Back to Journals » Drug Design, Development and Therapy » Volume 18
Network Pharmacology and Molecular Docking Identify the Potential Mechanism and Therapeutic Role of Scutellaria baicalensis in Alzheimer’s Disease
Received 3 December 2023
Accepted for publication 4 April 2024
Published 17 April 2024 Volume 2024:18 Pages 1199—1219
DOI https://doi.org/10.2147/DDDT.S450739
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Yutao Peng,1 Chanjuan Zhou2
1Department of Function, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan, People’s Republic of China; 2Department of Clinical Psychology, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan, People’s Republic of China
Correspondence: Chanjuan Zhou, Department of Clinical psychology, The First Affiliated Hospital, Hengyang Medical School, University of South China, 69 Chuan Shan Road, Hengyang, Hunan, 421001, People’s Republic of China
, Tel +86 13212694904
, Email [email protected]
Aim: Scutellaria baicalensis, a traditional Chinese medicinal herb renowned for its anti-inflammatory, antioxidant, and anti-tumor properties, has shown promise in alleviating cognitive impairment associated with Alzheimer’s disease. Nonetheless, the exact neuroprotective mechanism of Scutellaria baicalensis against Alzheimer’s disease remains unclear. In this study, network pharmacology was employed to explore the possible mechanisms by which Scutellaria baicalensis protects against Alzheimer’s disease.
Methods: The active compounds of Scutellaria baicalensis were retrieved from the TCMSP database, and their corresponding targets were identified. Alzheimer’s disease-related targets were obtained through searches in the GeneCards and OMIM databases. Cytoscape 3.6.0 software was utilized to construct a regulatory network illustrating the “active ingredient-target” relationships. Subsequently, the target genes affected by Scutellaria baicalensis in the context of Alzheimer’s disease were input into the String database to establish a PPI network. GO analysis and KEGG analysis were conducted using the DAVID database to predict the potential pathways associated with these key targets. Following this, the capacity of these active ingredients to bind to core targets was confirmed through molecular docking. In vitro experiments were then carried out for further validation.
Results: A total of 36 active ingredients from Scutellaria baicalensis were screened out, which corresponded to 365 targets. Molecular docking results demonstrated the robust binding abilities of Baicalein, Wogonin, and 5,2’-Dihydroxy-6,7,8-trimethoxyflavone to key target proteins (SRC, PIK3R1, and STAT3). In vitro experiments showed that the active components of Scutellaria baicalensis can inhibit STAT3 expression by downregulating the PIK3R1/SRC pathway in Neuro 2A cells.
Conclusion: In summary, these findings collectively suggest that Scutellaria baicalensis holds promise as a viable treatment option for Alzheimer’s disease.
Keywords: Scutellaria baicalensis, Alzheimer’s disease, network pharmacology, molecular docking
Introduction
Alzheimer’s disease is a prevalent neurodegenerative disorder, accounting for 50% to 75% of dementia cases, with no current cure available.1,2 Patients with Alzheimer’s disease exhibit clinical manifestations such as memory impairment, cognitive decline, behavioral disturbances, and impaired daily functioning.3,4 There is an urgent demand for effective treatments for Alzheimer’s disease. Presently, drugs targeting cholinergic and glutamatergic neurotransmission are the mainstay of Alzheimer’s treatment, offering symptom relief but with contentious neuroprotective effects.5,6 Consequently, the search for novel Alzheimer’s treatments remains imperative. Traditional Chinese medicine, with a history spanning over 2500 years in the Chinese healthcare system, has revealed numerous bioactive compounds within Chinese herbs that have demonstrated efficacy in treating various ailments. Of notable significance, traditional Chinese medicine has emerged as a promising approach for addressing the treatment of Alzheimer’s disease.7–9
Scutellaria baicalensis, an herb belonging to the platycodon family, is commonly used in the treatment of bacterial pneumonia, colds, and insomnia. Recently, numerous chemical, pharmacological, and clinical investigations have demonstrated its neuroprotective,10–12 anti-tumor,13 antioxidant,14,15 and anti-inflammatory16 properties. Notably, it has been reported that Scutellaria baicalensis attenuates neuronal loss and improves memory and neuroplasticity disorders in rats with Alzheimer’s disease.17,18 However, the comprehensive mechanism of Scutellaria baicalensis intervention in Alzheimer’s disease remains unclear, with limited research at the molecular level. Alzheimer’s disease is influenced by alterations in multiple gene expressions; therefore, drugs targeting a single pathway may not yield optimal therapeutic outcomes for Alzheimer’s disease. Network pharmacology offers a systematic approach to study the multi-component, multi-target, and multi-pathway mechanisms underlying drug treatment through network visualization diagrams based on existing biological data. This aligns with the holistic principles of traditional Chinese medicine, which emphasize overall disease management and provide novel insights and methodologies for studying complex disease mechanisms.19–21 Molecular docking employs computer technology to explore spatial structure interactions between drug molecules and targets, enabling the targeted design of appropriate drugs.22,23 In this study, we employed network pharmacology and molecular docking approaches to elucidate the target proteins involved along with related pathways and mechanisms underlying Scutellaria baicalensis intervention in Alzheimer’s disease.
