulating PI3K/AKT/mTOR signaling pathway. Research Support, Non-U.S. Gov’t. Exp Cell Res. 2018;364(1):84–94. doi:10.1016/j.yexcr.2018.01.033
49. Liu Y, Liu X, Hua W, et al. Berberine inhibits macrophage M1 polarization via AKT1/SOCS1/NF-kappaB signaling pathway to protect against DSS-induced colitis. Int Immunopharmacol. 2018;57:121–131. doi:10.1016/j.intimp.2018.01.049
50. Qi W, Lin C, Fan K, et al. Hesperidin inhibits synovial cell inflammation and macrophage polarization through suppression of the PI3K/AKT pathway in complete Freund’s adjuvant-induced arthritis in mice. Chem Biol Interact. 2019;306:19–28. doi:10.1016/j.cbi.2019.04.002
51. Lee H, Son YS, Lee MO, et al. Low-dose interleukin-2 alleviates dextran sodium sulfate-induced colitis in mice by recovering intestinal integrity and inhibiting AKT-dependent pathways. Research Support, Non-U.S. Gov’t. Theranostics. 2020;10(11):5048–5063. doi:10.7150/thno.41534
52. Xie X, Zhao M, Huang S, et al. Luteolin alleviates ulcerative colitis by restoring the balance of NCR(-)ILC3/NCR(+)ILC3 to repairing impaired intestinal barrier. Int Immunopharmacol. 2022;112:109251. doi:10.1016/j.intimp.2022.109251
53. Hwang YJ, Nam SJ, Chun W, et al. Anti-inflammatory effects of apocynin on dextran sulfate sodium-induced mouse colitis model. Research Support, Non-U.S. Gov’t. PLoS One. 2019;14(5):e0217642. doi:10.1371/journal.pone.0217642
54. Wan F, Zhong R, Wang M, et al. Caffeic Acid Supplement Alleviates Colonic Inflammation and Oxidative Stress Potentially Through Improved Gut Microbiota Community in Mice. Front Microbiol. 2021;12:784211. doi:10.3389/fmicb.2021.784211
55. Yao H, Yan J, Yin L, Chen W. Picroside II alleviates DSS-induced ulcerative colitis by suppressing the production of NLRP3 inflammasomes through NF-kappaB signaling pathway. Immunopharmacol Immunotoxicol. 2022;44(3):437–446. doi:10.1080/08923973.2022.2054425
56. Kim SJ, Kim MC, Um JY, Hong SH. The beneficial effect of vanillic acid on ulcerative colitis. Research Support, Non-U.S. Gov’t. Molecules. 2010;15(10):7208–7217. doi:10.3390/molecules15107208
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