Methods
Obtaining the Active Ingredients and Targets of Scutellaria baicalensis
The Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) database (https://tcmsp-e.com/tcmsp.php) was used to screen the main components of Scutellaria baicalensis. According to the TCMSP database recommendation, the main ingredients of Scutellaria baicalensis with “oral bioavailability (OB) > 30% and drug-likeness (DL) ≥ 0.18” were selected. Based on the data obtained by screening the TCMSP database and, at the same time, consulting relevant literature for supplementation, the active ingredients of Scutellaria baicalensis were finally determined. Subsequently, the 3D structures of all compounds were built using 3D Viewer and saved in mol2 file format for target prediction. Finally, target information was obtained from the TCMSP database based on feature data and physicochemical parameters, followed by the retrieval of SMILES files for these active ingredients from the PubChem database. These SMILES files are utilized in target prediction through the SwissTargetPrediction (STP) website (http://old.swisstargetprediction.ch/). The combined targets derived from both the TCMSP and STP databases represent the main targets of active ingredients in Scutellaria baicalensis.
Identification of Targets Associated with Alzheimer’s Disease
Initially, the keyword “Alzheimer” was employed to screen established therapeutic targets for Alzheimer’s disease from both the Online Mendelian Inheritance in Man (OMIM) (https://omim.org/) and GeneCards (https://www.genecards.org/).
Construction of Active Ingredient-Target Network
The Venn diagram online tool (https://bioinfogp.cnb.csic.es/tools/venny/) was utilized to identify the common targets of Scutellaria baicalensis and Alzheimer’s disease by intersecting the targets related to Alzheimer’s disease and those related to active ingredients. Subsequently, a Venn diagram was generated. The intersection genes represented potential targets of Scutellaria baicalensis for the treatment of Alzheimer’s disease. The target proteins for active ingredients and Alzheimer’s disease were predicted, and standardized naming (gene name) was applied. The selected Alzheimer’s disease targets were imported into the Cytoscape 3.7.1 software to generate an ingredient-target network map.
Construction of the Protein-Protein Interaction (PPI) Network and Identification of Core Targets
The obtained intersection target protein was uploaded to the STRING database, with the species selected as “Homo sapiens” to retrieve PPI information. The resulting data file in Tab Separated Values (TSV) format was exported and imported into the Cytoscape 3.8.2 software for visualization processing. Within Cytoscape, CytoHubba, a built-in plug-in, was utilized for network analysis based on 11 topology methods derived from shortest path algorithms, including degree, edge penetration component, maximum neighborhood component density, maximum group centrality, and six centralities (bottleneck, degree, betweenness, radiality, closeness, and stress). Among these methods, the maximum clique centrality (MCC) algorithm has been proven to be more accurate in predicting important targets. By employing the MCC topology algorithm in this study, key core targets were identified as crucial nodes representing Scutellaria baicalensis’s therapeutic effects against Alzheimer’s disease. Furthermore, key sub-networks of active ingredients from Scutellaria baicalensis were constructed within the PPI network, which plays a significant role.
Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway and Gene Ontology (GO) Enrichment Analysis
The obtained target genes were subjected to online analysis using the DAVID database, followed by importing them into Omicsbean software. The ID type was set as Gene Symbol, and the species was specified as “Homo sapiens”. Subsequently, GO enrichment and KEGG pathway analyses were conducted on the target proteins. The resulting output file was imported into an Excel worksheet for further screening of functions and pathways with a significance level of P < 0.05, which was then visualized in a bar chart.
Validation of Drug-Component-Target-Disease Network
The drug-component-target-disease network was created through the Cytoscape 3.7.1 software by combining the shared genes from the disease and drug datasets with the top 20 pathways identified through KEGG analysis.
Molecular Docking
Molecular docking is a process that simulates the docking posture of small molecules at the active sites of large molecules to predict affinity. In this study, AutoDock4 was employed for molecular docking analysis between the potential active ingredients of Scutellaria baicalensis and their targets. The active ingredients of Scutellaria baicalensis were obtained from the PubChem database in SDF format, and their 3D structure was constructed using ChemOffice software, followed by energy minimization and conversion to mol2 format. In terms of target proteins, their 3D structure was retrieved from the PDB database (http://wwwl.rcsb.org/) and processed using pymol 2.3 to remove water molecules and AutoDock4 to add hydrogen atoms. Both the compound and target were saved in pdbqt format for subsequent docking analysis with the V program. Finally, ligplot2.23 generated a 2D interaction diagram, while pymol 2.3 analyzed the 3D model diagram of the docking complex.
Cell Culture
The Neuro 2A cells, sourced from Procell Life Science & Technology Co., Ltd. in Wuhan, China, were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM). The growth medium consisted of 10% fetal bovine serum (FBS), 100 U/mL streptomycin, and 100 U/mL penicillin. Cultivation was carried out under conditions of 5% CO2 at 37 °C. Following this, the cells were subjected to treatment with Baicalein, Wogonin, and 5,2’-Dihydroxy-6,7,8-trimethoxyflavone, all at a concentration of 80 μM, for a duration of 12 hours.
Cell Activity Detection
Baicalein (CAS No. 491-67-8), Wogonin (CAS No. 632-85-9), and 5,2’-Dihydroxy-6,7,8-trimethoxyflavone (CAS No. 86926-52-5) were procured from Baoji Chenguang Biotechnology Co., Ltd. (China). To assess cell viability, we employed the Counting Kit-8 (CCK-8) assay. In brief, Neuro 2A cells were initially seeded into 96-well culture plates and allowed to incubate for 12 hours at 37°C. Subsequently, the cells underwent treatment with various concentrations of Baicalein, Wogonin, and 5.2’-Dihydroxy-6,7,8-trimethoxyflavone (ranging from 0 to 160 μM) for a duration of 24 hours. Following this treatment, a 10 µL aliquot of CCK-8 solution was added to each well and incubated for an additional hour at 37°C. The absorbance at 450 nm was then measured using a 96-well microplate reader (Bio-Tek Instruments Inc., Winooski, VT, USA). To ensure reliability and robustness of the experiment, each experiment was conducted independently at least three times.
Real-Time Quantitative PCR
TRIzol extraction kits (Invitrogen, America) were used to extract RNA from cells. A high-capacity cDNA reverse transcription kit (Takara, Kyoto, Japan) was used to create the first strand of complementary DNA. qRT-PCR was then done on an ABI7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) for 40 cycles (60°C for 1 min, 90°C for 15 sec, and 95°C for 3 min). The primer sequences for real-time PCR were as follows: PIK3R1-forward sequence, 5′-AAGAAGTTGAACGAGTGGTTGG-3′; reverse sequence, 5′-GCCCTGTTTACTGCTCTCCC-3′. SRC-forward sequence, 5′-AGAGTGCCCTATCCTGGGAT-3′; reverse sequence, 5′-AAAGTAGTCTTCCAGGAAGGCC-3′. STAT3-forward sequence, 5′-TGCAGTTTGGAAATAATGGTGA-3′; reverse sequence, 5′-CATGTCAAAGGTGAGGGACTC-3′. β-actin-forward sequence, 5′-TGAGGCCCAGAGCAAGAGA-3′; reverse sequence, 5′-TCGTCCCAGTTGGTGACGAT-3′. For mRNA, the internal control of qRT-PCR was β-actin. Melting curve analysis was used to assess the specificity of all PCR products. The 2−ΔΔCt method was used to determine relative gene expression. The experiments were conducted independently, ensuring the absence of pseudo-replication.
Small Interfering RNA Transfection
The specific small interfering RNAs (siRNAs) targeting SRC were synthesized by the GenePharma Company (Shanghai, China). The forward sequence was 5′-GGUUCACCAUCAAGUCAGATT-3′ and the reverse sequence was 5′-UCUGACUUGAUGGUGAACCTT-3′. Similarly, siRNAs against STAT3 and PIK3R1 were also synthesized with their respective sequences: STAT3 forward, 5′-GGACGACUUUGAUUUCAACTT-3′, reverse, 5′-GUUGAAAUCAAAGUCGUCCTG-3′; PIK3R1 forward, 5′-GAAAGGAGGAAAUAAACAAATT-3’ and reverse, 5’-UUUGUUAUUUCCUCCUUUCT-3’. Neuro2A cells at a confluency of 60–80% were seeded in standard medium in a 12-well plate with a volume of 1 mL. The following day, Lipofectamine™ RNAiMAX reagent (Invitrogen) was used to transfect the cells with siRNA duplexes at a final concentration of 20 nM as per the manufacturer’s instructions. After incubation for 72 hours, qRT-PCR analysis was performed to assess transfection efficiency. The experiments were conducted independently, ensuring the elimination of pseudo-replication.
Data Analysis
Data from 5 experiments were statistically analyzed using parametric methods (Welch-ANOVA and Welch t-test), and the distribution of the data was evaluated using QQ plots. Tukey post-hoc test is employed to examine multiple comparisons among different groups. The experiments were performed independently, and there is no pseudo-replication. All statistical analyses were carried out with the help of GraphPad software (version 8.0.1) and SPSS 24.0. A P value < 0.05 was considered statistically significant.
Results
Acquisition of Active Ingredients of Scutellaria baicalensis and Alzheimer’s Disease-Related Targets
The active ingredients of Scutellaria baicalensis were searched through the TCMSP database, and 36 compounds were obtained by setting OB ≥ 30% and DL ≥ 0.18 (Table 1). The TCMSP database was further used to obtain the corresponding targets of active ingredients, and 365 corresponding targets of active ingredients were obtained after summarization and deduplication. The keyword “Alzheimer’s disease” was retrieved in the GeneCards and OMIM databases. Then, the results of Alzheimer’s disease-related targets retrieved from these two databases were integrated. Duplicate data were eliminated during the screening process, resulting in a total of 2345 Alzheimer’s disease-related targets.
 |
Table 1 Active Ingredients in Scutellaria baicalensis |
Construction of the Compound-Target Network
Potential targets of Scutellaria baicalensis were mapped with Alzheimer’s disease-related targets, and a total of 176 intersection targets were obtained by the Venn platform (Figure 1). Then, active components and intersection targets were imported into the Cytoscape 3.8.2 software for visual analysis, and the diagram of the “compound-target network” was obtained (Figure 2). The analysis presented in Figure 2 reveals that certain targets are subject to modulation by multiple active compounds, whereas others exhibit responsiveness to only one compound (eg, RPS6KB2, SAE1, ROCK2, QPCT et al). Notably, SRC, PIK3R1, ESR1, EGFR, STAT3, and other targets demonstrate susceptibility to regulation by all of the compounds.
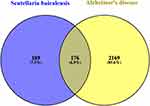 |
Figure 1 Intersection of target genes between the active ingredients of Scutellaria baicalensis and those linked to Alzheimer’s disease. |
 |
Figure 2 Active ingredients-target network construction. The dark green circular node represents active ingredients of Scutellaria baicalensis, and the other nodes represent the targets. |
Construction of the PPI Network
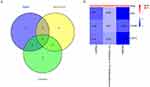
To elucidate the mechanism by which Scutellaria baicalensis protects against Alzheimer’s disease, we began by inputting the intersection targets into the String database. The species was specified as “Homo sapiens”, while the other parameters were left at their default settings. As shown in Figure 3A, the network linking drug targets and disease targets comprises 176 nodes and 302 edges. Notably, the network exhibited an average node degree of 10.4 and a local clustering coefficient of 0.900. Subsequently, we imported the network data into Cytoscape 3.7.2 software, where we employed the NetworkAnalyzer plug-in tool to generate a PPI network diagram (Figure 3B). This diagram was constructed based on the density values, or degrees, of the nodes within the network. Larger nodes in the diagram corresponded to genes with higher degrees. As shown in Figure 3C, the top 10 proteins were ranked by their degree centrality (Degree, Betweenness, and Closeness). Among them, SRC, PIK3R1, and STAT3 were determined by taking the intersection of targets obtained through these three methods (Figure 4A). Within Scutellaria baicalensis, Baicalein, Wogonin, and 5,2’-Dihydroxy-6,7,8-trimethoxyflavone had a significant correlation with these key targets. (Figure 4B). These proteins are of paramount importance in PPI and represent the core targets of Scutellaria baicalensis’ active ingredients in combating Alzheimer’s disease.
Pathway Enrichment Analysis
Next, GO and KEGG enrichment analyses were performed to identify biological functions and metabolic pathways associated with cross-targets. The bubble plot results obtained from the KEGG enrichment analysis illustrated in Figure 5 demonstrate the impact of Scutellaria baicalensis on Alzheimer’s disease. Out of the 166 identified pathways, we focused our comparative analysis on 20 of them. These pathways are closely associated with a range of pathophysiological processes, including Alzheimer’s disease, cancer, atherosclerosis, insulin resistance, and endocrine resistance. To visualize the key pathways and associated targets identified through KEGG enrichment analysis, we employed Cytoscape software to construct a “target-pathway” network diagram, which is depicted in Figure 6. Additionally, for a comprehensive perspective on metabolic pathways relevant to Alzheimer’s disease, we integrated data from KEGG and the Genome Encyclopedia to generate Figure 7.
 |
Figure 5 Enrichment analysis of KEGG biological pathways. |
 |
Figure 6 Target-pathway network diagram. |
Then, we imported the set of 176 targets into the Metascape platform and subsequently conducted a GO functional enrichment analysis to identify the top 10 entries of significance. The outcomes of the GO enrichment analysis unveiled a comprehensive range of molecular function (MF) pathways, totaling 163. These pathways encompassed critical functions such as identical protein binding, ATP binding, protein serine/threonine/tyrosine kinase activity, protein kinase activity, kinase activity, protein tyrosine kinase activity, heme binding, transmembrane receptor protein tyrosine kinase activity, RNA polymerase II transcription factor activity, and ligand-activated sequence-specific DNA binding. Regarding biological processes (BPs), the 711 pathways enriched by the common genes indicated that the therapeutic effects of Scutellaria baicalensis on Alzheimer’s disease involve diverse processes such as protein phosphorylation, response to xenobiotic stimulus, protein autophosphorylation, peptidyl-serine phosphorylation, transmembrane receptor protein tyrosine kinase signaling pathway, and positive regulation of the MAPK cascade. Furthermore, a total of 107 cellular components (CCs) were identified in the enrichment analysis. These components encompassed structures like the plasma membrane, cytoplasm, neuronal cell body, axon, and dendrite, as depicted in Figure 8.
 |
Figure 8 GO enrichment analysis. |
Molecular Docking Analysis
To investigate the interaction between Scutellaria baicalensis’ active ingredients and target genes, we employed Autodock and Pymol software for molecular docking. Specifically, we conducted docking simulations involving three critical targets and three active components of Scutellaria baicalensis, recording the corresponding binding energies (Figure 4B). The planar structures of Baicalein, 5,2’-Dihydroxy-6,7,8-trimethoxyflavone, and Wogonin in silico active compounds can be seen in Figure 9A–C, respectively. The results of molecular docking demonstrated the facile entry and binding of all three active ingredients within the active pocket regions of SRC, PIK3R1, and STAT3 proteins, as shown in Figures 10–12. The docking results of these active ingredients and three targets were refined via investigating the specific binding sites in the UCFS chimera. Specifically, in PIK3R1, Baicalein had hydrogen bonds with amino acid residues ASN-47 and ARG-34. In SRC, Baicalein and amino acid residues GLU-160, HIS-204, and LY-S155 had hydrogen bonds. In STAT3, Baicalein had hydrogen bonds with amino acid residues in TYR-657. The hydrogen bonds between Wogonin and the amino acid residues GLU-94 and HIS-96 were observed in PIK3R1. In SRC, Wogonin formed hydrogen bonds with amino acid residues of LYS-155. In STAT3, Wogonin had hydrogen bonds with amino acid residues of TYR-657. The compound 5,2’-Dihydroxy-6,7,8-trimethoxyflavone established hydrogen bonds with the amino acid residues located at HIS-96, LEU-132, PRO-98, and LEU-129 in PIK3R1. In SRC, 5,2’-Dihydroxy-6,7,8-trimethoxyflavone formed hydrogen bonds with amino acid residues of HIS-204 and LYS-206. In STAT3, 5,2’-Dihydroxy-6,7,8-trimethoxyflavone had hydrogen bonds with amino acid residues of THR-443. These findings further confirm that the active ingredients of Scutellaria baicalensis have a good binding effect on their targets.
 |
Figure 9 The planar structures of the in silico active ingredients. (A) Baicalein; (B) 5,2’-Dihydroxy-6,7,8-trimethoxyflavone; (C) Wogonin. |
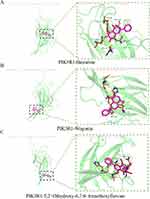 |
Figure 10 Molecular docking model of PIK3R1-active ingredients. (A) PIK3R1-Baicalein; (B) PIK3R1-Wogonin; (C) PIK3R1-5,2’-Dihydroxy-6,7,8- trimethoxyflavone. |
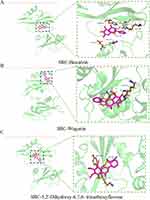 |
Figure 11 Molecular docking model of SRC-active ingredients. (A) SRC-Baicalein; (B) SRC-Wogonin; (C) SRC-5,2’-Dihydroxy-6,7,8- trimethoxyflavone. |
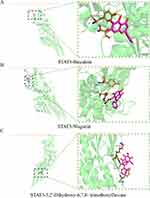 |
Figure 12 Molecular docking model of STAT3-active ingredients. (A) STAT3-Baicalein; (B) STAT3-Wogonin; (C) STAT3-5,2’-Dihydroxy-6,7,8- trimethoxyflavone. |
Effect of Scutellaria baicalensis’ Active Ingredients on the Expression of Target Genes
The emergence of several cell lines, such as the rat pheochromocytoma (PC12) cell line, adipose mesenchymal stem cell, SH-SY5Y cells, and Neuro 2A cells, has provided valuable models for studying Alzheimer’s disease-related processes and investigating the neuroprotective effects of various compounds.24 Among them, Neuro 2A cells have been widely used to study neuronal differentiation, cell signaling pathways, and Aβ metabolism during Alzheimer’s disease. These cells provide a valuable model for exploring the cellular mechanisms involved in Alzheimer’s disease pathology and evaluating the therapeutic potential of various compounds. To investigate the impact of active ingredients from Scutellaria baicalensis on the expression of their target genes, we conducted both qRT-PCR and CCK-8 assays. The results of the CCK-8 assay demonstrated that treatment with 80 μM Baicalein, Wogonin, and 5,2’-Dihydroxy-6,7,8-trimethoxyflavone did not induce cytotoxic effects in Neuro 2A cells (Figure 13A). Consequently, we proceeded with a concentration of 80 μM for subsequent experiments. Figure 13B–D showed that the active ingredients of Scutellaria baicalensis (Baicalein, Wogonin, and 5,2’-Dihydroxy-6,7,8-trimethoxyflavone) significantly decreased the expression levels of SRC, PIK3R1, and STAT3. STAT3 is known as a substrate of the non-receptor tyrosine kinase SRC.25 Our results also demonstrated that Baicalein, Wogonin, and 5,2’-Dihydroxy-6,7,8-trimethoxyflavone decreased STAT3 levels via inhibiting the PIK3R1/SRC pathway (Supplementary Figures 1–3). These results indicate that Scutellaria baicalensis’ active components can suppress the expression of their target genes in Neuro 2A cells, reinforcing the outcomes from network pharmacology and molecular docking analyses.
Discussion
As a commonly used traditional tonic medicine, Scutellaria baicalensis possesses neuroprotective, anti-tumor, and mood-enhancing properties.26,27 The anti-Alzheimer’s disease mechanism of Scutellaria baicalensis was elucidated in this study using network pharmacology and molecular docking techniques. Traditional Chinese medicine adopts a holistic perspective in its development. Network pharmacology serves as a systems biology approach for disease treatment by reflecting the concept of multi-component, multi-target, and multi-pathway regulation. Molecular docking utilizes computational simulations to design drugs with targeted effects. Therefore, the application of network pharmacology and molecular docking technology plays a crucial role in elucidating the therapeutic mechanisms underlying complex diseases treated with Scutellaria baicalensis. A total of 176 core targets were individually examined through functional enrichment analysis using the GO and KEGG databases. The results showed that protein binding was the most enriched item in GO analysis, indicating that Scutellaria baicalensis may interfere with Alzheimer’s disease progression by modulating protein-protein interactions. Among the 166 KEGG pathways analyzed, Alzheimer’s disease-related genes were predominantly enriched in pathways in the KEGG category, suggesting that Scutellaria baicalensis intervention in Alzheimer’s disease might exert its effects through regulating signaling pathways associated with Alzheimer’s disease.
The interference of active ingredients of Scutellaria baicalensis on neurodegenerative disease has been reported.18,28,29 Our study revealed that Baicalein, Wogonin, and 5,2’-Dihydroxy-6,7,8-trimethoxyflavone in Scutellaria baicalensis also exhibit significant intervention effects on Alzheimer’s disease. Molecular docking analysis further identified Baicalein, Wogonin, and 5,2’-Dihydroxy-6,7,8-trimethoxyflavone as key players in the intervention of Alzheimer’s disease by effectively binding to SRC, PIK3R1, and STAT3. These targets are crucial for the pharmacodynamic effects exerted by Scutellaria baicalensis’ active ingredients and play pivotal roles in the pathogenesis and progression of Alzheimer’s disease. It has been reported that suppression of SRC mitigates amyloid-associated microgliosis and attenuates the inflammation response in a murine model of Alzheimer’s disease.30,31 PIK3R1 induces the deposition of tau and Aβ and disrupts the insulin signaling pathway in the brain of patients with Alzheimer’s disease.32,33 Moreover, STAT3 inhibition protects against neuroinflammation and decreases β-amyloid levels and plaque burden in an Alzheimer’s disease model.34,35 Notably, our findings showed that the active ingredients of Scutellaria baicalensis inhibit STAT3 expression by downregulating the PIK3R1/SRC pathway. Collectively, these findings imply that Scutellaria baicalensis guards against Alzheimer’s disease in vitro through the inhibition of these specific targets.
Conclusion
The present study found that active ingredients of Scutellaria baicalensis protect against Alzheimer’s disease through inhibiting the PIK3R1/SRC/STAT3 pathway in Neuro 2A cells by integrating network pharmacology, molecular docking, and experimental verification. These findings provide evidence for the potential therapeutic effect of Scutellaria baicalensis on Alzheimer’s disease, offering valuable insights for future clinical drug development.
Data Sharing Statement
The corresponding author can provide access to the datasets used and/or analyzed in the current study upon request through a reasonable means of communication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ben-Shlomo Y, Darweesh S, Llibre-Guerra J, Marras C, San Luciano M, Tanner C. The epidemiology of Parkinson’s disease. Lancet. 2024;403(10423):283–292. PubMed PMID: 38245248. doi:10.1016/s0140-6736(23)01419-8
2. Diaz-Galvan P, Przybelski SA, Algeciras-Schimnich A, et al. Plasma biomarkers of Alzheimer’s disease in the continuum of dementia with Lewy bodies. Alzheimers Dement. 2024. PubMed PMID: 38329197. doi:10.1002/alz.13653
3. Florian H, Wang D, Arnold SE, et al. Tilavonemab in early Alzheimer’s disease: results from a Phase 2, randomized, double-blind study. Brain. 2023;146(6):2275–2284. PubMed PMID: 36730056; PubMed Central PMCID: PMCPMC10232284. doi:10.1093/brain/awad024
4. Landau SM, Lee J, Murphy A, et al. Individuals with Alzheimer’s disease and low tau burden: characteristics and implications. Alzheimers Dement. 2024. PubMed PMID: 38241084. doi:10.1002/alz.13609
5. O’Leary K. Modeling real-world data to repurpose drugs for Alzheimer’s disease. Nature Med. 2024. PubMed PMID: 38238602. doi:10.1038/d41591-024-00003-7
6. Nemy M, Dyrba M, Brosseron F, et al. Cholinergic white matter pathways along the Alzheimer’s disease continuum. Brain. 2023;146(5):2075–2088. PubMed PMID: 36288546; PubMed Central PMCID: PMCPMC10151179. doi:10.1093/brain/awac385
7. Liu C, Zhang L, Li Y, Li M, Han H, Wang K. Traditional Chinese Patent Medicine in the treatment of Alzheimer’s disease: a systematic review and network meta-analysis. Am J Chin Med. 2023;51(3):517–546. PubMed PMID: 36866797. doi:10.1142/s0192415x2350026x
8. Yan H, Feng L, Li M. The role of Traditional Chinese Medicine Natural Products in β-amyloid deposition and tau protein hyperphosphorylation in Alzheimer’s disease. Drug Des Devel Ther. 2023;17:3295–3323. PubMed PMID: 38024535; PubMed Central PMCID: PMCPMC10655607. doi:10.2147/dddt.S380612
9. Lei X, Xu H, Wang Y, et al. Integrating network pharmacology and component analysis to study the potential mechanisms of Qi-Fu-Yin Decoction in Treating Alzheimer’s disease. Drug Des Devel Ther. 2023;17:2841–2858. PubMed PMID: 37727255; PubMed Central PMCID: PMCPMC10506672. doi:10.2147/dddt.S402624
10. Seo HW, Ha TY, Ko G, et al. Scutellaria baicalensis attenuated neurological impairment by regulating programmed cell death pathway in ischemic stroke mice. Cells. 2023;12(17). PubMed PMID: 37681864; PubMed Central PMCID: PMCPMC10486384. doi:10.3390/cells12172133
11. Delerue T, Fátima Barroso M, Dias-Teixeira M, Figueiredo-González M, Delerue-Matos C, Grosso C. Interactions between Ginkgo biloba L. and Scutellaria baicalensis Georgi in multicomponent mixtures towards cholinesterase inhibition and ROS scavenging. Food Res Int. 2021;140:109857. PubMed PMID: 33648175. doi:10.1016/j.foodres.2020.109857
12. Huang J, Zhang X, Yang X, et al. Baicalin exerts neuroprotective actions by regulating the Nrf2-NLRP3 axis in toxin-induced models of Parkinson’s disease. Chem Biol Interact. 2024;387:110820. PubMed PMID: 38016618. doi:10.1016/j.cbi.2023.110820
13. Liu H, Liu H, Zhou Z, et al. Scutellaria baicalensis enhances 5-fluorouracil-based chemotherapy via inhibition of proliferative signaling pathways. Cell Commun Signal. 2023;21(1):147. PubMed PMID: 37337282; PubMed Central PMCID: PMCPMC10278337. doi:10.1186/s12964-023-01156-7
14. Zhang S, Lv H, Cai X, et al. Effects of the compound extracts of Caprifoliaceae and Scutellaria baicalensis Georgi on the intestinal microbiota and antioxidant function. Front Microbiol. 2023;14:1289490. PubMed PMID: 38282732; PubMed Central PMCID: PMCPMC10822692. doi:10.3389/fmicb.2023.1289490
15. Jalili S, Panji M, Mahdavimehr M, et al. Enhancing anti-amyloidogenic properties and antioxidant effects of Scutellaria baicalensis polyphenols through novel nanoparticle formation. Int J Biol Macromol. 2024;262(Pt 1):130003. PubMed PMID: 38325696. doi:10.1016/j.ijbiomac.2024.130003
16. Gao L, Zhao JX, Qin XM, Zhao J. The ethanol extract of Scutellaria baicalensis Georgi attenuates complete Freund’s adjuvant (CFA)-induced inflammatory pain by suppression of P2X3 receptor. J Ethnopharmacol. 2023;317:116762. PubMed PMID: 37301308. doi:10.1016/j.jep.2023.116762
17. Zhang H, Liu QQ, Ding SK, Li H, Shang YZ. Flavonoids from stems and leaves of Scutellaria baicalensis Georgi improve composited Aβ-induced Alzheimer’s disease model rats’ memory and neuroplasticity disorders. Comb Chem High Throughput Screen. 2023;26(8):1519–1532. PubMed PMID: 36200197. doi:10.2174/1386207325666221003092627
18. Wang R, Shen X, Xing E, Guan L, Xin L. Scutellaria baicalensis stem-leaf total flavonoid reduces neuronal apoptosis induced by amyloid beta-peptide (25–35). Neural Regen Res. 2013;8(12):1081–1090. PubMed PMID: 25206402; PubMed Central PMCID: PMCPMC4145899. doi:10.3969/j.issn.1673-5374.2013.12.003
19. Jiashuo WU, Fangqing Z, Zhuangzhuang LI, Weiyi J, Yue S. Integration strategy of network pharmacology in Traditional Chinese Medicine: a narrative review. J Tradit Chin Med. 2022;42(3):479–486. PubMed PMID: 35610020; PubMed Central PMCID: PMCPMC9924699. doi:10.19852/j.cnki.jtcm.20220408.003
20. Zhao L, Zhang H, Li N, et al. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J Ethnopharmacol. 2023;309:116306. PubMed PMID: 36858276. doi:10.1016/j.jep.2023.116306
21. Li X, Liu Z, Liao J, Chen Q, Lu X, Fan X. Network pharmacology approaches for research of Traditional Chinese Medicines. Chin J Nat Med. 2023;21(5):323–332. PubMed PMID: 37245871. doi:10.1016/s1875-5364(23)60429-7
22. Yang WG, Sun A, Zhu R, Liu N, He WJ, Liu LL. Exploration of artemisinin against IgA nephropathy via AKT/Nrf2 pathway by bioinformatics and experimental validation. Drug Des Devel Ther. 2023;17:1679–1697. PubMed PMID: 37309415; PubMed Central PMCID: PMCPMC10257916. doi:10.2147/dddt.S403422
23. Qi D, Li H, Liang C, et al. Herb-drug interaction of Xingnaojing injection and Edaravone via pharmacokinetics, mixed inhibition of UGTs, and molecular docking. Phytomedicine. 2023;112:154696. PubMed PMID: 36764095. doi:10.1016/j.phymed.2023.154696
24. Vicente-Zurdo D, Rosales-Conrado N, León-González ME. Unravelling the in vitro and in vivo potential of selenium nanoparticles in Alzheimer’s disease: a bioanalytical review. Talanta. 2024;269:125519. PubMed PMID: 38086100. doi:10.1016/j.talanta.2023.125519
25. Li X, Mak VCY, Zhou Y, et al. Deregulated Gab2 phosphorylation mediates aberrant AKT and STAT3 signaling upon PIK3R1 loss in ovarian cancer. Nat Commun. 2019;10(1):716. PubMed PMID: 30755611; PubMed Central PMCID: PMCPMC6372715. doi:10.1038/s41467-019-08574-7
26. Xiang L, Gao Y, Chen S, Sun J, Wu J, Meng X. Therapeutic potential of Scutellaria baicalensis Georgi in lung cancer therapy. Phytomedicine. 2022;95:153727. PubMed PMID: 34535372. doi:10.1016/j.phymed.2021.153727
27. Limanaqi F, Biagioni F, Busceti CL, Polzella M, Fabrizi C, Fornai F. Potential antidepressant effects of Scutellaria baicalensis, Hericium erinaceus and Rhodiola rosea. Antioxidants. 2020;9(3). PubMed PMID: 32178272; PubMed Central PMCID: PMCPMC7139475. doi:10.3390/antiox9030234
28. Liu QQ, Ding SK, Zhang H, Shang YZ. The molecular mechanism of Scutellaria baicalensis georgi stems and leaves flavonoids in promoting neurogenesis and improving memory impairment by the PI3K-AKT-CREB signaling pathway in rats. Comb Chem High Throughput Screen. 2022;25(5):919–933. PubMed PMID: 33966617. doi:10.2174/1386207324666210506152320
29. Shengkai D, Yazhen S. Flavonoids from stems and leaves of Scutellaria baicalensis georgi regulate the brain tau hyperphosphorylation at multiple sites induced by composited Aβ in rats. CNS Neurol Disord Drug Targets. 2022;21(4):367–374. PubMed PMID: 34455972. doi:10.2174/1871527320666210827112609
30. Dhawan G, Combs CK. Inhibition of Src kinase activity attenuates amyloid associated microgliosis in a murine model of Alzheimer’s disease. J Neuroinflammation. 2012;9:117. PubMed PMID: 22673542; PubMed Central PMCID: PMCPMC3388011. doi:10.1186/1742-2094-9-117
31. Portugal CC, Almeida TO, Socodato R, Relvas JB. Src family kinases (SFKs): critical regulators of microglial homeostatic functions and neurodegeneration in Parkinson’s and Alzheimer’s diseases. FEBS J. 2022;289(24):7760–7775. PubMed PMID: 34510775. doi:10.1111/febs.16197
32. Li H, Liu H, Lutz MW, Luo S. Novel genetic variants in TP37, PIK3R1, CALM1, and PLCG2 of the neurotrophin signaling pathway are associated with the progression from mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis. 2023;91(3):977–987. PubMed PMID: 36530083; PubMed Central PMCID: PMCPMC9905310. doi:10.3233/jad-220680
33. Qian XH, Liu XL, Chen SD, Tang HD. Identification of immune hub genes associated with braak stages in Alzheimer’s disease and their correlation of immune infiltration. Front Aging Neurosci. 2022;14:887168. PubMed PMID: 35619939; PubMed Central PMCID: PMCPMC9129065. doi:10.3389/fnagi.2022.887168
34. Reichenbach N, Delekate A, Plescher M, et al. Inhibition of Stat3-mediated astrogliosis ameliorates pathology in an Alzheimer’s disease model. EMBO Mol Med. 2019;11(2). PubMed PMID: 30617153; PubMed Central PMCID: PMCPMC6365929. doi:10.15252/emmm.201809665
35. Choi M, Kim H, Yang EJ, Kim HS. Inhibition of STAT3 phosphorylation attenuates impairments in learning and memory in 5XFAD mice, an animal model of Alzheimer’s disease. J Pharmacol Sci. 2020;143(4):290–299. PubMed PMID: 32507685. doi:10.1016/j.jphs.2020.05.009
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.